Abstract
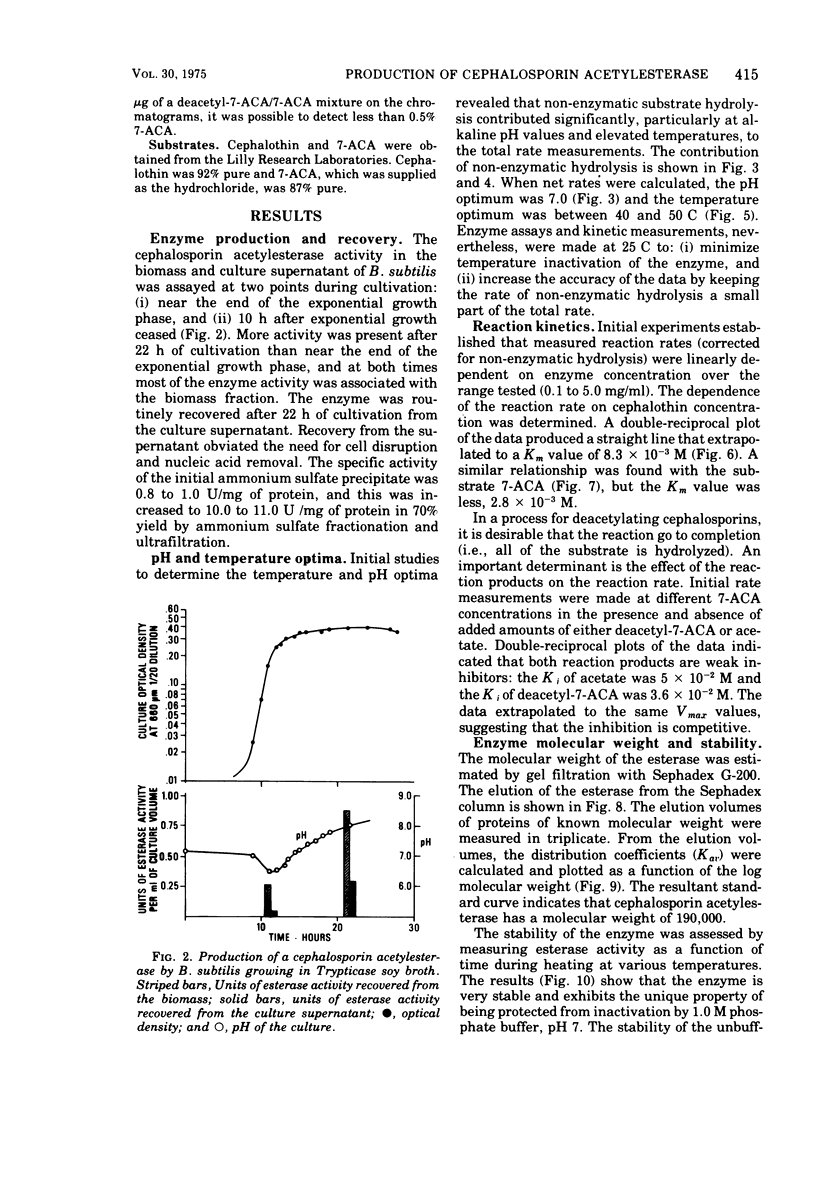
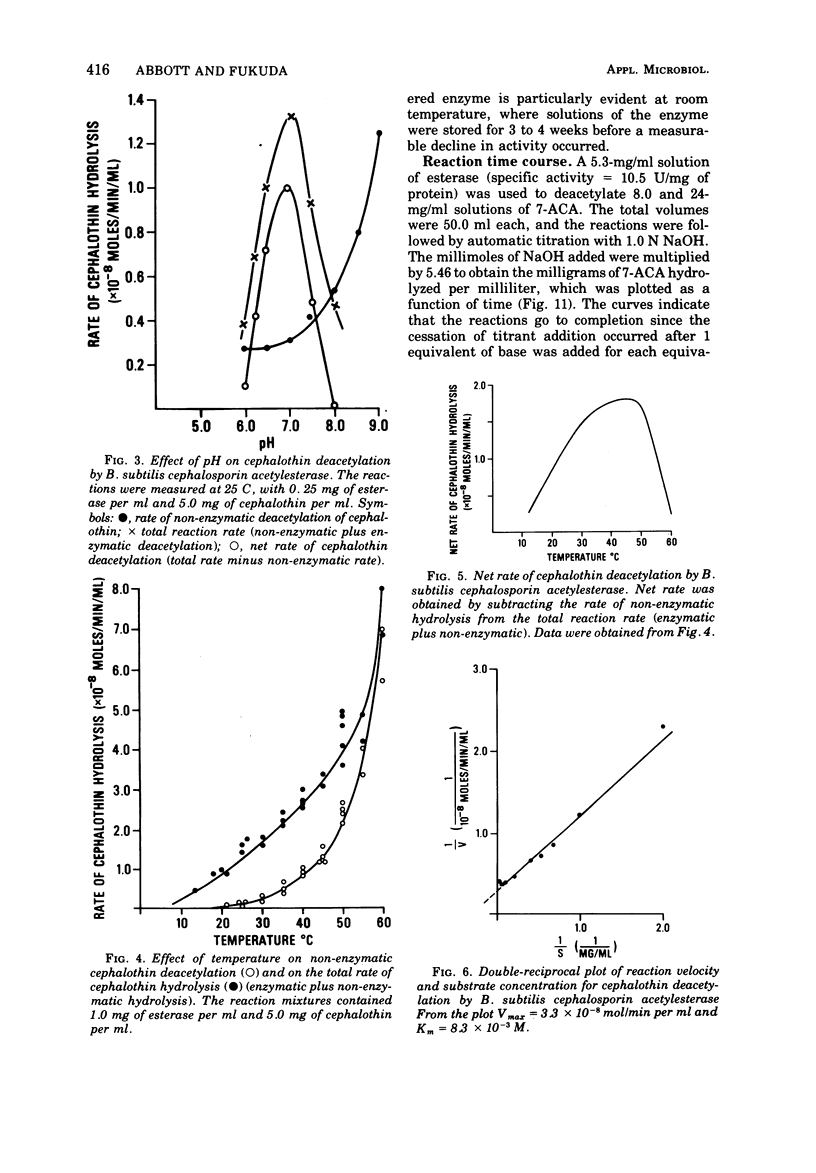
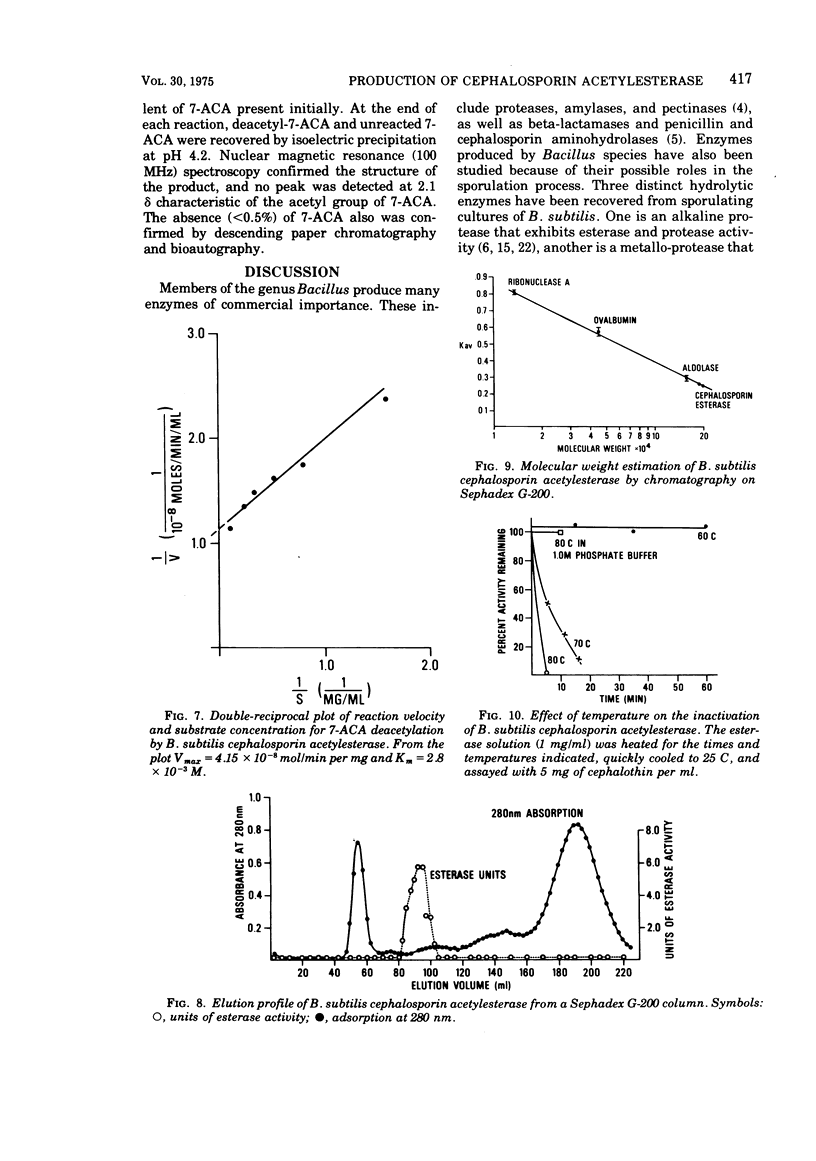
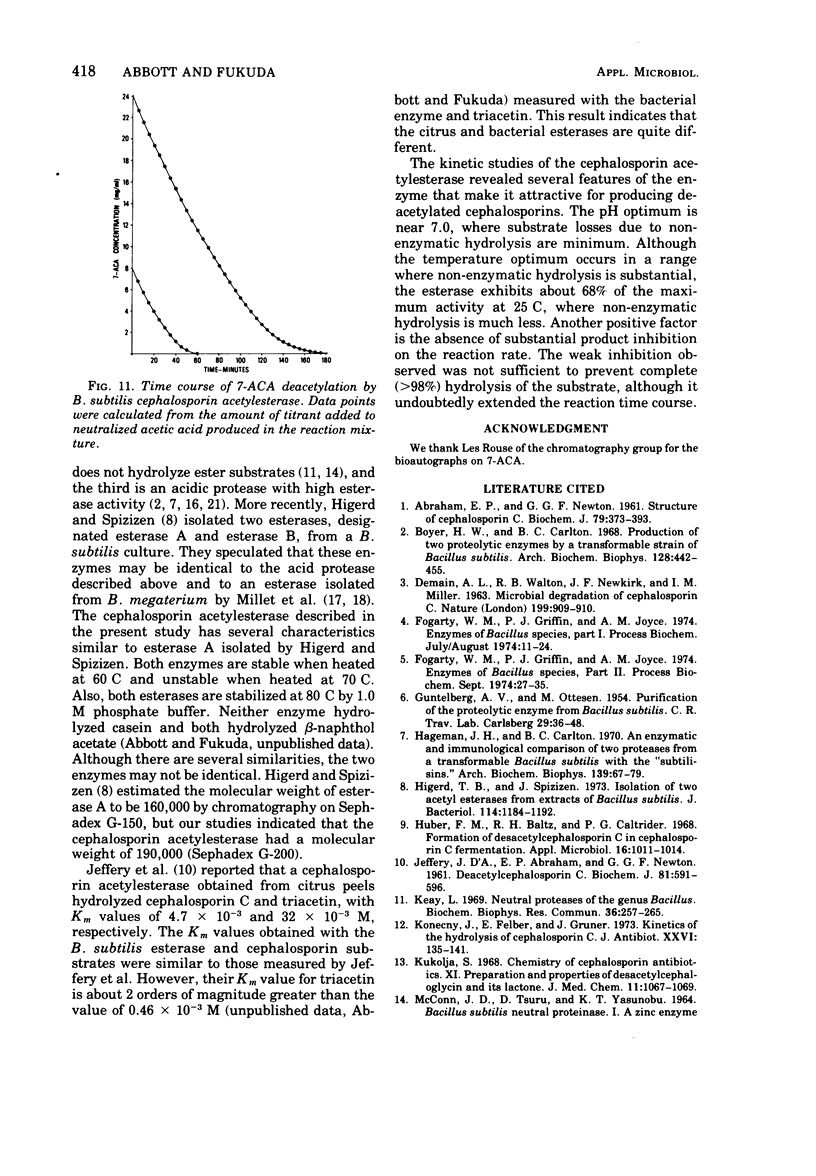
An esterase that deacetylates cephalosporins was recovered from the supernatant of a Bacillus subtilis culture. It was partially purified by ammonium sulfate fractionation and ultrafiltration. The enzyme had a temperature optimum between 40 and 50 C and a pH optimum of 7.0. The molecular weight was estimated by gel filtration to be 190,000. The enzyme was very stable and retained greater than 80% of its activity after storage in solution at 25 C for 1 month. The esterase exhibited Michaelis-Menton kinetics with the substrates 7-aminocephalosporanic acid (7-ACA) and 7-(thiophene-2-acetamido)cephalosporanic acid (cephalothin); the Km values were 2.8 × 10-3 and 8.3 × 10-3 M, respectively. The products of 7-ACA deacetylation were weak competitive inhibitors, and a Ki value of 5.0 × 10-2 M was determined for acetate and of 3.6 × 10-2 M for deacetyl-7-ACA. Weak product inhibition did not prevent the deacetylation reaction from going to completion. A 5-mg/ml solution of partially purified