Abstract
We compared survival in patients with anaplastic astrocytoma (AA) treated with adjuvant procarbazine, lomustine, and vincristine (PCV) with survival in patients treated with temozolomide. A retrospective analysis was made of patients with newly diagnosed AA treated with adjuvant postradiotherapy chemotherapy. Outcome analysis included progression-free survival and overall survival. The following prognostic factors were taken into account: patient age, extent of resection, performance status, presence of contrast enhancement in pre-surgical imaging, and type of adjuvant treatment. Among 109 AA patients, 49 were treated with PCV and 60 with temozolomide. The treatment groups were well matched for pretreatment characteristics, except for the presence of contrast enhancement. Age, extent of surgery, performance status, and presence of contrast enhancement were statistically significant prognostic factors according to the Cox model analysis of survival. Type of adjuvant chemotherapy was not a significant factor, either for progression-free survival or for overall survival. Hematological toxicity, nonhematological toxicity grades 3–4, and premature discontinuation due to toxicity were observed in 9%, 3% to 5%, and 37%, respectively, of cases in the PCV group versus 4% to 5%, 0, and 0, respectively, in the temozolomide group. Although the present study was not randomized, it was well designed, and it reports on two homogeneous and consecutive series of patients, for whom histology was verified to obtain survival data only for patients with AA following the recent WHO 2000 classification. Even if no survival advantage has been demonstrated for temozolomide versus PCV, we conclude that temozolomide should be preferred because of its greater tolerability.
Keywords: adjuvant chemotherapy, anaplastic astrocytoma, clinical trials, PCV, temozolomide
According to the 2004 report from the Central Brain Tumor Registry of the United States (CBTRUS, 2004), the incidence of primary brain tumors is estimated at 14 per 100,000 person-years; 40% of these tumors are gliomas. Novel treatment modalities, such as concomitant radiotherapy and chemotherapy, are now considered standard for glioblastomas (Stupp et al., 2005). However, as anaplastic astrocytomas (AA)3 account for 8.3% of all CNS gliomas, their low incidence makes it difficult to perform phase 3 studies and establish new standards of care. Moreover, most of the studies on AA performed in the past (Levin et al., 1990, 2003; Prados et al., 1999a, b) also included anaplastic oligodendrogliomas and anaplastic oligoastrocytomas, which have a higher chemosensitivity and better prognosis (Donahue et al., 1997), and all the studies were performed before the 2000 WHO classification was applied (Kleihues et al., 2002), thus precluding a reliable comparison of findings from studies made before with those made after the year 2000. We report findings from the analysis of large series of patients treated with different types of adjuvant chemotherapy, for whom a rereview was made on the basis of the 2000 WHO classification to provide data indicating the more promising type of therapeutic management currently available and to yield parameters for future studies.
Materials and Methods
Patient Eligibility
To be considered eligible for the present study, patients were required to participate in the two subsequent prospective trials on adjuvant treatment for AA performed in the Neuro-Oncology Unit of Padova University Hospital. As it is difficult to make a histologic diagnosis of AA, and in view of the disagreement between pathologists in >30% of samples (McDonald et al., 2005; See and Gilbert, 2004), a central pathology rereview (M.G., P.I., and C.G.) was performed to avoid the bias of subjective interpretation and interobserver variation. The diagnosis of AA was based on a very strict application of the criteria of the WHO classification (Kleihues et al., 2002). Accordingly, all cases with the following were confirmed as AA: increased cellularity, nuclear atypia, marked mitotic activity, absence of microvascular proliferation, or necrosis. The diagnosis was made on the basis of both morphological aspects and immunohistochemical staining. Microscopic features confirming a diagnosis of AA included the following: cells with irregular, angulated, dark, and hyperchromatic nuclei, often with scant and sometimes gemistocytic cytoplasm, with marked, diffuse staining that was positive for glial fibrillary acidic protein (GFAP). Marked variations were found in cellularity and mitotic activity: Lesions with low cellularity, at least one or two mitoses, or an MIB1 labeling index greater than 4% to 5%, as well as more mitotically active tumors with greater cellularity, were included. As oligodendroglioma and mixed oligoastrocytoma may exhibit sparse GFAP staining, usually because of the presence of reactive astrocytes or because of the astrocytic component of the tumor, respectively, the presence of GFAP staining does not rule out the presence of an oligodendroglioma component. Special care was therefore taken to rule out other signs, such as microgemistocytes, gliofibrillary oligodendrocytes, and protoplasmic astrocytes, all of which suggest that the tumor is of oligodendroglial origin. No widely accepted histological criteria are available for establishing the extent to which oligodendroglial elements should be present in a predominantly astrocytic lesion for it to be considered an oligoastrocytoma; an arbitrary cutoff of more than 25% of oligodendroglial elements was consequently chosen for the rereview.
