Abstract
The emergence of temozolomide as an effective alkylating agent with little acute toxicity or cumulative myelosuppression has led to protracted courses of chemotherapy for many patients with gliomas. Secondary, or treatment-related, myelodysplasia (t-MDS) and acute myelogenous leukemia (t-AML) are life-threatening complications of alkylating chemotherapy and have been reported in patients with primary brain tumors. We describe a case of temozolomide-related t-MDS/AML and discuss the clinical features of this condition. Administration of an alkylating agent in patient populations with long median survivals must be undertaken with an understanding of the potential for this treatment complication.
Keywords: brain neoplasm, glioblastoma, myelodysplastic syndrome, recurrent glioma, secondary leukemia, secondary myelodysplastic syndrome, temozolomide, treatment complication
Temozolomide is an orally administered analog of dacarbazine whose activity is mediated primarily via DNA methylation at the O6 position of guanine (Brada et al., 1999). It is relatively well tolerated and is increasingly administered in clinical studies over prolonged periods to patients with gliomas and several other malignancies (Grewal et al., 2005; Stupp et al., 2005). Myelodysplastic syndrome is a clonal hematopoietic disorder that may be caused by cytotoxic chemotherapy and radiation therapy (RT).3 We report a second case of treatment-related myelodysplasia (t-MDS) and treatment-related acute myelogenous leukemia (t-AML) in a patient who received temozolomide, and we review the clinical picture of t-MDS/AML.
Case Study
A 66-year-old woman developed mental confusion and was found to have a right temporoparietal brain mass in 2000. Two years before, she had been treated with lumpectomy and 6120 cGy of adjuvant RT for early-stage breast cancer. Subtotal resection of the temporal lobe mass revealed anaplastic oligodendroglioma. She received adjuvant involved-field irradiation to 6000 cGy, followed by one cycle of adjuvant chemotherapy with PCV (procarbazine, CCNU [lomustine: 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea], and vincristine): lomustine, 110 mg/m2 orally on day 1; procarbazine, 60 mg/m2 orally on days 8–21; and vincristine, 1.4 mg/m2, capped at 2 mg, intravenously on days 8 and 29). She developed excessive myelosuppression during the first cycle, and further PCV chemotherapy was withheld. Disease progression was detected six months after initial diagnosis, and the mass was again resected. Carmustine-impregnated wafers were placed in the resection cavity. She was started on temozolomide at 150 mg/m2 daily for 5 days, every 28 days. After 22 cycles, her platelet count decreased to a nadir of 55,000/μl (normal range, 140,000–400,000/μl), and the white blood cell count and hematocrit remained normal, although her erythrocyte mean corpuscular volume was elevated at 109 fl (normal, 81–91 fl). Temozolomide was withheld until the platelet count had normalized and then was restarted with a treatment interval of every four months. Prior to cycle 26, she was found to be pancytopenic with the appearance of nucleated red cells, schistocytes, poi-kilocytosis, and polychromasia on a peripheral blood smear. A bone marrow biopsy in August 2004 revealed a hypocellular marrow with relative erythroid hyper-plasia, marked megaloblastic and dysplastic changes, a left-shifted myeloid series, and scattered micromega-karyocytes, consistent with myelodysplasia. Flow cytometry revealed an abnormal CD11b/CD16/CD13/CD10 myeloid maturation pattern with an abnormal population of CD71-negative, glycophorin-positive erythroid cells, also consistent with myelodysplasia. Less than 5% of the marrow elements were CD34+ myeloblasts. Multiple complex cytogenetic abnormalities were noted, including deletion of chromosomes 1, 5, 6, 7, 11, and 16; rearrangement of chromosomes 3, 9, 11, and 12; and the presence of one or two unidentified marker chromosomes. These findings were suggestive of alkylating-agent-induced t-MDS. She was treated with induction chemotherapy consisting of idarubicin and cytarabine. She progressed to refractory anemia with excess blasts, and subsequently to t-AML. Various salvage chemotherapy regimens were administered, including FLAG (fludarabine, high-dose cytarabine and granulocyte colony-stimulating factor), mitoxantrone with etoposide, and gemtuzumab. She succumbed to t-AML 13 months after diagnosis of t-MDS.
