Abstract
Temozolomide has established activity in the treatment of recurrent glioblastoma multiforme (GBM). Caelyx (liposomal doxorubicin) has established activity in a broad range of tumors but has not been extensively evaluated in the treatment of GBM. Phase 1 data suggest that temozolomide and Caelyx can be combined safely at full dose. In this phase 2 study, combination temozolomide (200 mg/m2 orally, days 1–5) and Caelyx (40 mg/m2 i.v., day 1) was given every 4 weeks to a cohort of 22 patients with recurrent GBM, who received a total of 109 cycles (median 3.5 cycles). The median age of the patients was 55 years (range, 31–80 years), and 17 were male. All patients had received radiotherapy, but only 2 had received prior chemotherapy. One patient (5%) had a complete response, 3 patients (14%) had a partial response, and 11 patients (50%) had stable disease. The median time to progression for the cohort was 3.2 months (range, 1–13 months). Median overall survival was 8.2 months (range, 1–16+ months). Seven patients (32%) were progression free at 6 months. Hematological toxicity included grade 3/4 neutropenia in 4 patients (18%) and grade 3/4 thrombocytopenia in 4 patients (18%). Grade 3 nonhematologic toxicity included rash in 3 patients (14%), nausea and vomiting in 1 patient (4%), hypersensitivity reaction to Caelyx in 3 patients (14%), and palmar-plantar toxicity in 1 patient (4%). We conclude that the combination of temozolomide and Caelyx is well tolerated, results in a modest objective response rate, but has encouraging disease stabilization in the treatment of recurrent GBM.
Primary brain tumors represent approximately 2% of all adult cancer deaths, with approximately 13,000 persons dying each year in the United States (Jemal et al., 2002). Glioblastoma multiforme (GBM)2 is the most common malignant primary brain tumor and is associated with a poor outcome. The median survival is usually less than 1 year from diagnosis (Friedman et al., 2000; Stupp et al., 2001). Despite initial treatment with surgery and radiotherapy, most patients relapse within 6 to 12 months (Levin, 1999; Surawicz et al., 1999; Walker et al., 1980).
Treatment in the recurrent setting may include palliative chemotherapy. Phase 2 trials of single-agent or combination salvage chemotherapy regimens have included agents such as nitrosoureas, taxanes, and temozolomide. These regimens have reported objective responses but have had minimal impact on survival (Brada et al., 2001; Burton and Prados, 1999; Kapelle et al., 2001; Rosenthal et al., 2000; Wong et al., 1999; Yung et al., 2000).
Temozolomide is a second-generation imidazotetrazine derivative that is well tolerated orally and has notable activity in GBM, anaplastic astrocytoma, and mixed astrocytoma/anaplastic oligodendromas. Several studies have established the efficacy of temozolomide as a single agent in recurrent GBM in terms of response, progression-free survival (PFS), and improved quality of life (Brada et al., 2001; Harris et al., 2001; Stupp et al., 2001; Yung et al., 1999).
Caelyx (Schering Canada, Pointe Claire, Quebec) is a liposomal formulation of doxorubicin in which the drug is encapsulated in liposomes (Stealth liposomes [Sequus Pharmaceuticals, Menlo Park, Calif.]). Currently, it has an established role in the management of breast and ovarian cancer. Preclinical data has demonstrated the efficacy of Caelyx in mouse brain tumor models (Koukourakis et al., 2002; Sharma et al., 1997; Siegal et al., 1995). A single clinical study of patients with recurrent high-grade gliomas demonstrated that this drug is well tolerated, with significant disease stabilization and prolongation of disease-free survival (Fabel et al., 2001). The major side effects found were palmar-plantar erythrodysesthesia and myelosuppression. No cardiac toxicity was observed.
The combination of temozolomide and Caelyx was examined in a phase 1 study, and results indicate that both agents can safely be given at full dose (Volm et al., 2000). In view of the established efficacy of temozolomide and the potential pharmacokinetic benefits of Caelyx, we chose to evaluate the efficacy and toxicity of temozolomide and Caelyx in patients with recurrent GBM in a multi-institutional open-label phase 2 trial.