All patients underwent surgery and external beam radiation therapy to limited fields (60 Gy/30 fractions) and adjuvant chemotherapy. Enrollment criteria were as follows: age, 18 years or older; Karnofsky performance status of ⩾60 on starting chemotherapy; adequate laboratory test values, with normal liver function (serum bilirubin less than 1.5-fold the upper limit of the normal range and aspartate aminotransferase or alanine aminotransferase less than three times the upper limit of the normal laboratory range, alkaline phosphatase less than twofold the upper limit of the normal laboratory value, and total bilirubin <1.5 mg/dl) and with blood urea nitrogen and serum creatinine less than 1.5-fold the upper limit of the normal laboratory range; and normal hemogram, including absolute neutrophil count of ⩾1500/mm3 and platelets of ⩾100,000/mm3. Patients with childbearing potential had to use effective contraception, and a normal level of beta human chorionic gonadotropin was required for fertile females. Patients who were pregnant or breast-feeding were considered ineligible, as were patients who had previously received any type of cytotoxic therapy, presented active infection or other uncontrolled diseases, exhibited psychiatric disturbances, or had a history of cancer other than resected nonmelanoma skin cancer or carcinoma in situ of the uterine cervix. If progression was found, the patient was taken off the study and the case followed for survival. Where appropriate, patients were offered alternative treatments. If no evidence of progression was observed during adjuvant chemotherapy, cases were followed with physical and neurological evaluations every three months for one year, and then every four months up to progression. Neuroimaging was performed at each of these follow-up examinations and/or when symptoms or clinical deterioration indicated that it was required. Progression was defined as an increase of 25% or more in enhancing tumor size on consecutive MRIs (from one scan to the next), or any new tumoral areas (Macdonald et al., 1990).
All patients signed a form giving their fully informed consent to take part in the study, which was approved by the Institutional Review Board of Padova University Hospital, Italy, and conducted according to the principles of the Declaration of Helsinki and the rules of Good Clinical Practice.
Treatment
PCV Regimen
On treatment day 1, oral lomustine (1-[2-chloroethyl]-3- cyclohexyl-1-nitrosourea; also termed CCNU) was given at a dose of 110 mg/m2. On day 8 through day 21, oral procarbazine was given at a dose of 60 mg/m2 per day. On days 8 and 29, i.v. vincristine was given at a dose of 1.4 mg/m2 (maximum total dose, 2.0 mg). Patients were treated every six weeks for one year.
Dose Modifications
CCNU was delayed for one week in cases of a leukocyte level of <2.5 × 109 per liter, polymorphonuclear leukocyte level of <1.5 × 109 per liter, or platelet level of <100 × 109 per liter. In cases of persistent leukopenia at <2.5 × 109 leukocytes per liter, neutropenia at <1.5 × 109 neutrophil cells per liter, or thrombocytopenia at <100 × 109 blood platelets per liter, treatment was delayed for a further week. Treatment was restored only when leukopenia, granulocytopenia, or thrombocytopenia levels returned to normal. If a cycle was delayed for two weeks because of hematological toxicity, CCNU and procarbazine doses were reduced by 25%. CCNU and procarbazine doses were reduced by 25% in cases of grade 3 or 4 leukopenia, granulocytopenia, or thrombocytopenia. In cases of allergic skin reaction due to procarbazine, this drug was discontinued. The vincristine dose was reduced by 50% in cases of grade 2 neurotoxicity or stopped in cases of grade 3 or greater neurotoxicity, but was neither adjusted nor delayed in cases of neutrocytopenia or thrombocytopenia.
Temozolomide Regimen
Temozolomide was administered for a maximum of one year or until unacceptable toxicity or tumor progression occurred. Patients received temozolomide (150 mg/m2 per day) for five days every four weeks in the first cycle (750 mg/m2 per cycle). In the absence of grade 3 or 4 hematologic toxicity, doses for the subsequent cycle were increased to 200 mg/m2per day for 5 days (1000 mg/m2 per cycle). Repeat cycles were administered according to the schedule only if, before the first day of the next cycle, the absolute neutrophil count was ⩾1500/μl and the platelet count was ⩾ 100,000/μl.
Dose Modifications
Patients were closely monitored for toxicity in all cycles. Hematological evaluation was repeated at days 21 and 28, whereas biochemistry was assessed only once per cycle, preferably on day 21. The subsequent cycle could be administered only if, at day 28, neutrophils were ⩾ 1500/μl and platelets ⩾100,000/μl; otherwise, chemotherapy was postponed for one or two weeks. In cases of grade 3 or greater hematological toxicity or reversible grade 3 nonhematological toxicity (except for nausea/vomiting), temozolomide was reduced by 25%. In cases in which grade 4 hematological or grade 3 nonhematological toxicity reappeared notwithstanding dose reductions or in cases of nonhematological grade 4 toxicity, chemotherapy was interrupted.
