Abstract
This study was conducted to determine the maximum tolerated dose and dose-limiting toxicity of irinotecan (CPT-11) administered every 3 weeks to adults with progressive malignant glioma who were treated with enzyme-inducing antiepileptic drug (EIAED) therapy, and to compare the pharmacokinetics with those in patients not on EIAED therapy treated at the recommended phase 2 dose for other cancers. The CPT-11 dose was 350 mg/m2 i.v. every 3 weeks and remained fixed in patients not on EIAED therapy, but the dose was escalated by 50-mg/m2 increments in patients on EIAED therapy. CPT-11 and its metabo-lites SN-38, SN-38 glucuronide (SN-38G), and APC (7-ethyl-10[4-N-(5 aminopentanoic acid)-1-piperidine]-carbonyloxycamptothecin) were characterized in both groups. Patients on EIAEDs received 350 to 800 mg/m2 of CPT-11. Dose-limiting toxicity was due to grade 3 diarrhea despite maximal doses of loperamide. The systemic levels of CPT-11, APC, SN-38G, and SN-38 were all lower in the EIAED group. There was a moderate-to-fair relationship between CPT-11 dose and the area under the curve (AUC) for CPT-11 and APC over the dosage range of 350 to 800 mg/m2, but no relationship between CPT-11 dose and the AUC for SN-38 or SN-38G. At the 750-mg/m2 dose, the AUC for CPT-11 (21.6 μg × h/ml) matched the AUC (21.6 μg × h/ml) in the non-EIAED group treated with 350 mg/m2 of CPT-11. We conclude that the recommended phase 2 dose of CPT-11 for patients on EIAEDs is 750 mg/m2 when given every 3 weeks. A phase 2 study of patients with recurrent malignant glioma is ongoing to assess the efficacy of CPT-11 when the dose is stratified according to the use of EIAEDs.
The treatment of recurrent malignant gliomas using available chemotherapy drugs is ineffective for the prolongation of life in the majority of patients. The median time to tumor progression after treatment for patients with recurrent glioblastoma multiforme (GBM)3 is only 8 to 12 weeks, and fewer than 20% of patients are progression-free by 6 months (Hess et al., 1999). Patients with anaplastic gliomas other than GBM fare better, but fewer than half are progression-free at 6 months (Hess et al., 1999). The efficacy of chemotherapy for glioma has been limited because of the low activity of available antineoplastic agents, the compromised delivery of these agents to at least partially privileged intracranial sites, and the emergence or de novo presence of resistance to these agents. Irinotecan (CPT-11), a drug that is active against colon carcinoma, has been used to treat recurrent glioma. Preclinical activity of CPT-11 has been promising, with in vitro activity noted against a panel of xenografts derived from ependymoma, childhood and adult high-grade astrocytoma, and medulloblastoma (Hare et al., 1997). A phase 2 study of CPT-11 for malignant glioma was conducted at Duke University Medical Center using doses and schedules recommended for colon cancer (Friedman et al., 1999). The results suggested clinical efficacy with less toxicity, particularly diarrhea, than has been seen in other cancer patient populations. This low toxicity may be due to the treatment of many glioma patients with enzyme-inducing antiepileptic drug (EIAED) therapy, which could alter the exposure to CPT-11 in this population. However, reduced exposure to drug may also reduce efficacy.
CPT-11 (Mr anhydrous freebase, 587) is oxidized in the liver by the highly inducible CYP3A4, yielding 2 less active metabolites, APC (7-ethyl-10[4-N-(5 aminopentanoic acid)-1-piperidine]-carbonyloxycamptothecin [Mr 619]) and NPC (7-ethyl-10[4-(piperidine)-1-amino] carbonyloxycamptothecin [Mr 519]) (Haaz et al., 1998; Santos et al., 2000). Additionally, CPT-11 is bioactivated by hepatic and tissue carboxylesterases (predominately hCE2) to the potent topoisomerase-I inhibitor SN-38 [Mr anhydrous base, 393] (Humerickhouse et al., 2000; Khanna et al., 2000; Slatter et al., 1997). SN-38 is excreted unchanged or conjugated by the highly polymorphic and inducible uridine-diphosphoglucuronosyl transferase (UGT1A1)-generating SN-38 glucuronide [Mr 569] (Iyer et al., 1998). Subsequently, CPT-11 and its metabolites are exported from the hepatocytes by specific apical, membrane-bound transporters. The canalicular, multi-specific organic anion transporter multidrug resistance protein 2 (MRP2) reportedly is responsible for the biliary transport of the inactive carboxylate forms of CPT-11 and SN-38 as well as the lactone and carboxylate forms of SN-38 glucuronide (Chu et al., 1998). SN-38 has recently been identified as a substrate for the canalicular, ATP-binding cassette (ABC) transporter (ABCP, ABCG2), the breast cancer resistant protein (BCRP), and mitoxantrone-resistance half-transporter (MXR) (Brangi et al., 1999; Maliepaard et al., 2001).