Patients and Methods
Patient Eligibility
Eligible patients were ⩾18 years old and had histologically proven GBM and unequivocal evidence of tumor recurrence or progression at first relapse by contrast-enhanced MRI or CT. Eligibility criteria included Eastern Cooperative Oncology Group performance status 0–2, serum creatinine ⩽1.5 times the upper limit of normal, granulocytes ⩾1500/dl, platelets ⩾100,000/dl, AST ⩽2.5 times the upper limit of normal, total bilirubin within normal limits, completion of radiotherapy more than 4 weeks previously, left ventricular ejection fraction ⩾ lower limit of normal, and completion of any treatment more than 4 weeks previously. Patients had to have contrast-enhanced imaging within 3 weeks prior to study entry.
Patients were excluded if they had received prior chemotherapy for recurrent disease, had experienced severe intercurrent medical illness or symptomatic heart disease, had not recovered from surgery, or were pregnant or breastfeeding. Women of childbearing potential had to be using adequate contraceptive methods while on study and for 2 months after completion of treatment. If clinically possible, patients were to have been on a stable dose of steroid 1 week prior to treatment. The respective Institutional Ethics Committees approved the protocol, and patients were required to give informed consent.
Study Design
The study was a multi-institutional, open-label phase 2 study. Caelyx was given intravenously at a dose of 40 mg/m2 diluted in 100 ml 5% dextrose on day 1. The initial infusion was administered more slowly because of the occasional acute reaction to the first dose. Five percent of the total dose was given over 15 min, and if this rate was tolerated, the infusion rate was doubled. The remaining infusion was completed over 60 min for a total infusion time of 90 min. Further courses of Caelyx could be infused over an hour if no reactions occurred during the first dose. If the patient experienced an infusion reaction, the infusion was ceased, and appropriate premedications such as an antihistamine and/or steroid were given. The infusion was then recommenced at a lower rate.
Temozolomide was administered orally on wakening in a fasting state at a dose of 200 mg/m2 every day for 5 consecutive days every 4 weeks for up to 12 months. All doses were rounded up to the nearest 5 mg to accommodate capsule strength. Capsules of temozolomide were available in 5-mg, 20-mg, 100-mg, and 250-mg strengths. Patients were instructed not to have any food for 2 h after temozolomide. Water was allowed during the fasting period. Temozolomide was taken with a glass of water over as short a time as was possible. Patients were instructed to swallow capsules whole and in rapid succession and were told not to chew capsules. If vomiting occurred during the course of treatment, no re-dosing of the patient was allowed before the next scheduled dose.
Treatment with Caelyx and temozolomide was administered every 28 days on an outpatient basis until disease progression or a dose-limiting toxicity occurred. Toxicity and dose modifications were based on National Cancer Institute Common Toxicity Criteria version 2 (NCI, 1999). Criteria for retreatment were an absolute neutrophil count (ANC) >1500 cells/dl, platelet >100,000/dl, hemoglobin 10 g/dl, liver function test values ⩽2 times the upper limit of normal, creatinine <1.5 times the upper limit of normal, total bilirubin within normal limits, and all other toxicity resolved to baseline or grade 1 except palmar-plantar erythrodysesthesia.
Dose Modifications
The doses of temozolomide and Caelyx administered for subsequent cycles were determined by the nadir ANC or platelet count on day 1. For patients with nadir ANC of 500 to 999 cells/dl and nadir platelet count of 50 to 74,999 cells/dl or nadir platelet count of 25 to 49,999 cells/dl alone, temozolomide and Caelyx were both reduced by 25%. If the nadir ANC was less than 500 cells/dl or nadir platelet count was less than 25,000 cells/dl, both drugs were reduced by 50%.
If patients experienced any grade ⩾2 palmar-plantar toxicity, treatment was withheld for an extra week. If grade 1 toxicity persisted after a 2-week delay, Caelyx was reduced by 25%. If grade 3 or 4 toxicity persisted beyond 2 weeks, patients discontinued Caelyx and continued temozolomide if clinically indicated. For grades 3 and 4 nonhematological toxicity, treatment was withheld until the toxicity improved to grade 1 or less, and both Caelyx and temozolomide doses were reduced by 25%. All patients who developed grade 4 toxicity were taken off study.