Prophylactic antiemetics were prescribed if considered necessary. Brain edema was treated by using the lowest possible dose of corticosteroids. Administration of colony-stimulating factors, allowed only for rescue from grade 4 neutropenia, was suspended as early as possible.
Off-Study Criteria
Patients were withdrawn from the study for the following reasons: (a) progressive disease, (b) unacceptable toxicity even at reduced doses of chemotherapy, (c) refusal to continue therapy, and (d) noncompliance with protocol requirements.
Statistical Analysis
Progression-free survival (PFS), PFS at six months (PFS-6), and overall survival were calculated according to the Kaplan and Meier (1958) method with S-PLUS software (MathSoft, Inc., Seattle, Wash.), and differences in progression and survival according to prognostic factors were evaluated with the log-rank test. Parameters possibly correlated with disease progression and survival were age, KPS at diagnosis, extent of surgery (biopsy vs. partial resection vs. gross total resection), and presence or absence of contrast enhancement before surgery.
Multivariate analysis with the Cox (1972) model, used to assess truly independent prognostic factors, was performed only on variables for which P < 0.05 was obtained at univariate analysis.
PFS was evaluated from the diagnosis to progression, and survival was calculated as median survival time (MST) from the diagnosis of AA to death for any reason.
Results
In the two protocols, a total of 109 patients with AA were treated with adjuvant chemotherapy, 49 patients being treated with PCV and 60 with temozolomide. The pretreatment characteristics were well balanced between treatment groups (Table 1), and the only statistically significant difference found between the two groups was for presence of contrast enhancement. The median follow-up of patients was 37 months.
Table 1.
Characteristics of patients*
| Characteristic | Total | PCV | Temozolomide | P |
|---|---|---|---|---|
| Patients | 109 | 49 (45) | 60 (55) | |
| Gender | M = 59 (54)
F = 50 (46) |
M = 29 (59)
F = 20 (41) |
M = 30 (50)
F = 30 (50) |
0.34 |
| Age** | 39.3 ± 11.16 | 38 ± 11.6 | 40.2 ± 10.7 | 0.32 |
| Range | 18–70 | 18–66 | 18–70 | |
| KPS** | 86.3 ± 10.7 | 86.7 ± 8.3 | 86 ± 12.4 | 0.67 |
| Median | 90 | 90 | 90 | |
| Range | 60–100 | 60–100 | 60–100 | |
| Extent of resection | ||||
| Biopsy | 17 (16) | 9 (18) | 8 (13) | 0.37 |
| Partial | 48 (44) | 18 (37) | 30 (50) | |
| Total | 44 (40) | 22 (45) | 22 (37) | |
| Enhancement | ||||
| Absent | 63 (58) | 35 (71.4) | 28 (46.7) | 0.01 |
| Present | 46 (42) | 14 (28.6) | 32 (53.3) | |
Abbreviation: PCV, procarbazine, CCNU, and vincristine.
Numbers in parentheses are percents.
Mean ± SD.
Progression-Free Survival
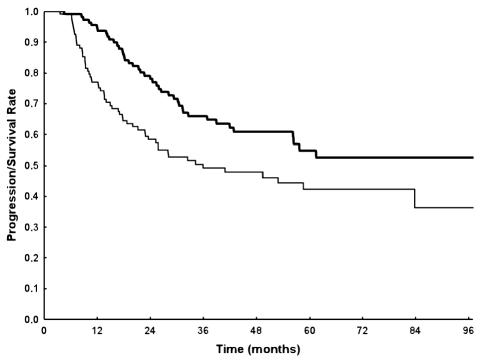
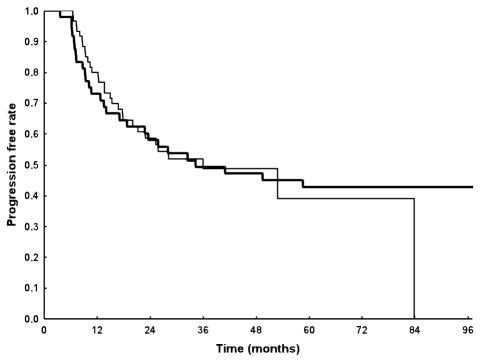
The median PFS for the entire population was 36 months (95% CI, 24 months–NR [upper limit not reached]); 58% (CI, 50%–69%) of the patients were progression free at two years and 49% (CI, 40%–60%) at three years (Fig. 1). In the PCV group, the median PFS was 34.4 months; 58% (CI, 46%–74%) and 49% (CI, 37%–66%) of patients were progression free at two and three years, respectively. In the temozolomide group, the median PFS was 36.1 months; 59% (CI, 47%–73%) and 49% (CI, 37%–65%) of patients were progression free at two and three years, respectively. According to univariate analysis, the following characteristics were significantly predictive of PFS: age (<40, 40–50, and >50 years of age; P = 0.01), KPS (<80 vs. ⩾80; P = 0.00001), contrast enhancement (present vs. absent, P = 0.02), and extent of surgery (biopsy vs. partial vs. macroscopic radical, P = 0.001). Findings from multivariate analysis confirmed that PFS was significantly correlated with age (P = 0.01), KPS (P = 0.0008), and extent of surgery (P = 0.006), but not with contrast enhancement. Type of adjuvant chemotherapy was not found to influence PFS (Fig. 2). Findings from the analysis of prognostic factors are presented in Table 2.