Recent preclinical and clinical studies have demonstrated that EIAEDs can alter the metabolism and elimination of CPT-11 and its metabolites. Pretreatment of rats with the antiepileptic drug phenobarbital, an inducer of UGT1A1 and CYP3A4, has been shown to enhance the formation of SN-38G and decrease the area under the concentration-time curve (AUC) for both CPT-11 and SN-38. However, the non-EIAED valproic acid increased the AUC of SN-38, presumably by inhibiting the glucuronidation of SN-38 (Gupta et al., 1997). In contrast to the induction of UGT1A1 and CYP3A4, phenobarbital has recently been shown not to increase the expression of MRP2 in rat hepatocytes (Hagenbuch et al., 2001). A recent study of CPT-11 in patients with malignant gliomas receiving EIAEDs indicated significantly lower systemic exposure (decreased AUC) to CPT-11, SN-38, and SN-38G compared to historical controls (Friedman et al., 1999).
Based upon the background above, the North American Brain Tumor Consortium (NABTC) began a phase 1 study of CPT-11 in patients on EIAEDs to determine the optimal phase 2 dose when given every 3 weeks and to compare the pharmacokinetics with those in patients not on EIAEDs treated at the recommended phase 2 dose for other cancers. A phase 2 study has begun based upon the results of this trial.
Patients and Methods
Study Population and Patient Eligibility
Patients 18 years or older with histologically confirmed diagnosis of a progressive or recurrent malignant glioma were eligible to participate, provided they had measurable disease, a Karnofsky performance status of 60 or greater, and acceptable hematologic, liver, and renal function. The latter required an absolute neutrophil count ⩾1500/mm3, a platelet count of ⩾100,000/mm3, serum creatinine <1.5 mg/dl, serum bilirubin <1.5 mg/dl, and AST <3 times the institutional upper normal limits. Patients in the phase 1 portion (group B) of this study had undergone no more than 2 prior chemotherapy regimens, including 1 prior adjuvant therapy and 1 prior regimen for recurrent tumor, or 2 prior regimens for recurrent disease. Patients not on EIAED (group A) received a fixed dose of 350 mg/m2 and were considered part of an expanded phase 2 trial that will be reported separately. These patients had undergone no more than one prior chemotherapy regimen, either as adjuvant chemotherapy or for recurrent disease. In both groups, the interval from prior irradiation or chemotherapy had to be at least 4 weeks, and 6 weeks if prior nitrosourea therapy had been used.
The study was designed to include women and minorities but was not designed to measure differences of intervention effects. No exclusion was allowed based upon race. Patients were excluded if they were pregnant or breast feeding, if they had severe nonmalignant systemic disease or active infection, or if they had any uncontrolled medical conditions that, in the judgment of the investigator, would make the patient inappropriate for entry. Patients previously treated with CPT-11, topotecan, or other topoisomerase 1 inhibitors were also excluded. All patients were provided with and had to sign a written informed consent approved by the local institutional review board at each institution before treatment, informing them of the investigational nature of this study.
Treatment
Patients who met eligibility requirements and signed an informed consent received an intravenous infusion of CPT-11 over a 90-min period every 3 weeks. The initial dose was 350 mg/m2 in both groups. Patients in group A (not on EIAEDs) continued to receive this dose throughout treatment until tumor progression, unacceptable toxicity, or completion of 12 treatment cycles. Patients in group B (on EIAEDs) were treated in groups of 3, beginning at the initial dose. If the first group of 3 patients did not experience dose-limiting toxicity (DLT), a subsequent group of 3 additional patients was enrolled, and the dose was increased by 50-mg/m2 increments. Cohorts of 3 patients were thus enrolled until the DLT was determined. Up to 3 patients were enrolled simultaneously. These patients were observed for DLT for at least 3 weeks from the first day of treatment before new patients were enrolled at the next higher dose. The following dose-escalation rules were used. Three patients were studied at the first dose level. If none of these 3 experienced DLT, then the dose was escalated to the next dose in 3 subsequent patients, but if 1 of 3 patients experienced DLT, then 3 more patients were enrolled at the same dose. If none of these 3 patients experienced DLT, then the dose was escalated to the next dose in 3 subsequent patients. If 1 or more of the additional 3 patients experienced DLT, the maximum tolerated dose (MTD) was considered to have been exceeded, and 3 more patients were treated at the next lower dose (if only 3 were treated at that dose). If 2 or more patients experienced a DLT, the MTD was considered to have been exceeded.
A treatment cycle was considered to be 1 infusion and a 3-week evaluation period. Doses assigned were not allowed to be escalated, and patients remained on that dose until tumor progression, unacceptable toxicity, or completion of 12 treatment cycles.
The definition of DLT for group B patients was based upon the National Cancer Institute (NCI) Common Toxicity Criteria scale (NCI, 1999) and included the following:
Hematologic toxicity: Grade 4 neutropenia lasting > 5 days; neutropenic fever (defined as grade 4 neutropenia with > grade 2 fever); neutropenic infection; or grade 4 thrombocytopenia.
Diarrhea: Grade ⩾3 diarrhea despite maximal intensive supportive treatment with loperamide. Guidelines for loperamide use were to initiate drug at the earliest sign of a poorly formed stool or at the occurrence of 1 to 2 more bowel movements than usual in 1 day. Loperamide was provided to patients at the initial treatment visit.