Patients received a 5-hydroxytryptamine serotonin antagonist at the same time as they took their temozolomide and 8 mg of intravenous dexamethasone as an antiemetic prior to the administration of Caelyx. Prophylactic haematopoietic growth factors were not permissible, but could be used for febrile neutropenic episodes.
Patient Evaluation
Patients who received at least 1 course of chemotherapy were eligible for response evaluation. Performance status evaluation, clinical examination, neurological evaluation, hematological assessments, and clinical chemistry tests were performed monthly. Tumor status was evaluated by an independent radiologist every 2 months with a repeat MRI scan using MacDonald criteria (MacDonald et al., 1990). A complete response (CR) was the disappearance of all enhancing tumor on scans at least 1 month apart, discontinuation of steroids, and stable or improved neurological status. A partial response (PR) was defined as a decrease of ⩾50% in the product of the 2 largest perpendicular diameters of the enhancing lesion on scans at least 1 month apart, stable or reduced dose of steroids, and stable or improved neurological status. Progressive disease was a ⩾25% increase in size of the product of the largest perpendicular diameters of enhancing tumor or a neurological assessment of “definitely worse” or any new tumor on scans. All other assessments were considered stable disease (SD).
Statistical Methods
This study was designed to evaluate the safety and efficacy of Caelyx used in combination with temozolomide in the treatment of recurrent GBM. The primary response variable was the response rate. Sample size was based on the Minimax design proposed by Simon for a 2-stage phase 2 trial (Simon, 1989). Parameters that must be specified for this design are P0, a response rate of no interest (i.e., we would definitely reject the investigation drugs for further development if the true response rate were less than P0); P1, a desirable response rate (i.e., we would definitely accept the drugs for further testing if the true response rate were greater than P1); alpha, the probability of accepting an ineffective drug; and beta, the probability of rejecting an effective drug. The following set of parameters were selected by taking into consideration that temozolomide alone has an objective response rate (CR+PR) of 20% to 30% in patients with GBM: P0 = 0.30, P1 = 0.5, alpha = 0.10, beta = 0.10. These choices of parameters lead to the following sample sizes: 22 patients were to be enrolled in the first stage, and the trial was to stop if there were fewer than 7 responses. Otherwise, the trial was to continue until a total of 46 patients were enrolled. The drug combination was to be rejected for further development if 17 or fewer responses were observed in 46 patients. We used the exact binomial distribution to calculate 95% confidence intervals. Kaplan-Meier curves for progression-free and overall survival were analyzed.
Results
Patient Characteristics
Between March 8, 2001, and August 27, 2002, 23 patients were enrolled onto this study. One patient had a grade 4 hypersensitivity reaction within minutes of commencing the first dose of Caelyx and did not receive any further Caelyx. This patient has not been included in the efficacy analysis but has been included in the toxicity analysis. One patient had received 1 course of low-dose temozolomide during whole-brain irradiation, and 1 patient had 1 course of temozolomide for “recurrence” prior to a second surgical resection, which pathologically was proven to be radiation necrosis. The patient then developed pathologically proven tumor recurrence some months later prior to enrollment. Both these patients were included in both the toxicity and efficacy analysis. Thus 22 patients were evaluable for response and toxicity.
Patient characteristics are shown in Table 1. All patients had undergone prior surgery and radiotherapy before developing recurrent GBM. Three patients had a second surgical resection at initial relapse and entered the study on progression. The median age was 55 years (range, 31–80 years), and 17 patients (77 %) were men. The median Eastern Cooperative Oncology Group performance status was 1 (range, 0 – 2). The 22 patients received a total of 109 cycles. The median number of cycles administered was 4 cycles (range, 2–12).
Table 1.
Patient characteristics
| Characteristics | No. of Patients |
|---|---|
| Eligible patients | 23 (22 evaluable) |
| Sex | |
| Male | 17 |
| Female | 6 |
| Age, years | |
| Median | 55 |
| Range | 31–80 |
| ECOG performance | |
| 0 | 8 |
| 1 | 10 |
| 2 | 4 |
| Prior chemotherapy | 2 (9%) |
| Prior radiotherapy | 22 |
| No. of cycles of chemotherapy | |
| Median | 4 |
| Range | 2–12 |
The abbreviation used is as follows: ECOG, Eastern Cooperative Oncology Group.