Fig. 1.
Progression-free survival (thin line) and overall survival (thick line) of the study series.
Fig. 2.
Progression-free survival according to treatment. Thick line: patients treated with adjuvant PCV; thin line: patients treated with adjuvant temozolomide.
Table 2.
Median time to progression, in months, based on prognostic factors
| Treatment | Prognostic Factor (CI) | ||
|---|---|---|---|
| Patient Age | |||
| <40 | 40–50 | >50 | |
| Temozolomide | |||
| Number of patients | 30 | 20 | 10 |
| TTP (CI) | 84 (53–NA) | 18 (15–NA) | 10 (7–NA) |
| PCV | |||
| Number of patients | 31 | 8 | 9 |
| TTP (CI) | 59 (19–NA) | 74 (34–NA) | 14 (11–NA) |
| Total | |||
| Number of patients | 61 | 28 | 19 |
| TTP (CI) | 84 (41–NA) | 25 (17–NA) | 12 (9–NA) |
| KPS | ||
|---|---|---|
| <80 | ⩾80 | |
| Temozolomide | ||
| Number of patients | 9 | 51 |
| TTP (CI) | 14 (8–NA) | 52 (28–NA) |
| PCV | ||
| Number of patients | 5 | 43 |
| TTP (CI) | 7 (2–NA) | 59 (26–NA) |
| Total | ||
| Number of patients | 14 | 94 |
| TTP (CI) | 8 (7–21) | 59 (34–NA) |
| Enhancement | ||
|---|---|---|
| Absent | Present | |
| Temozolomide | ||
| Number of patients | 28 | 32 |
| TTP (CI) | 53 (28–NA) | 23 (15–NA) |
| PCV | ||
| Number of patients | 34 | 14 |
| TTP (CI) | 99 (26–NA) | 13 (10–NA) |
| Total | ||
| Number of patients | 62 | 46 |
| TTP (CI) | 99 (34–NA) | 19 (14–NA) |
| Extent of surgery | |||
|---|---|---|---|
| Biopsy | Partial Resection | Total | |
| Temozolomide | |||
| Number of patients | 8 | 30 | 22 |
| TTP (CI) | 12 (9–NA) | 26 (18–NA) | 53 (53–NA) |
| PCV | |||
| Number of patients | 9 | 17 | 22 |
| TTP (CI) | 10 (9–NA) | 28 (14–NA) | 99 (41–NA) |
| Total | |||
| Number of patients | 17 | 47 | 44 |
| TTP (CI) | 10 (9–NA) | 26 (19–NA) | 84 (53–NA) |
Abbreviations: CI, 95% confidence interval; NA, not achieved; PCV, procarbazine, CCNU, and vincristine; TTP, time to progression.
Survival
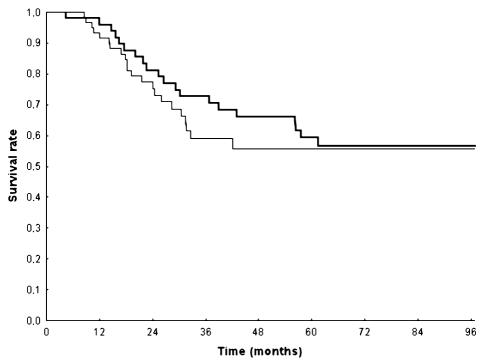
Median survival has not yet been obtained; 78% (CI, 70%–86%) of patients were alive at two years and 66% (CI, 57%–76%) at three years (Fig. 1). In the PCV group, the MST has not yet been reached, and 83% (CI, 73%–94%) and 74% (CI, 63%–88%) of patients were alive at two and three years, respectively. In the temozolomide group, the MST has not yet been attained; 75% (CI, 65%–87%) and 59% (CI, 47%–75%) of patients were alive at two and three years, respectively.