Nausea or vomiting: Grade >3 despite maximal antiemetic therapy. All patients were treated with dexamethasone 10 mg i.v. and either ondansetron or granisetron before infusion of CPT-11. Ativan (Wyeth-Ayerst Pharmaceuticals, Collegeville, Penn.) or Compazine (GlaxoSmithKline, Research Triangle Park, N.C.) was also allowed at the discretion of the treating physician.
Other nonhematologic toxicity: Grade >3 toxicity attributable to CPT-11 therapy.
Failure to recover: Failure to recover sufficiently from toxicity to be eligible for retreatment with CPT-11 within 28 days of the start of the first cycle of CPT-11 treatment.
The definition of the MTD was the dose level at which 0/3 or 1/6 patients experienced DLT, with at least 2/3 or 2/6 patients experiencing DLT at the next higher dose.
Patient Monitoring and Toxicity Assessment
Complete physical and neurological examinations, including Karnofsky performance status, were performed every other cycle. Weekly complete blood counts with differential and platelets were obtained throughout the course of treatment. Creatinine, BUN, total bilirubin, AST, and serum electrolyte counts were obtained before each cycle. Magnetic resonance imaging of the brain was done every other cycle to assess response. Patients with stable or responding disease received the same dose at the next cycle or a reduced dose if adverse events were observed in the current cycle. If a patient experienced a DLT, the dose of the subsequent cycle was reduced by one dose level (50 mg/m2). If a toxicity was thought to be directly related to CPT-11, subsequent doses were not re-escalated, even if there was minimal or no toxicity at the reduced dose. A new course of treatment could begin if the absolute neutrophil count was ⩾1500/mm2 and the platelet count was ⩾100,000/mm3 and any other treatment-related toxicity was ⩽grade 1. If, after a 1-week delay, toxicity was ⩽grade 1, treatment resumed. If the toxicity did not resolve in 1 week, a second week’s delay was allowed, but retreatment required a reduction of 1 dose level. If retreatment had to be held off for more than 2 weeks, or if the administered dose would be ⩽200 mg/m2, the patient was removed from the study. Routine prophylactic use of hematologic growth factors was not allowed in the first cycle of therapy. Therapeutic use in cycles complicated by neutropenic infection was allowed at the discretion of the treating physician. Because of the possibility of lacrimation, diaphoresis, flushing, abdominal cramping, diarrhea, or other symptoms of early cholinergic syndrome in patients receiving CPT-11, we allowed subcutaneous or intravenous administration of atropine, both therapeutically and prophylactically as needed.
Patients were removed from study if tumor progression was determined by MRI. In this phase 1 trial, the definition of progression was a 25% increase in the sum of the products of all measurable disease over the smallest sum observed, or clear worsening of any evaluable disease, or the appearance of any new lesion. Failure to return for evaluation due to death or deteriorating condition was considered to represent progression.
Pharmacokinetic Evaluation
Sample Collection
Heparinized blood samples (7 ml) were drawn via venipuncture or through an indwelling i.v. heparin lock. One milliliter of blood was withdrawn from the heparin lock and discarded before sample collection at the following times: before drug administration (baseline), 45 min into the infusion, and at the end of the infusions, then 15, 30, 60, and 90 min and 2, 3, 4, 6, 8, 10, and 24 h after the end of infusion on day 1 of a patient’s first cycle, for a total of 14 samples per patient.
Blood samples were immediately centrifuged. Plasma was removed and frozen at ⩽20°C for subsequent analysis by high-performance liquid chromatography (HPLC) for total concentrations of CPT-11 and SN-38 as well as for concentrations of SN-38 glucuronide (SN-38G) and APC. The total time of frozen storage was less than 1 year. The long-term stability of total CPT-11 and SN-38 in plasma has been documented to be at least 2 years when samples were stored at ⩽20°C.
Analytical Methods
Plasma samples were analyzed for total concentrations of CPT-11, APC, and SN-38 by using a validated and sensitive HPLC method (Saltz et al., 1996). In brief, the plasma specimen was mixed with the internal standard (IS, camptothecin) in acidified ace-tonitrile to precipitate plasma proteins, and the mixture was incubated for 15 min at 40°C to convert the analytes to their respective lactones. After addition of triethylamine buffer (pH 4.2), the sample was centrifuged. The supernatant was transferred to an amber vial for injection (60 μl) onto the HPLC system. Chromatographic separation was achieved with a Zorbax-SB-C8 column (MacMod Analytical, Inc., Chadds Ford, Penn.) and a mobile phase consisting of 25:75 (v/v) acetonitrile/0.025 triethylamine buffer (pH 4.2). The fluorescence detector was operated at an excitation wavelength of 372 nm; the CPT-11 and APC were monitored at an emission wavelength of 425 nm; SN-38 and the IS were monitored at 535 nm. To determine the concentrations of SN-38 glucuronide, a separate portion of each plasma sample was hydrolyzed via the addition of a β-glucuronidase solution. The conversion reaction was terminated by precipitating the proteins using an acidified acetonitrile solution of the IS. The conversion of SN-38G to total SN-38 was 100%. The remainder of the procedure was repeated as described above.