Discussion
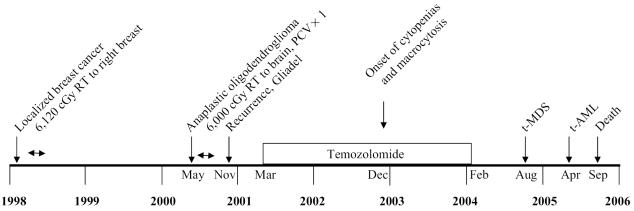
We report the occurrence of t-MDS/AML in a patient with localized breast cancer and malignant glioma after external beam radiation therapy for both tumors, one cycle of PCV, and 25 cycles of temozolomide chemotherapy. The time course of events is depicted in Fig. 1. We believe that procarbazine and lomustine contributed little to the accumulation of alkylator-related DNA damage, as only one cycle was administered, although the unusually severe myelosuppression following the single cycle of PCV may have reflected a predisposition to the myelotoxic effects of chemotherapy. In addition, RT for her breast cancer and brain tumor may have played a role, although the potential contribution of RT to the induction of t-MDS/AML is controversial. The individual contribution of each treatment modality remains undetermined in this patient, although the exposure to temozolomide may have played a role in the development of t-MDS/AML.
Fig. 1.
Time line of events from initial diagnosis of breast cancer to the onset of acute leukemia. Months are shown for key dates.
The paucity of t-MDS/AML cases in recipients of temozolomide chemotherapy is likely related to the relatively recent introduction of the agent and the poor prognosis associated with malignant gliomas. Temozolomide is also used for patients with anaplastic oligodendrogliomas and is increasingly used for the treatment of patients with clinically or radiographically progressive low-grade gliomas (Grewal et al., 2005; Hoang-Xuan et al., 2004). Both of these tumors can be associated with survival of several years. Although the relative value of the duration of various treatment regimens is not well understood (e.g., six months vs. up to two years), the median time to best response in patients with low-grade oligodendrogliomas is 12 months (Hoang-Xuan et al., 2004), and there is a tendency to administer protracted courses of therapy when patients achieve stable disease and there is minimal short-term toxicity. With the increasing use of temozolomide in these patients, more cases of t-MDS/AML will likely emerge.
t-MDS/AML has been described following therapy of primary brain tumors with classic alkylating agents, including nitrosoureas or procarbazine, RT alone, or combined chemotherapy and RT (Genot et al., 1983; Perry et al., 1998). In a review including 28 case reports of patients with primary brain tumors who developed t-AML, 15 were adults with a median age of 30 years (range, 23–59 years), and all received a nitrosourea. The median latency between the start of therapy and the diagnosis of t-AML was 2.5 years (range, 8 months to 4.5 years) (Perry et al., 1998). The only prior case report of temozolomide-associated MDS described a 44-year-old woman with recurrent anaplastic astrocytoma who developed t-MDS eight months after starting temozolomide (Su et al., 2005). However, this patient had previously received four cycles of nimustine.
MDS and AML are related clonal stem cell disorders that occur sporadically in older adults (80% of cases) and iatrogenically (20% of cases) in patients treated with alkylating agents, topoisomerase II inhibitors (e.g., epipodophyllotoxins), or RT. In 75% of cases, patients develop t-MDS, and disease then progresses over a median interval of four months to t-AML (Smith et al., 2003). t-AML is diagnosed when myeloblasts exceed 20% (Harris et al., 1999). Cytogenetic changes involving deletions of chromosomes 5 and 7 are present in 85% of cases with alkylating agent exposure, although several subsets of genetic changes are known (Pedersen- Bjergaard, 2005; Smith et al., 2003). The target of the chromosome 5 deletion is not known, but there is a strong correlation between it and inactivating mutations of the TP53 gene. The target of the chromosome 7 deletion is inactivation of the AML1 tumor suppressor gene. Topoisomerase type II inhibitors, such as etoposide, produce t-MDS/AML with balanced rearrangements involving chromosome 11.