esterase completely hydrolyzed (>99.5%) a 24-mg/ml solution of 7-ACA in 3 h. Because of the kinetic properties and excellent stability, this enzyme may be useful in an immobilized form to prepare large quantities of deacetylated cephalosporin derivatives.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer H. W., Carlton B. C. Production of two proteolytic enzymes by a transformable strain of Bacillus subtilis. Arch Biochem Biophys. 1968 Nov;128(2):442–455. doi: 10.1016/0003-9861(68)90050-7. [DOI] [PubMed] [Google Scholar]
- DEMAIN A. L., WALTON R. B., NEWKIRK J. F., MILLER I. M. MICROBIAL DEGRADATION OF CEPHALOSPORIN C. Nature. 1963 Aug 31;199:909–910. doi: 10.1038/199909a0. [DOI] [PubMed] [Google Scholar]
- GUNTELBERG A. V., OTTESEN M. Purification of the proteolytic enzyme from Bacillus subtilis. C R Trav Lab Carlsberg Chim. 1954;29(3-4):36–48. [PubMed] [Google Scholar]
- Hageman J. H., Carlton B. C. An enzymatic and immunological comparison of two proteases from a transformable Bacillus subtilis with the "subtilisins". Arch Biochem Biophys. 1970 Jul;139(1):67–79. doi: 10.1016/0003-9861(70)90045-7. [DOI] [PubMed] [Google Scholar]