The lower limits of quantitation of CPT-11 (expressed as the freebase, Mr 587), SN-38 (expressed as monohydrate, Mr 410), and APC (Mr 618) were 1.28 ng/ml, 0.480 ng/ml, and 0.960 ng/ml, respectively. The assay precision for CPT-11 [APC values in brackets], expressed as the coefficient of variation (% CV) of the estimated concentrations of quality control samples, was 4.9% [8.3%], 4.1% [9.3%], and 5.4% [7.0%], respectively, for the low (12.8 [1.20] ng/ml), medium (160 [12.0] ng/ml), and high (3200 [320] ng/ml) concentrations of CPT-11 [APC] and 10.8%, 3.5%, and 5.1%, respectively, for the low (1.2 ng/ml), medium (12.0 ng/ml), and high (320 ng/ml) concentrations of SN-38. Assay accuracy, expressed as the ratio (or percent) of the estimated to theoretical quality control standard concentrations, averaged 96% to 99% for CPT-11 [98%–101% for APC] and 96% to 101% for SN-38.
Pharmacokinetic Analyses
CPT-11, APC, SN-38, and SN-38G plasma concentrations were analyzed by non-compartmental methods. The time intervals relative to the start of the CPT-11 infusion and the actual sample times were used for the calculations of the time-to-peak curve and the plasma AUC. The maximal plasma concentration for CPT-11 was defined as the concentration achieved at the end of the 90-min infusion. The maximal plasma concentrations for APC, SN-38, and SN-38G were determined by visual inspection of each individual’s plasma concentration-versus-time profile. Elimination rate constants were estimated by linear regression of the last 2 data points on the terminal log linear portion of the concentration-time curve. The terminal half-life (t1/2) was calculated by dividing 0.693 by the elimination rate constant. The AUC was calculated by using the linear trapezoidal rule up to the last measurable data point (for AUC0 – 24), then extrapolated to infinity (AUC). The systemic clearance for CPT-11 was determined by dividing the dose (in milligrams freebase of CPT-11 per meter squared) by the AUC. A metabolic ratio, estimated as the ratio of AUCSN-38 or AUCSN-38 + AUCSN-38G to AUCCPT-11, was used as a measure of the relative extent of the conversion of CPT-11 to SN-38. The relative extent of metabolism of CPT-11 to APC was estimated as AUCAPC/AUCCPT-11 and the relative extent of glucuronidation of SN-38 as the ratio of AUCSN-38 to AUCSN-38G.
Statistical Considerations
The primary end points for this study were to describe toxicity and pharmacokinetics of CPT-11 in a dose-escalating phase 1 study and to define a recommended phase 2 dose. The dose for patients on EIAED therapy was escalated as described earlier, and the DLT, MTD, and safety were evaluated. When the dose escalation scheme described was used, the probability that the dose would be escalated to the next level, based on the true rate of DLT at the current dose, is given by the following:
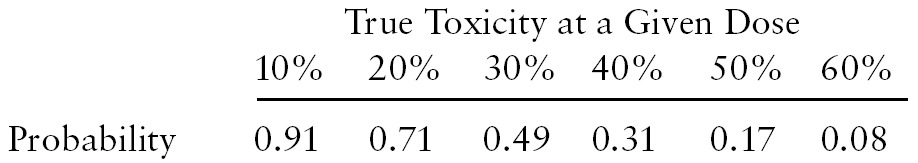 |
Thus, if the true underlying proportion of DLT was 50% at any dose, there was a 17% chance of escalating to the next higher dose.
Pharmacokinetic variables for all compounds are reported as mean values ± SD. The relationships between the CPT-11 dose administered (mg/m2) and AUCs were analyzed by Spearman’s correlation coefficient and linear regression analysis. Interpatient differences in the kinetics were assessed by the coefficient of variation, expressed as the ratio of the SD to the observed mean. Differences between the 2 groups with respect to the kinetic variables were evaluated by using an unpaired 2-tailed t test. Two-tailed probability values of less than 0.05 were regarded as statistically significant. Kinetic variables were also compared to those obtained from the Mayo Clinic trial that included 6 patients with non-CNS malignancies who received 340 mg/m2 of CPT-11 (Pitot et al., 2000). Relative metabolic ratios were then compared with those reported in 3 recent trials.
Results
Patient Characteristics
A total of 48 patients were enrolled in the phase 1 study (group B, Table 1). Three patients did not receive any drug, and 2 patients were ineligible, but treated. These 2 patients are included in the toxicity assessments. The median age was 47 years (range 19 – 77). The majority (37/48) of patients had recurrent GBM, and most had been treated with 1 or more prior chemotherapy regimens. Patients enrolled in the phase 2 study (group A) will be described in more detail in a separate report dealing with response to CPT-11. However, the median age was 53 years, and 75% were patients with recurrent GBM, treated with no more than 1 prior chemotherapy regimen.
Table 1.