Univariate analysis indicated that age (<40, 40–50, and >50 years of age; P = 0.002), KPS (<80 vs. ⩾80; P = 0.0001), presence of contrast enhancement (P = 0.0001), and extent of surgery (biopsy vs. partial resection vs. macroscopic radical; P = 0.0002) were significantly correlated to overall survival. Multivariate analysis confirmed that age (P = 0.03), KPS (P = 0.0003), presence of contrast enhancement (P = 0.004), and extent of surgery (P = 0.0003) were independent predictors of survival. No correlation was found between type of chemotherapy regimen administered and survival (Fig. 3). Findings from the analysis of prognostic factors are presented in Table 3.
Fig. 3.
Survival according to treatment. Thick line: patients treated with adjuvant PCV; thin line: patients treated with adjuvant temozolomide.
Table 3.
Median survival, in months, according to prognostic factors
| Treatment | Prognostic Factor (CI*) | ||
|---|---|---|---|
| Patient Age | |||
| <40 | 40–50 | >50 | |
| Temozolomide | |||
| Number of patients | 30 | 20 | 10 |
| MST (CI) | NA | 31 (26–NA) | 16 (12–NA) |
| PCV | |||
| Number of patients | 32 | 8 | 9 |
| MST (CI) | NA (56–NA) | NA (61–NA) | 28 (25–NA) |
| Total | |||
| Number of patients | 62 | 28 | 19 |
| MST (CI) | NA (58–NA) | 61 (30–NA) | 25 (15–NA) |
| KPS | ||
|---|---|---|
| <80 | ⩾80 | |
| Temozolomide | ||
| Number of patients | 9 | 51 |
| MST (CI) | 18 (10–NA) | NA |
| PCV | ||
| Number of patients | 5 | 44 |
| MST (CI) | 17 (15–NA) | NA (61–NA) |
| Total | ||
| Number of patients | 14 | 95 |
| MST (CI) | 17 (15–26) | NA |
| Enhancement | ||
|---|---|---|
| Absent | Present | |
| Temozolomide | ||
| Number of patients | 28 | 32 |
| MST (CI) | NA | 31 (24–NA) |
| PCV | ||
| Number of patients | 35 | 14 |
| MST (CI) | NA | 30 (33–NA) |
| Total | ||
| Number of patients | 63 | 46 |
| MST (CI) | NA | 30 (26–61) |
| Extent of surgery | |||
|---|---|---|---|
| Biopsy | Subtotal | Total | |
| Temozolomide | |||
| Number of patients | 8 | 30 | 22 |
| MST (CI) | 14 (11–NA) | 42 (26–NA) | NA |
| PCV | |||
| Number of patients | 9 | 18 | 22 |
| MST (CI) | 39 (26–NA) | 60 (30–NA) | NA |
| Total | |||
| Number of patients | 17 | 48 | 44 |
| MST (CI) | 26 (14–NA) | 56 (30–NA) | NA |
Abbreviations: CI, 95% confidence interval; MST, median survival time; NA, not achieved; PCV, procarbazine, CCNU, and vincristine.
Toxicity
All toxicities were recorded and graded according to Common Terminology Criteria for Adverse Events, version 3.0 (http://ctep.cancer.gov/forms/CTCAEv3.pdf).
There were neither drug-related deaths nor life-threatening events. A total of 161 cycles of PCV were administered (median, 3 cycles; range, 1–6 cycles). The most frequent grade 3/4 toxicity was hematological, observed in 9% of the cycles. Nonhematological grade 3/4 toxicity was mainly neurological (3% of cycles) or hepatic (5% of cycles). Procarbazine was withheld because of a skin allergy in three patients. Vincristine was reduced to 50% because of neurological toxicity in 15 patients (31.9%). Procarbazine and lomustine dosages were reduced by 25% in 18% of the cycles, respectively. Adjuvant chemotherapy was interrupted for prolonged hematological and nonhematological toxicity in 37% of the patients.
A total of 642 cycles of temozolomide were administered (median, 10 cycles; range, 3–12 cycles). The toxicity was mainly hematological, with grade 3/4 leukopenia reported in 4% cycles and thrombocytopenia grade 3/4 in 5% of cycles. Twenty-two cycles (3.4%) were reduced consequent to hematological toxicity. No patients interrupted adjuvant chemotherapy because of toxicity.