- Higerd T. B., Spizizen J. Isolation of two acetyl esterases from extracts of Bacillus subtilis. J Bacteriol. 1973 Jun;114(3):1184–1192. doi: 10.1128/jb.114.3.1184-1192.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber F. M., Baltz R. H., Caltrider P. G. Formation of desacetylcephalosporin C in cephalosporin C fermentation. Appl Microbiol. 1968 Jul;16(7):1011–1014. doi: 10.1128/am.16.7.1011-1014.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEFFERY J. D., ABRAHAM E. P., NEWTON G. G. Deacetylcephalosporin C. Biochem J. 1961 Dec;81:591–596. doi: 10.1042/bj0810591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay L. Neutral proteases of the genus Bacillus. Biochem Biophys Res Commun. 1969 Jul 23;36(2):257–265. doi: 10.1016/0006-291x(69)90323-4. [DOI] [PubMed] [Google Scholar]
- Konecny J., Felber E., Gruner J. Kinetics of the hydrolysis of cephalosporin C. J Antibiot (Tokyo) 1973 Mar;26(3):135–141. doi: 10.7164/antibiotics.26.135. [DOI] [PubMed] [Google Scholar]
- Kukolja S. Chemistry of cephalosporin antibiotics. XI. Preparation and properties of desacetylcephaloglycin and its lactone. J Med Chem. 1968 Sep;11(5):1067–1069. doi: 10.1021/jm00311a035. [DOI] [PubMed] [Google Scholar]