The development of t-MDS/AML is related to the specific DNA-damaging agent, dose, therapy duration, and patient age. Alkylating agents produce t-MDS/AML with a latency of several years (median, 55 months) following exposure, and the risk rises with increasing age (Smith et al., 2003). As already noted, it appears that patients with primary brain tumors treated with nitro-soureas may develop t-MDS/AML after a shorter latency period, which suggests the possibility of a synergistic effect with RT or a unique property of these alkylating agents. There are no known factors other than age and duration of therapy to predict which patients might be at higher risk of t-MDS/AML.
The risk of t-MDS is low but not negligible. In clinical trials of alkylating therapy, the rate has been 0.25% to 1% per year beginning two years after the start of therapy and decreasing seven years after the end of therapy (Pedersen-Bjergaard, 2005). Chronic oral alkylating therapy for Hodgkin’s disease produced a 13% incidence of t-MDS/AML (Pedersen-Bjergaard et al., 1987).
The clinical features of t-MDS/AML are a result of bone marrow failure. Symptomatic anemia is the most common presentation, but easy bruising and repeated infections may also be prominent. The complete blood count reveals persistent or worsening pancytopenia. An elevated mean corpuscular volume is common, but this finding is also seen during chemotherapy without t-MDS/AML. Bone marrow aspiration and biopsy are performed to confirm the clinical suspicion of t-MDS/AML.
Patients who develop t-MDS/AML are treated with supportive care, including growth factor support, transfusion of blood products, and administration of antibiotics. 5-Azacytidine is approved for the treatment of MDS, and thalidomide can reduce transfusion requirements in a subset of patients with primary MDS. Primary treatment is marrow ablative chemotherapy followed by allogeneic bone marrow transplant (Ballen et al., 1997; Rogers et al., 2001). Studies of transplantation suggest a 20%–40% chance of long-term, disease-free survival. Options for patients without matched related donors include a matched volunteer donor, cord blood transplantation (Ballen, 2005), or haploidentical (i.e., mismatched family member) transplant. New approaches to treatment include decitabine; lenalidomide (Revlimid; Celgene, Summit, N.J.), an immunomodulatory relative of thalidomide; PTK787, an oral VEGF (vascular endo-thelial growth factor) tyrosine kinase inhibitor; and the proteasome inhibitor bortezomib. Despite these interventions, the median survival is nine months for patients with t-MDS and seven months for those with t-AML. Patients with chromosome 5 and 7 abnormalities have a worse prognosis than do those without this finding.
Conclusion
Protracted administration of an alkylating agent must be undertaken with an understanding of the risk of long-term treatment complications. This is most relevant for patients with 1p-deleted anaplastic oligodendrogliomas and low-grade gliomas whose tumors may be stable over several years. There is variation in the risk of t-MDS/AML based on the specific DNA-damaging agent, and at present no accurate information is available on the incidence with temozolomide. Many brain tumor patients also receive radiation and nitrosoureas, which makes it difficult to determine the contribution of a single agent. Until the specific risk is better understood, however, consideration of the duration of therapy, particularly in older patients, will be important factors in neuro-oncology practice.
Footnotes
This work was supported in part by the MGH Brain Tumor Research Fund.
Abbreviations used are as follows: CCNU, lomustine: 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea; PCV, procarbazine, CCNU (lomustine), and vincristine; RT, radiation therapy; t-AML, treatment-related acute myelogenous leukemia; t-MDS, treatment-related myelodysplasia.