Discussion
Randomized, controlled trials and meta-analyses have demonstrated that chemotherapy is of value in the management of patients with malignant glioma (Fine et al., 1993; Stewart, 2002; Stupp et al., 2005). However, evidence for the subgroup of patients with AA is weak. Adjuvant PCV after radiation and surgery has been widely used in the treatment of AA (Levin et al., 1990, 2003; Prados et al., 1999a, b), although no randomized trial on a large number of patients has demonstrated the advantage of PCV over single-agent BCNU in patients with anaplastic gliomas. In 1999, a retrospective study (Prados et al., 1999a), comparing (1) data from 257 AA patients (11% with an oligodendroglial component) from three Radiation Therapy Oncology Group (RTOG) studies who had been treated with BCNU adjuvant to radiotherapy with (2) data from 175 AA patients (23% with an oligodendroglial component) from the RTOG 9404 study who had been treated with adjuvant PCV, found that survival outcomes in the two treatment arms were similar. In recent years, temozolomide has been used as standard therapy for patients with recurrent anaplastic glioma because temozolomide has a better toxicity profile and is considered comparable to the nitrosourea regimen (Dinnes et al., 2002; Yung et al., 1999). In 1998, the RTOG started a randomized trial comparing temozolomide with BCNU in an adjuvant setting, but the study is still ongoing (protocol 9813 [http://www.rtog.org]).
In view of the paucity of data on patients with this rare disease, the main aim of the present study was to determine whether temozolomide has advantages over PCV in this subset of patients. Although the study was not randomized, it was made on two consecutive cohorts of patients that were homogeneous for histology, prognostic characteristics, and radiotherapy. Ideally, decisions regarding adjuvant chemotherapy should be based on findings made in well-designed, appropriately sized, prospective phase 3 studies. However, it may prove difficult to accrue patients for this type of study because of physician or patient bias, temozolomide being preferred because of its greater tolerability. Although the present study was not randomized, it was well designed, and it reports on two homogeneous and consecutive series of patients in whom histology was verified to obtain survival data only for patients with AA according to the recent WHO 2000 classification; it may therefore be a useful basis for future studies on this disease. The process of diagnosing and grading gliomas is difficult and somewhat subjective; to avoid any bias from subjective interpretation, we performed a centralized review. Despite our strict application of histologic criteria proposed in the 2000 WHO classification, we cannot rule out that patients who, elsewhere, might have been classified as having low-grade astrocytoma, oligoastrocytoma, or glioblastoma may have been included in the present series. In general, diagnostic reliability is further decreased in cases for which histologic material is limited, because AA frequently evolves from a less malignant precursor lesion and may transform into glioblastoma. Accordingly, areas with different tumor grade may coexist in the same mass, thus increasing the risk of sampling errors.
The favorable survival figures observed in our series may raise the question of whether patients with low-grade tumors were also included. It should therefore be emphasized that survival reported in the present study is only slightly better than survival reported in other recent studies in the literature (See and Gilbert, 2004), and that most patients in our series were in recursive partitioning analysis class I or II, with an estimated median survival of almost five years and three years, respectively (Curran et al., 1993). On the other hand, the use of the 2000 WHO classification (Kleihues et al., 2002), which allowed the identification of the presence of microvascular proliferation without necrosis as an adequate criterion for upgrading a grade III astrocytoma to grade IV (glioblastoma multiforme), makes any comparison with historical controls (Curran et al., 1993) particularly troublesome. Therefore, an apparent improvement in survival figures of patients with AA diagnosed according to the 2000 WHO classification criteria may result from a differently defined population rather than from misdiagnosis, and this should be taken into account when assessing the activity of novel chemotherapy regimens.
In the present study, a large percentage (58%) of non-enhancing tumors was observed. AA has been considered a typically enhancing tumor (Buckner, 2003), and the presence of contrast enhancement in our study was less frequent than in studies of patients with AA classified before 2000. The degree of enhancement, which is correlated with the biological and clinical behavior of the tumor, increases proportionally with the degree of anaplasia. Again, this difference may reflect disagreement between neuropathologists and the limited reproducibility of neuropathological criteria for accurately classifying gliomas. In fact, the cellularity and mitotic activity of AA varied considerably in our series. Furthermore, the upgrading of diagnosis to glioblastoma for patients with microvascular proliferation, according to 2000 WHO classification criteria, excludes a group of patients with a more aggressive tumor that typically presents contrast enhancement.
The presence of contrast enhancement was significantly greater in the temozolomide-treated group than in the PCV-treated group (53% vs. 29%; P = 0.01). This difference may have masked an advantage of temozolomide treatment because the presence of enhancement, considered the expression of a greater aggressiveness, can influence survival. In patients without enhancement, median survival has not yet been reached, whereas in patients with enhancement, the median survival was 30.5 months. However, it is unlikely that the presence of enhancement could have masked an advantage of temozolomide over PCV because MST was 31 months in the 32 temozolomide-treated patients with enhancement and 30 months in the 14 PCV-treated patients with enhancement, no significant difference being found between the groups for this parameter.