- MCCONN J. D., TSURU D., YASUNOBU K. T. BACILLUS SUBTILIS NEUTRAL PROTEINASE. I. A ZINC ENZYME OF HIGH SPECIFIC ACTIVITY. J Biol Chem. 1964 Nov;239:3706–3715. [PubMed] [Google Scholar]
- Millet J. Caractérisation d'une endopeptidase cytoplasmique chez Bacillus megaterium en voie de sporulation. C R Acad Sci Hebd Seances Acad Sci D. 1971 Mar 29;272(13):1806–1809. [PubMed] [Google Scholar]
- Millet J. Characterization of proteinases excreted by Bacillus subtilis Marburg strain during sporulation. J Appl Bacteriol. 1970 Mar;33(1):207–219. doi: 10.1111/j.1365-2672.1970.tb05245.x. [DOI] [PubMed] [Google Scholar]
- Millet J., Kerjan P., Aubert J. P., Szulmajster J. Proteolytic conversion in vitro of B. subtilis vegetative RNA polymerase into the homologous spore enzyme. FEBS Lett. 1972 Jun 1;23(1):47–50. doi: 10.1016/0014-5793(72)80281-3. [DOI] [PubMed] [Google Scholar]
- Morin R. B., Jackson B. G., Mueller R. A., Lavagnino E. R., Scanlon W. B., Andrews S. L. Chemistry of cephalosporin antibiotics. XV. Transformations of penicillin sulfoxide. A synthesis of cephalosporin compounds. J Am Chem Soc. 1969 Mar 12;91(6):1401–1407. doi: 10.1021/ja01034a023. [DOI] [PubMed] [Google Scholar]