Characteristics of the 48 group B patients enrolled and receiving EIAEDs
| Characteristic | Median (range), Percentage, or Number |
|---|---|
| Median Age (years) | 47 (19–77) |
| Karnofsky Performance Status (%) | |
| 100 | 11.3 |
| 90 | 25.8 |
| 80 | 30.6 |
| 70 | 21.0 |
| 60 | 11.3 |
| Sex M:F (%) | 66.1:33.9 |
| Histology (number of patients) | |
| Glioblastoma multiforme | 37 |
| Anaplastic astrocytoma | 7 |
| Anaplastic oligodendroglioma | 1 |
| Anaplastic mixed glioma | 1 |
| Oligodendroglioma | 2 |
| Prior Chemotherapy Regimens (%) | |
| None | 17.7 |
| One | 58.2 |
| Two | 24.2 |
Toxicity
The dose of CPT-11 ranged from 350 to 800 mg/m2. Dose-limiting toxicity was grade 3 diarrhea despite maximal loperamide support and was noted in 2/6 patients at a dose of 800 mg/m2. A third patient treated at this dose also had grade 3 diarrhea but did not receive maximal loperamide support. The recommended phase 2 dose is 750 mg/m2. No treatment-related deaths were reported. Table 2 describes the most common types of toxicity reported in the study.
Table 2.
Reported grade 3 toxicity in cycle 1, indicating definite relationship to drug1
| Dose, m2 (No. of patients treated) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Toxicity | 450 (6) | 500 (3) | 550 (3) | 600 (6) | 650 (6) | 700 (3) | 750 (6) | 800 (6) |
| Diarrhea | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 3 |
| Granulocytopenia2 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 |
| Lymphocytopenia | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Leukopenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Nausea | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
There were no instances of grade 4 toxicity reported.
Grade 3 but not dose limiting.
There were 42 patients evaluable for response by MRI. All had progressed at the time of this report. Although it was not a response study, there were no reports of any patients who had an objective response (>50% decrease in size of tumor). The median progression-free survival time measured from the date of registration into the study was 6 weeks (6 weeks for GBM and 9 weeks for non-GBM). Ten patients were progression-free for 13 weeks or longer, and 3 patients were progression free for 6 months or longer.
Pharmacokinetic Results
Blood samples were obtained from patients during their first course of treatment. Sixteen patients were nonevaluable because of incomplete sample collections. Pharmacokinetic variables were characterized for 56 patients: 22 patients in group A (non-EIAEDs), who were treated with 350 mg/m2 of CPT-11, and 34 patients in group B (EIAEDs), who were treated with doses ranging from 350 to 800 mg/m2.
The pharmacokinetic characteristics for CPT-11, APC, SN-38, and SN-38G are summarized in Tables 3 and 4. Comparisons between the 2 groups at the 350-mg/m2 dose level are displayed in Table 5, which also includes the pharmacokinetic characteristics from the Mayo Clinic trial that included 6 patients with non-CNS malignancies who received 340 mg/m2 of CPT-11 (Pitot et al., 2000). Overall, the kinetic variables in patients receiving no anticonvulsants or steroids in our trial are similar to those of non-CNS malignancy patients. Unfortunately, kinetic characteristics were available for only 2 patients in the EIAED group at the 350-mg/m2 dose level. However, a comparison of the kinetics of the 2 groups revealed that the peak concentrations and AUCs of CPT-11 and SN-38 were substantially decreased in the EIAED-treated patients. Although not as substantial, a quantitative decrease in peak concentrations and AUCs for APC and SN-38G was also observed. CPT-11 clearance values (29.3 ± 7.11 l/h/m2, n = 34) for the EIAED group were increased 2-fold compared to those for the non-EIAED group (14.2 ± 4.12 l/h/m2, n = 7) or by 1.5-fold compared to those for patients receiving only dexamethasone and/or non-EIAEDs (20.1 ± 5.72 l/h/m2, n = 15).
Table 3.
Pharmacokinetic parameters of CPT-111
| Group | Dose (mg/m2) | No. of Patients | Cpmax (μg/ml) | t1/2 (h) | AUC0–24 (μg × h/ml) | AUC2 (μg × h/ml) | CL (liters/h/m2) | CV3 (%) |
|---|---|---|---|---|---|---|---|---|
| A | 350 | 22 | 3.3 (0.50) | 6.7 (1.30) | 17.3 (5.22) | 18.4 (5.73) | 18.2 (5.86) | 32.2 |
| B | 350 | 2 | 2.4 (0.50) | 7.5 (0.57) | 9.2 (0.14) | 9.7 (0.07) | 31.6 (0.07) | 0.22 |
| B | 400 | 3 | 2.7 (0.50) | 7.7 (0.88) | 10.7 (3.34) | 11.2 (3.62) | 33.8 (12.6) | 37.1 |
| B | 450 | 5 | 3.3 (0.50) | 5.9 (0.34) | 12.5 (2.81) | 12.7 (2.84) | 31.9 (6.78) | 21.3 |
| B | 500 | 3 | 4.2 (0.55) | 5.3 (0.54) | 19.3 (0.59) | 19.7 (0.61) | 22.1 (0.67) | 3.00 |
| B | 550 | 2 | 3.9 (0.07) | 5.4 (1.27) | 16.2 (4.24) | 16.5 (4.10) | 29.9 (7.42) | 24.8 |
| B | 600 | 4 | 4.0 (0.43) | 6.1 (0.75) | 16.8 (2.34) | 17.1 (2.51) | 31.1 (4.80) | 15.4 |
| B | 650 | 4 | 4.4 (1.39) | 6.4 (0.40) | 19.5 (4.89) | 20.4 (4.99) | 29.1 (6.84) | 23.5 |
| B | 700 | 3 | 5.6 (1.74) | 6.2 (3.08) | 25.6 (7.01) | 26.9 (7.97) | 24.1 (7.68) | 31.9 |
| B | 750 | 4 | 4.3 (1.03) | 5.4 (0.93) | 21.2 (5.80) | 21.6 (5.83) | 33.1 (9.31) | 28.1 |
| B | 800 | 4 | 6.0 (1.28) | 6.0 (0.63) | 27.3 (3.50) | 27.9 (3.81) | 25.3 (3.23) | 12.8 |
Abbreviations used are as follows: AUC, area under the curve; CL, systemic clearance; Cpmax, maximal plasma concentration; CV, coefficient of variation; t1/2, terminal half-life (harmonic
mean).