Age and performance status were confirmed as highly significant prognostic factors, in agreement with other reports (Buckner, 2003). Extent of resection is still a widely debated issue. On analyzing the outcomes of a mixed group of 85 patients with anaplastic glioma (66 AA and 19 anaplastic oligoastrocytoma) treated between 1993 and 1999, Tortosa et al. (2003) found no significant difference between the survival of patients who underwent gross complete resection and that of patients who underwent partial resection or biopsy. A recent interim report (Laws et al., 2003) on a series of 147 patients with grade III gliomas showed that patients who underwent resection had a consistent survival advantage with respect to patients that received biopsy only (MST, 87 weeks vs. 52 weeks). In another series consisting of a more homogeneous group of 78 AA patients treated between 1993 and 2000, Cohen et al. (2002) found that extent of tumor resection, based on T2 volume, was an independent predictor of increased survival. Our findings confirm that extent of surgery has a statistically significant impact on both PFS and overall survival. High-grade gliomas can present as mixed grade III and IV tumors, and the risk of identifying a mass as AA when it also contains areas of glioblastoma is inversely correlated with the amount of histologic material available. A diagnosis based on biopsy or on partial removal is thus less reliable than a diagnosis based on an entire surgical specimen. In our series, biopsies or partial removals were more frequent in the temozolomide-treated group than in the PCV-treated group, suggesting that the effect of temozolomide may have been masked by a higher rate of misdiagnosed glioblastoma multiforme. However, we consider this unlikely, because the difference between the two groups was slight (biopsy and partial removal, 63% vs. 55%) and was not statistically significant (P = 0.38), and it may simply have been related to a less subjective evaluation of the extent of removal in the recently treated group of patients (e.g., more frequent use of postoperative neuroimaging to assess residual disease). In our study, no significant difference was observed either in terms of progression or in terms of survival between PCV and temozolomide treatment. However, temozolomide should now always be considered first-line therapy for this disease in view of temozolomide’s greater tolerability. In fact, in our series, grade 3/4 hematological toxicity was 9%, and discontinuation of treatment was due to failure to return to hematological normal levels, which occurred in 37% in the PCV group, whereas these rates were 4% to 5% (hematological toxicity) and 0 (premature discontinuation) in the temozolomide group. The two-year survival rate of 78% to 83% observed in the present trial is higher than the 25% to 31% previously reported for patients with AA undergoing radiotherapy alone (MRC, 2001). Although the aforementioned considerations concerning changes in the WHO classification and selection bias could in part explain this large difference, in the absence of large randomized trials addressing this issue, our data seem to further support the addition of chemotherapy to radiotherapy as first-line treatment for patients with anaplastic astrocytoma.
Although our understanding of anaplastic astrocytoma has greatly improved, the prognosis for patients with the disease is still poor, and there is a need for large, cooperative, and randomized trials using concomitant treatment and/or new schedules. Ideally, uncertainties concerning histological classification will be overcome by a classification based on genetic profile (such as 1p/19q loss) predicting the prognosis and the chemora-diosensitivity of the tumors, thus allowing better tailoring of treatment choices.
Acknowledgments
The authors are grateful to Gruppo Italiano Cooperativo di Neuro-Oncologia for its support and to Dr. Valeria Blatt for her help in manuscript preparation.
Footnotes
Abbreviations used are as follows: AA, anaplastic astrocytoma; CCNU, 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (lomustine); MST, median survival time; PCV, procarbazine, CCNU, and vincristine; GFAP, glial fibrillary acidic protein; MST, median survival time; PFS, progression-free survival; PFS-6, progression-free survival at six months; RTOG, Radiation Therapy Oncology Group.