Response Rates and Overall Survival
Table 2 summarizes the response to therapy. Of the 22 evaluable patients, 1 patient (5%) achieved a CR, 3 patients (14%) achieved a PR, and 11 patients (50%) had SD. The overall response rate was 19% (95% confidence interval, 5%–40%). The median overall survival was 8 months (range, 1–16+ months). The median duration of response was 10.5 months (range 2–16+ months), and the median duration of SD was 5.4 months (range 2.3–8.6 months). Nine patients (32%) progressed after 2 cycles. One patient continues to be in CR at 16+ months. The median PFS for the cohort was 3.6 months (range, 1–16+ months). The 6-month PFS was 32%. At last analysis, 10 patients are alive, and 12 are dead. Figures 1a and 1b show Kaplan-Meier progression and overall survival curves.
Table 2.
Response rates in 22 patients
| Response | No. of Patients (%) |
|---|---|
| Complete response | 1 (5%) |
| Partial response | 3 (14%) |
| Stable disease | 11 (50%) |
| Progressive disease | 7 (32%) |
Fig. 1.
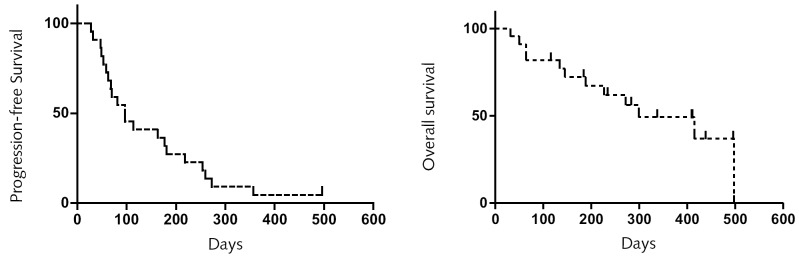
Kaplan-Meier survival curves. Progression-free survival curve (left) and overall survival curve (right).
Toxicity
All adverse events are listed in Table 3. Overall, the combination of temozolomide and Caelyx was well tolerated. There were no treatment-related deaths. Hematological toxicity included grade 3/4 neutropenia in 4 patients (18%) and grade 3/4 thrombocytopenia in 3 patients (14%). Two episodes of febrile neutropenia were documented. The most common nonhematological toxicities were rash/dry skin in 10 patients (45%), lethargy in 13 patients (59%), nausea or vomiting in 10 patients (45%), and mucositis in 11 patients (50%). Palmar-plantar toxicity was seen in 5 patients (28%), but only 1 patient had grade 3 toxicity. Four patients had a hypersensitivity reaction to Caelyx.
Table 3.
National Cancer Institute Common Toxicity Criteria version 2 toxicity*
| Grade 1–2 (%) | Grades 3–4 (%) | |
|---|---|---|
| Thrombocytopenia | 2 (9%) | 4 (18%) |
| Neutropenia | 2 (9%) | 4 (18%) |
| Anemia | 2 (9%) | 0 (0%) |
| Rash/dry skin | 7 (31%) | 3 (14%) |
| Palmar-plantar changes | 4 (18%) | 1 (4%) |
| Nausea/vomiting | 9 (41%) | 1 (4%) |
| Mucositis | 11 (50%) | 0 (0%) |
| Reaction to Caelyx | 1 (4%) | 3 (14%) |
| Lethargy | 12 (53%) | 1 (4%) |
| Diarrhea | 5 (23%) | 0 (0%) |
Four patients (18%) were discontinued from the trial for (1) an attempt at curative re-resection after achieving a good PR, (2) grade 4 allergic reaction to Caelyx, (3) grade 4 febrile neutropenia, and (4) a sudden death at home of unknown cause. An additional patient developed a left below-knee deep vein thrombosis but continued on the trial while receiving antithrombolytic treatment.
Discussion
In this study, we evaluated the efficacy and tolerability of combination temozolomide and Caelyx in patients with recurrent GBM. Although the use of temozolomide in the setting of recurrent GBM is well established, the role of Caelyx in this tumor type remains undefined. Our interest in using Caelyx in the treatment of recurrent GBM stems from a number of sources.