Values reported are means (±SD) unless otherwise indicated.
Average percent extrapolation between the AUC0-24 and AUC (3%).
Percent CV related to CL.
Table 4.
Pharmacokinetic parameters of CPT-11 metabolites*
|
CPT-11 Dose (mg/m2)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolite Profile | 350 (group A) | 350 (group B) | 400 | 450 | 500 | 550 | 600 | 650 | 700 | 750 | 800 |
| APC | |||||||||||
| tmax (h) | 3.16 (0.93) | 2.00 (0) | 2.5 (0.87) | 1.95 (0.33) | 2.25 (0.25) | 2.59 (0.12) | 2.27 (0.27) | 2.63 (1.53) | 2.91 (0.37) | 2.44 (0.66) | 2.27 (0.43) |
| Cpmax (ng/ml) | 527 (284) | 414 (30.4) | 729 (283) | 644 (301) | 1106 (610) | 1006 (33.9) | 1532 (780) | 1090 (382) | 1461 (693) | 1816 (1259) | 1578 (557) |
| t1/2 (h) | 6.5 (1.52) | 7.6 (1.13) | 7.8 (1.62) | 6.5 (0.80) | 5.2 (0.41) | 5.8 (0.71) | 6.01 (1.50) | 6.8 (1.03) | 6.5 (4.17) | 5.4 (0.67) | 5.7 (0.98) |
| AUC0 – 24 (μg × h/ml) | 4.8 (2.91) | 3.1 (0.28) | 5.6 (3.15) | 4.3 (2.35) | 8.0 (2.86) | 7.6 (0.71) | 10.8 (3.9) | 8.9 (2.89) | 14 (7.73) | 12.2 (6.64) | 11.0 (3.72) |
| AUC (μg × h/ml) | 5.3 (3.27) | 3.4 (0.28) | 6.2 (3.76) | 4.6 (2.54) | 8.3 (2.89) | 7.9 (0.85) | 11.3 (3.8) | 9.5 (3.02) | 15.8 (8.44) | 12.6 (6.93) | 11.6 (4.19) |
| AUCAPC/AUCCPT-11 (%)# | 28.8 | 35.1 | 55.4 | 36.2 | 42.1 | 47.9 | 66.1 | 46.6 | 58.7 | 58.3 | 41.6 |
| SN-38 | |||||||||||
| tmax (h) | 1.78 (0.55) | 1.13 (0.53) | 2.00 (0.87) | 1.45 (0.44) | 1.67 (0.14) | 1.59 (0.12) | 1.66 (0.24) | 2.40 (1.69) | 1.25 (0.43) | 1.60 (0.63) | 1.85 (0.46) |
| Cpmax (ng/ml) | 41.1 (26.6) | 21.5 (7.35) | 32.9 (24.1) | 20.6 (12.8) | 33.2 (5.64) | 22.8 (1.13) | 21.6 (8.36) | 21.1 (8.91) | 40.5 (20.5) | 26.1 (5.09) | 40.2 (14.2) |
| t1/2 (h) | 14.9 (7.92) | 16.0 (4.03) | 23.1 (9.33) | 11.6 (3.22) | 8.5 (0.51) | 11.1 (1.48) | 14.5 (8.26) | 17.2 (16.8) | 10.4 (11.0) | 9.8 (3.44) | 11.9 (6.98) |
| AUC0 – 24 (ng × h/ml) | 314 (220) | 123 (38.9) | 212 (182) | 136 (90.7) | 211 (64.8) | 133 (24.0) | 161 (46.6) | 154 (65.2) | 257 (145) | 162 (45.4) | 230 (48.2) |
| AUC (ng × h/ml)2 | 558 (602) | 168 (33.9) | 365 (377) | 166 (127) | 229 (76.6) | 156 (23.3) | 296 (161) | 278 (167) | 409 (304) | 190 (58.6) | 300 (95.3) |
| AUCSN-38/AUCCPT-11(%)# | 3.1 | 1.7 | 3.3 | 1.3 | 1.2 | 1.0 | 1.7 | 1.4 | 1.5 | 0.9 | 1.1 |
| AUCSN-38 + AUCSN-38G/AUC CPT-11 (%)# | 11.8 | 11.0 | 21.1 | 7.6 | 7.6 | 7.3 | 11.5 | 7.5 | 9.5 | 8.5 | 6.7 |
| SN-38G | |||||||||||
| tmax (h) | 2.11 (0.63) | 1.90 (0.14) | 1.86 (0.62) | 1.41 (0.38) | 1.92 (0.14) | 1.84 (0.12) | 1.90 (0.27) | 2.17 (0.88) | 1.75 (0.25) | 1.93 (0.41) | 1.66 (0.14) |