References
- Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol. 2003;30:10–14. doi: 10.1053/j.seminoncol.2003.11.031. [DOI] [PubMed] [Google Scholar]
- CBTRUS, Central Brain Tumor Registry of the United States (2004) Primary Brain Tumors in the United States: Statistical Report, 1997–2001 Available at http://www.cbtrus.org/2004-2005/2004-2005.html
- Cohen ZR, Suki D, Shi W, Weinberg JS, DeMonte F, McCutcheon IE, Hassenbusch SJ, Gokaslan ZL, Rhines LD, Sawaya R, Lang FF. Surgical resection of anaplastic astrocytoma: Prognostic factors and outcome. Neuro-Oncology. 2002;4:367. (abstract 216) [Google Scholar]
- Cox D. Regression models and life-tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- Curran WJ, Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, Chang CH, Rotman M, Asbell SO, Krisch RE, Nelson DF. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- Dinnes J, Cave C, Huang S, Milne R. A rapid and systematic review of the effectiveness of temozolomide for the treatment of recurrent malignant glioma. Br J Cancer. 2002;86:501–505. doi: 10.1038/sj.bjc.6600135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue B, Scott CB, Nelson JS, Rotman M, Murray KJ, Nelson DF, Banker FL, Earle JD, Fischbach JA, Asbell SO, Gaspar LE, Markoe AM, Curran W. Influence of an oligodendroglial component on the survival of patients with anaplastic astrocytomas: A report of Radiation Therapy Oncology Group 83-02. Int J Radiat Oncol Biol Phys. 1997;38:911–914. doi: 10.1016/s0360-3016(97)00126-0. [DOI] [PubMed] [Google Scholar]
- Fine HA, Dear KB, Loeffler JS, Black PM, Canellos GP. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71:2585–2597. doi: 10.1002/1097-0142(19930415)71:8<2585::aid-cncr2820710825>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. [DOI] [PubMed] [Google Scholar]
- Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A, Lillehei KO, Bernstein M, Brem H, Sloan A, Berger MS, Chang S. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: Data from the Glioma Outcomes Project. J Neurosurg. 2003;99:467–473. doi: 10.3171/jns.2003.99.3.0467. [DOI] [PubMed] [Google Scholar]
- Levin VA, Silver P, Hannigan J, Wara WM, Gutin PH, Davis RL, Wilson CB. Superiority of post-radiotherapy adjuvant chemotherapy with CCNU, procarbazine, and vincristine (PCV) over BCNU for anaplastic gliomas: NCOG 6G61 final report. Int J Radiat Oncol Biol Phys. 1990;18:321–324. doi: 10.1016/0360-3016(90)90096-3. [DOI] [PubMed] [Google Scholar]
- Levin VA, Hess KR, Choucair A, Flynn PJ, Jaeckle KA, Kyritsis AP, Yung WK, Prados MD, Bruner JM, Ictech S, Gleason MJ, Kim HW. Phase III randomized study of postradiotherapy chemotherapy with combination alpha-difluoromethylornithine-PCV versus PCV for anaplastic gliomas. Clin Cancer Res. 2003;9:981–990. [PubMed] [Google Scholar]
- Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- McDonald JM, See SJ, Tremont IW, Colman H, Gilbert MR, Groves M, Burger PC, Louis DN, Giannini C, Fuller G, Passe S, Blair H, Jenkins RB, Yang H, Ledoux A, Aaron J, Tipnis U, Zhang W, Hess K, Aldape K. The prognostic impact of histology and 1p/19q status in anaplastic oligodendroglial tumors. Cancer. 2005;104:1468–1477. doi: 10.1002/cncr.21338. [DOI] [PubMed] [Google Scholar]
- MRC Medical Research Council. Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: A Medical Research Council trial. J Clin Oncol. 2001;19:509–518. doi: 10.1200/JCO.2001.19.2.509. [DOI] [PubMed] [Google Scholar]
- Prados MD, Scott C, Curran WJ, Jr, Nelson DF, Leibel S, Kramer S. Procarbazine, lomustine, and vincristine (PCV) chemotherapy for anaplastic astrocytoma: A retrospective review of Radiation Therapy Oncology Group protocols comparing survival with carmustine or PCV adjuvant chemotherapy. J Clin Oncol. 1999a;17:3389–3395. doi: 10.1200/JCO.1999.17.11.3389. [DOI] [PubMed] [Google Scholar]
- Prados MD, Scott C, Sandler H, Buckner JC, Phillips T, Schultz C, Urtasun R, Davis R, Gutin P, Cascino TL, Greenberg HS, Curran WJ., Jr A phase 3 randomized study of radiotherapy plus procarbazine, CCNU, and vincristine (PCV) with or without BUdR for the treatment of anaplastic astrocytoma: A preliminary report of RTOG 9404. Int J Radiat Oncol Biol Phys. 1999b;45:1109–1115. doi: 10.1016/s0360-3016(99)00265-5. [DOI] [PubMed] [Google Scholar]
- See SJ, Gilbert MR. Anaplastic astrocytoma: Diagnosis, prognosis, and management. Semin Oncol. 2004;31:618–634. doi: 10.1053/j.seminoncol.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Stewart LA. Chemotherapy in adult high-grade glioma: A systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Tortosa A, Vinolas N, Villa S, Verger E, Gil JM, Brell M, Caral L, Pujol T, Acebes JJ, Ribalta T, Ferrer I, Graus F. Prognostic implication of clinical, radiologic, and pathologic features in patients with anaplastic gliomas. Cancer. 2003;97:1063–1071. doi: 10.1002/cncr.11120. [DOI] [PubMed] [Google Scholar]
- Yung WK, Prados MD, Yaya-Tur R, Rosenfeld SS, Brada M, Friedman HS, Albright R, Olson J, Chang SM, O’Neill AM, Friedman AH, Bruner J, Yue N, Dugan M, Zaknoen S, Levin VA. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group (erratum in J. Clin. Oncol. [1999] 17, 3693). J. Clin. Oncol. 1999;17:2762–2771. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]