The ability of chemotherapy to cross the blood-brain barrier to reach brain tumors has been an ongoing therapeutic issue. Lipophilic agents have the potential to minimize this problem and to improve the safety profile. We have previously reported a phase 2 evaluation of a novel morpholino anthracycline (MX2), which demonstrated preclinical evidence of improved blood-brain barrier penetration because of its lipophilic properties. MX-2 proved to be active and well tolerated in patients with high-grade gliomas (Clarke et al., 1999).
Doxorubicin is known to be effective in high-grade glioma cell lines and tumor models, but its efficacy is limited by its low lipid solubility. Caelyx, which is doxorubicin encapsulated in liposomes (Stealth liposomes), has demonstrated enhanced drug exposure and improved therapeutic activity in rat brain tumor models when compared to doxorubicin alone (Koukourakis et al., 2002; Sharma et al., 1997; Siegal et al., 1995). In phase 1 studies the toxicity profile of doxorubicin was found to differ from that of the free drug alone (Uziely et al., 1995). The main types of toxicity found were palmar-plantar syndrome and stomatitis. Caelyx may ameliorate the toxicity of doxorubicin while maintaining its efficacy. A solitary clinical study suggests that Caelyx produced significant and prolonged disease stabilization in patients with high-grade gliomas (Fabel et al., 2001).
Temozolomide has predictable bioavailability and minimal toxicity, and the combination of Caelyx and temozolomide is particularly appealing given the documented efficacy of temozolomide coupled with the pre-clinical data recommending Caelyx in the treatment of GBM. In addition, studies have demonstrated that the drugs can be combined safely at full dose with little toxicity (Volm et al., 2000).
Our study was terminated early because the objective response rate (CR+PR) did not reach that required according to the study design. At least 7 responders were required in the first 22 patients to warrant continuation of the study. We observed 4 objective responses (18%). This compares with that documented for temozolomide alone in the treatment of recurrent GBM but does not suggest that the combination is more active than single agent temozolomide (Brada et al., 2001; Harris et al., 2000; Stupp, et al., 2001; Yung et al., 1999). However, the addition of stable disease to objective responses results in an overall response rate of 68%. This response rate also compares favorably with the response rates reported in studies examining combinations of temozolomide and other agents. (Britten et al., 1999; Gander et al., 1999; Patel et al., 2000).
Of interest, the 6-month PFS was 32%. This compares favorably with the data from single agent temozolomide GBM studies, in which the 6-month PFS is approximately 20% (Brada et al., 2001; Harris et al., 2001; Khan et al., 2002; Yung et al., 1999). This study was not statistically powered to examine 6-month PFS, nor was it the primary objective of this study. However, this data suggests that the addition of Caelyx to temozolomide may provide significant benefits in terms of disease stabilization and PFS.
There are obvious limitations with our trial. Like all studies, ours was limited by the well-documented difficulty of defining a radiological response (Gilbert, 2001). The radiological assessments were performed by independent neuroradiologists, and all objective responses were reviewed and confirmed by the investigators. Similarly, few patients had documented histologic proof of recurrent disease. However, all patients were required to have unequivocal disease progression based on MRI or CT scan appearances. Finally, the number of patients remains relatively small, and our findings may not be truly representative of the combination’s efficacy.
Nevertheless, this study is the first to examine the combination of Caelyx and temozolomide in the treatment of recurrent GBM. Preclinical and early phase clinical data suggested that this might be an active and tolerable regimen. Our data confirms the tolerability of the regimen and identifies a modest number as an objective response rate. However, a number of secondary end points, including disease stabilization and the 6-month PFS, are more encouraging. Further studies of this combination appear warranted.
Footnotes
Abbreviations used are as follows: ANC, absolute neutrophil count; CR, complete response; AST, aspartate transferase; GBM, glioblastoma multiforme; MX-2, morphalino-anthracycline; PFS, progression-free survival; PR, partial response; SD, stable disease.