| Cpmax (ng/ml) | 120 (61.4) | 115 (32.5) | 207 (105) | 105 (33.1) | 179 (37.5) | 173 (42.4) | 198 (71.5) | 140 (60.2) | 232 (110) | 169 (76.6) | 196 (66.1) |
| t1/2 (h) | 15.1 (6.31) | 16.3 (8.13) | 17.5 (9.40) | 12.3 (2.80) | 9.1 (1.98) | 9.5 (0.92) | 12.8 (5.71) | 14.6 (8.58) | 11.0 (4.75) | 12.3 (3.89) | 11.1 (3.16) |
| AUC0 – 24 (ng × h/ml) | 1099 (663) | 690 (154) | 1355 (824) | 625 (295) | 1134 (181) | 944 (295) | 1422 (551) | 870 (214) | 1719 (1007) | 1401 (1117) | 1301 (404) |
| AUC (ng × h/ml) | 1589 (1045) | 894 (134) | 1997 (1695) | 793 (437) | 1270 (168) | 1052 (308) | 1671 (516) | 1257 (361) | 2145 (1379) | 1640 (1234) | 1570 (517) |
| AUCSN-38/AUCSN-38G (%)# | 35.1 | 18.8 | 18.3 | 20.9 | 18.0 | 14.7 | 17.7 | 22.1 | 19.1 | 11.6 | 19.1 |
Abbreviations used are as follows: AUC, area under the curve; Cpmax, maximal plasma concentration; tmax, time to peak; T1/2 (h), terminal half-life (harmonic mean).
Values reported are mean (± SD).
Average % extrapolation between the AUC0 – 24 and AUC (APC [7%]; SN-38 [46%]; SN-38G [27%]).
Table 5.
Pharmacokinetic comparisons between patients on EIAEDs and patients not on EIAEDs receiving CPT-11 at the 350-mg/m2 dose level*
| EIAED group B | Non-EIAED group A | ||
|---|---|---|---|
| PK Parameter | n = 2 | None (n = 7) | ±Steroid (n = 15) |
| CPT-11 (Freebase) | |||
| Cpmax (μg/ml) | 2.4# | 3.7 (3.4)§ | 3.1# |
| t1/2 (h) | 7.5 | 6.8 (12)§ | 6.7 |
| AUC (μg × h/ml) | 9.7# | 22.9 (23)§ | 16.2# |
| CL (l/h/m2) | 31.6# | 14.2 (12)§ | 20.1# |
| APC | |||
| Cpmax (ng/ml) | 414 | 619 (ND) | 484 |
| t1/2 (h) | 7.6 | 6.7 (ND) | 6.4 |
| AUC (μg × h/ml) | 3.4 | 6.2 (ND) | 4.6 |
| SN-38 | |||
| Cpmax (ng/ml) | 21.5# | 63.8 (56)§ | 30.5# |
| t1/2 (h) | 16.0 | 16.2 (21) § | 14.3 |
| AUC (ng × h/ml) | 168# | 768 (714)§ | 319# |
| SN-38G | |||
| Cpmax (ng/ml) | 115 | 145 (168)§ | 109 |
| t1/2 (h) | 16.3 | 13.8 (17)§ | 15.8 |
| AUC (ng × h/ml) | 894 | 1944 (2329)* | 1424 |
Abbreviations used are as follows: APC, 7-ethyl-10[4-N-(5 aminopentanoic acid)-1-piperidine]-carbonyloxycamptothecin; AUC, area under the curve; CL, systemic clearance; Cpmax, maximal plasma concentration; EIAED, enzyme-inducing antiepileptic drug, ND = not done; PK, pharmacokinetic; t1/2, terminal half-life.
Mean values for 2 patients receiving EIAED therapy; 7 patients who did not receive EIAED medications, non-EIAED medications, or steroids; and 15 patients who received non-EIAED medications with or without steroids.
P < 0.05 versus group A patients receiving no steroids.
Non-CNS malignancy patients (340 mg/m2 CPT-11 every 3 weeks, n = 6) from Mayo Clinic Study (Pitot et al., 2000).