- O'CALLAGHAN C. H., MUGGLETON P. W. THE FORMATION OF METABOLITES FROM CEPHALOSPORIN COMPOUNDS. Biochem J. 1963 Nov;89:304–308. doi: 10.1042/bj0890304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestidge L., Gage V., Spizizen J. Protease activities during the course of sporulation on Bacillus subtilis. J Bacteriol. 1971 Sep;107(3):815–823. doi: 10.1128/jb.107.3.815-823.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPPAPORT H. P., RIGGSBY W. S., HOLDEN D. A. A BACILLUS SUBTILIS PROTEINASE. I. PRODUCTION, PURIFICATION, AND CHARACTERIZATION OF A PROTEINASE FROM A TRANSFORMABLE STRAIN OF BACILLUS SUBTILIS. J Biol Chem. 1965 Jan;240:78–86. [PubMed] [Google Scholar]
- Willner D., Rossomano V. Z., Sprancmanis V. An improved preparation of desacetylcephaloglycin. J Antibiot (Tokyo) 1973 Mar;26(3):179–180. doi: 10.7164/antibiotics.26.179. [DOI] [PubMed] [Google Scholar]
- Yamana T., Tsuji A., Kanayama K., Nakano O. Comparative stabilities of cephalosporins in aqueous solution. J Antibiot (Tokyo) 1974 Dec;27(12):1000–1002. doi: 10.7164/antibiotics.27.1000. [DOI] [PubMed] [Google Scholar]