References
- Brada M, Hoang-Xuang K, Rampling R, Dietrich P.-Y, Dirix LY, Macdonald D, Heimans JJ, Zonnenberg BA, Bravo-Marques JM, Henriksson R, Stupp R, Yue N, Bruner J, Dugan M, Rao S, Zaknoen S. Multicentre phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol. 2001;12:259–266. doi: 10.1023/a:1008382516636. [DOI] [PubMed] [Google Scholar]
- Britten CD, Rowinsky EK, Baker SD, Agarwala SS, Eckardt JR, Barrington R, Diab SG, Hammond LA, Johnson T, Villalona-Calero M, Fraass U, Statkevich P, Von Hoff DD, Eckhardt SG. A Phase I and pharmacokinetic study of temozolomide and cisplatin in patients with advanced solid malignancies. Clin Cancer Res. 1999;5:1629–1637. [PubMed] [Google Scholar]
- Burton E, Prados M. New chemotherapy options for the treatment of malignant gliomas. Curr Opin Oncol. 1999;11:157–161. doi: 10.1097/00001622-199905000-00003. [DOI] [PubMed] [Google Scholar]
- Clarke K, Basser RL, Underhill C, Mitchell P, Bartlett J, Cher L, Findlay M, Dalley D, Pell M, Byrne M, Geldard H, Hill JS, Maher D, Fox RM, Green MD, Kaye AH. KRN8602 (MX2-hydochloride): An active new agent for the treatment of recurrent high-grade glioma. J Clin Oncol. 1999;17:2579–2584. doi: 10.1200/JCO.1999.17.8.2579. [DOI] [PubMed] [Google Scholar]
- Fabel K, Dietrich J, Hau P, Wismeth C, Winner B, Przywara S, Steinbrecher A, Ullrich W, Bogdahn U. Long-term stabilization in patients with malignant glioma after treatment with liposomal doxorubicin. Cancer. 2001;92:1936–1942. doi: 10.1002/1097-0142(20011001)92:7<1936::aid-cncr1712>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000;6:2585–2597. [PubMed] [Google Scholar]
- Gander M, Leyvraz S, Decosterd L, Bonfanti M, Marzolini C, Shen F, Lienard D, Perey L, Colella G, Biollaz J, Lejeune F, Yarosh D, Belanich M, D’Incalci M. Sequential administration of temozolomide and fotemustine: Depletion of O6-alkyl guanine-DNA transferase in blood lymphocytes and in tumours. Ann Oncol. 1999;10:831–838. doi: 10.1023/a:1008304032421. [DOI] [PubMed] [Google Scholar]
- Gilbert MR. Neuro-oncology clinical trials: Promise and pitfalls. Ann Oncol. 2001;12:149–150. doi: 10.1093/oxfordjournals.annonc.a000239. [DOI] [PubMed] [Google Scholar]
- Harris MT, Rosenthal MA, Ashley DL, Cher L. An Australian experience with temozolomide for the treatment of recurrent high grade glioma. J Clin Neurosci. 2001;8:325–327. doi: 10.1054/jocn.2000.0809. [DOI] [PubMed] [Google Scholar]
- Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- Kappelle AC, Postma TJ, Taphoorn MJB, Groeneveld GJ, van den Bent MJ, van Groeningen CJ, Zonnenberg BA, Sneeuw KCA, Heimans JJ. PCV chemotherapy for recurrent glioblastoma multiforme. Neurology. 2001;56:118–120. doi: 10.1212/wnl.56.1.118. [DOI] [PubMed] [Google Scholar]
- Khan RB, Raizer JJ, Malkin MG, Bazylewicz KA, Abrey LE. A phase II study of extended low-dose temozolomide in recurrent malignant gliomas. Neuro-Oncology. 2002;4:39–43. doi: 10.1215/15228517-4-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukourakis MI, Koukouraki S, Fezoulidis I, Kelekis N, Kyrias G, Archimandritis S, Karkavitsas N. High intratumoural accumulation of Stealth liposomal doxorubicin (Caelyx) in glioblastomas and in metastatic brain tumours. Br J Cancer. 2000;83:1281–1286. doi: 10.1054/bjoc.2000.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin VA. Chemotherapy for brain tumors of astrocytic and oligodendroglial lineage: The past decade and where we are heading. Neuro-Oncol. 1999;1:69–80. doi: 10.1093/neuonc/1.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant gliomas. J Clin Oncol. 1994;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- NCI. National Cancer Institute (1999) Common Toxicity Criteria version 2.0. Available at https://webapps.ctep.nci.nih.gov/ctcv2/plsql/ctc000w$.startup. [PubMed]