References
- Ballen KK. New trends in umbilical cord blood transplantation. Blood. 2005;105:3786–3792. doi: 10.1182/blood-2004-10-4125. [DOI] [PubMed] [Google Scholar]
- Ballen KK, Gilliland DG, Guinan EC, Hsieh CC, Parsons SK, Rimm IJ, Ferrara JL, Bierer BE, Weinstein HJ, Antin JH. Bone marrow transplantation for therapy-related myelodysplasia: Comparison with primary myelodysplasia. Bone Marrow Transplant. 1997;20:737–743. doi: 10.1038/sj.bmt.1700971. [DOI] [PubMed] [Google Scholar]
- Brada M, Judson I, Beale P, Moore S, Reidenberg P, Statkevich P, Dugan M, Batra V, Cutler D. Phase I dose-escalation and pharmacokinetic study of temozolomide (SCH 52365) for refractory or relapsing malignancies. Br J Cancer. 1999;81:1022–1030. doi: 10.1038/sj.bjc.6690802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genot JY, Krulik M, Poisson M, van Efferterre R, Renoux M, Audebert AA, Canuel C, Smadja N, Debray J. Two cases of acute leukemia following treatment of malignant glioma. Cancer. 1983;52:222–226. doi: 10.1002/1097-0142(19830715)52:2<222::aid-cncr2820520207>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Grewal J, Nestor V, Fink K. Long term use of monthly temozolomide in patients with oligodendroglioma: Feasibility and tolerability of two years of therapy. Neurology. 2005;64:A25. (abstract) [Google Scholar]
- Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November 1997. J. Clin. Oncol. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- Hoang-Xuan K, Capelle L, Kujas M, Taillibert S, Duffau H, Lejeune J, Polivka M, Crinière E, Marie Y, Mokhtari K, Carpentier AF, Laigle F, Simon JM, Cornu P, Broët P, Sanson M, Delattre JY. Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol. 2004;22:3133–3138. doi: 10.1200/JCO.2004.10.169. [DOI] [PubMed] [Google Scholar]
- Pedersen-Bjergaard J. Insights into leukemogenesis from therapy-related leukemia. N Engl J Med. 2005;352:1591–1594. doi: 10.1056/NEJMe048336. [DOI] [PubMed] [Google Scholar]
- Pedersen-Bjergaard J, Specht L, Larsen SO, Ersboll J, Struck J, Hansen MM, Hansen HH, Nissen NI. Risk of therapy-related leukaemia and preleukaemia after Hodgkin’s disease. Relation to age, cumulative dose of alkylating agents, and time from chemotherapy. Lancet. 1987;2:83–88. doi: 10.1016/s0140-6736(87)92744-9. [DOI] [PubMed] [Google Scholar]
- Perry JR, Brown MT, Gockerman JP. Acute leukemia following treatment of malignant glioma. J Neurooncol. 1998;40:39–46. doi: 10.1023/a:1006175831785. [DOI] [PubMed] [Google Scholar]
- Rogers LR, Janakiraman N, Kasten-Sportes C, Rosenblum ML. Therapy-related myelodysplastic syndrome (t-MDS) in a patient with anaplastic astrocytoma: Successful treatment with allogeneic bone marrow transplant. J Neurooncol. 2001;53:55–59. doi: 10.1023/a:1011878214861. [DOI] [PubMed] [Google Scholar]
- Smith SM, Le Beau MM, Huo D, Karrison T, Sobecks RM, Anastasi J, Vardiman JW, Rowley JD, Larson RA. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: The University of Chicago series. Blood. 2003;102:43–52. doi: 10.1182/blood-2002-11-3343. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Su YW, Chang MC, Chiang MF, Hsieh RK. Treatment-related myelodysplastic syndrome after temozolomide for recurrent high-grade glioma. J Neurooncol. 2005;71:315–318. doi: 10.1007/s11060-004-2028-0. [DOI] [PubMed] [Google Scholar]