- Patel VJ, Elion GB, Houghton PJ, Keir S, Pegg AE, Johnson SP, Dolan ME, Bigner DD, Friedman HS. Schedule-dependent activity of temozolomide plus CPT-11 against a human central nervous system tumor-derived xenograft. Clin Cancer Res. 2000;6:4154–4157. [PubMed] [Google Scholar]
- Rosenthal MA, Gruber ML, Glass J, Nirenberg A, Finlay J, Hochster H, Muggia FM. Phase II study of combination taxol and estramustine phosphate in the treatment of recurrent glioblastoma multiforme. J Neurooncol. 2000;47:59–63. doi: 10.1023/a:1006426215005. [DOI] [PubMed] [Google Scholar]
- Sharma US, Sharma A, Chau RI, Straubinger RM. Liposome-mediated therapy of intracranial brain tumors in a rat model. Pharm Res. 1997;14:992–998. doi: 10.1023/a:1012136925030. [DOI] [PubMed] [Google Scholar]
- Siegal T, Horowitz A, Gabizon A. Doxorubicin encapsulated in sterically stabilized liposomes for the treatment of a brain tumor model: Biodistribution and therapeutic efficacy. J Neurosurg. 1995;83:1029–1037. doi: 10.3171/jns.1995.83.6.1029. [DOI] [PubMed] [Google Scholar]
- Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- Stupp R, Gander M, Leyvraz S, Newlands E. Current and future developments in the use of temozolomide for the treatment of brain tumours. Lancet Oncol. 2001;2:552–560. doi: 10.1016/S1470-2045(01)00489-2. [DOI] [PubMed] [Google Scholar]
- Surawicz TS, McCarthy BJ, Kupelian V, Jukich PJ, Bruner JM, Davis FG. Descriptive epidemiology of primary brain and CNS tumors: Results from the Central Brain Tumor Registry of the United States, 1990–1994. Neuro-Oncol. 1999;1:14–25. doi: 10.1093/neuonc/1.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uziely B, Jeffers S, Isacson R, Kutsch K, Wei-Tsao D, Yehoshua Z, Libson E, Muggia FM, Gabizon A. Liposomal doxorubicin: Antitumor activity and unique toxicities during two complementary phase I studies. J Clin Oncol. 1995;13:1777–1785. doi: 10.1200/JCO.1995.13.7.1777. [DOI] [PubMed] [Google Scholar]
- Volm M, Oratz R, Pavlick A, Farrell K, Lee J, Muggia F. A phase I study of liposomal doxorubicin and temozolomide in patients with advanced cancer. Proc Am Soc Clin Oncol. 2000;19:223a. (abstract 872) [Google Scholar]
- Walker MD, Green SB, Byar DP, Alexander E, Jr, Batzdorf U, Brooks WH, Hunt WE, MacCarty CS, Mahaley MS, Jr, Mealey J, Jr, Owens G, Ransohoff J, 2nd, Robertson JT, Shapiro WR, Smith KR, Jr, Wilson CB, Strike TA. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303:1323–1329. doi: 10.1056/NEJM198012043032303. [DOI] [PubMed] [Google Scholar]
- Wong ET, Hess KR, Gleason MI, Jaeckle KA, Kyritsis AP, Prados MD, Levin VA, Yung WKA. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572. doi: 10.1200/JCO.1999.17.8.2572. (comment) [DOI] [PubMed] [Google Scholar]
- Yung W.K.A, Albright RE, Olson J, Fredericks R, Fink K, Prados MD, Brada M, Spence A, Hohl RJ, Shapiro W, Glantz M, Greenberg H, Selker RG, Vick NA, Rampling R, Friedman H, Phillips P, Bruner J, Yue N, Osoba D, Zaknoen S, Levin VA. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Can. 2000;83:588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung WKA, Prados MD, Yaya-Tur R, Rosenfeld SS, Brada M, Friedman HS, Albright R, Olson J, Chang SM, O’Neil AM, Friedman AH, Bruner J, Yue N, Dugan M, Zaknoen S, Levin VA. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol. 1999;17:2762–2771. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]