The EIAED patients were empirically categorized into 4 groups (phenytoin, carbamazepine, phenobarbital, and combination EIAEDs) for the purposes of comparison (Table 6). The non-EIAED drugs, particularly gabapentin, had a small but statistically significant effect on the clearance of CPT-11 when compared to the clearance values for patients not receiving steroids or antiepileptic agents. Six patients were receiving valproic acid in combination with a non-EIAED. For those patients receiving valproic acid, CPT-11 clearance values (19 ± 2.98 l/h/m2) were unchanged compared to the clearance values for the other group A patients (18 ± 16.69 l/h/m2, P = 0.73). However, SN-38 AUCs (185 ± 49.94 ng × h/ml) were significantly lower for the patients receiving valproic acid compared to the SN-38 AUCs (552 ± 306 ng × h/ml, P = 0.01) for patients receiving other non-EIAEDs ± steroids. SN-38G AUCs of the 2 groups were not different (1113 ± 395 vs. 1713 ± 1182 ng × h/ml, P = 0.24). Likewise, there was no difference in the AUC values for CPT-11 and SN-38 for those patients receiving dexamethasone alone compared to the no-anticonvulsant group.
Table 6.
Influence of anticonvulsants on CPT-11 clearance
| Group A (non-EIAED group, n = 22) | Mean (±SD) CL (l/h/m2) |
|---|---|
| Steroid + Non-EIAEDs (n = 11) | 21.2 (±5.74) |
| Steroid only (n = 4) | 17.0 (±5.05) |
| None (n = 7) | 14.2 (±4.12) |
| Group B (EIAED group, n = 34) | |
| Phenytoin ± Steroid ± Non-EIAEDs (n = 17) | 30.1 (±7.40) |
| Carbamazapine ± Steroid ± Non-EIAEDs (n = 9) | 28.1 (±7.92) |
| Phenobarbital ± Steroid ± Non-EIAEDs (n = 2) | 23.6 (±0.99) |
| Combination EIAEDs (n = 6) | 30.9 (±5.82) |
Abbreviations used are as follows: CL, systemic clearance; EIAED, enzyme-inducing antiepileptic drug.
The relationship between CPT-11 dose and systemic exposure (AUC) was relatively linear (r2 = 0.64, P = 4.53E−06) over the dosage range of 350 to 800 mg/m2. Likewise, the clearance of CPT-11 was dose independent (r2 = 0.035). However, no proportionate increase was observed in the SN-38 or SN-38G AUC nor in the dose-normalized AUCs with increasing dosages of CPT-11 (r2 = 0.04). Additionally, there was a poor linear relationship between CPT-11 dose and APC systemic exposure (r2 = 0.35). The pharmacodynamic assessment of correlation between (a) CPT-11, APC, and SN-38 systemic exposure, (b) NCI grade of diarrhea, and (c) percent change in neutrophil count showed no correlations in either of the treatment groups.
At the MTD of 750 mg/m2, the AUC for CPT-11 (21.6 μg × h/ml) matched the AUC (18 μg × h/ml) in the group of patients receiving no anticonvulsants treated with 350 mg/m2 of CPT-11. The mean percent change in neutrophil count (nadir-baseline count/baseline count) was 62.4% (± 27%) for the non-EIAED group (350 mg/m2) and 58.2% (± 18%) for the EIAED group treated with 750 mg/m2 of CPT-11. There was considerable variability observed for the SN-38 AUCs at all of the dose levels. The mean AUC for SN-38 at the MTD was not statistically different from the SN-38 AUC at the 350-mg/m2 dose level for the non-EIAED patient group.
Discussion
The goals of this study were to define the toxicity and pharmacokinetics of CPT-11 in patients receiving EIAED therapy and to establish a recommended phase 2 dose. For patients receiving non-EIAEDs, a fixed dose of 350 mg/m2 of CPT-11 was administered i.v. every 3 weeks. Dose escalations in the EIAED patient groups ranged from 350 to 800 mg/m2. The DLT at the 800-mg/m2 dose level was grade 3 diarrhea despite maximal doses of loperamide.
EIAEDs altered the pharmacokinetics of CPT-11 and its metabolites. At the comparative dose level of 350 mg/m2, peak concentrations and the AUC of CPT-11, APC, SN-38, and SN-38G were substantially lower in the EIAED patient group. A moderate-to-fair linear relationship existed between CPT-11 dose and AUC over the dosage range of 350 to 800 mg/m2. No relationship was observed between CPT-11 dose and the AUCs for SN-38 or SN-38G. The clearance of CPT-11 was dose independent. The clearance of CPT-11 in the EIAED group (29.3 ± 7.11 l/h/m2) was increased 1.6-fold compared to the clearance for the non-EIAED group (18.2 l/h/m2). The majority of the patients in the EIAED group were receiving phenytoin with or without dexamethasone. CPT-11 clearance values were highest among the patients receiving phenytoin or carbamazepine (30.1 and 28.1 l/h/m2, respectively) and were lowest among those receiving phenobarbital (23.6 l/h/m2). The relative influence of individual anticonvulsant agents and their dose effect on the pharmacokinetics of CPT-11 in glioma patients are also being evaluated in a larger database from 4 NCI-sponsored trials in glioma patients (Reid et al., 2001). As was observed in our study, the findings from the larger database suggest that the AUCs for CPT-11 and SN-38 were not altered by dexamethasone, a reported inducer of CYP3A4 and glucuronosyl transferase activity (McCune et al., 2000; Sutherland et al., 1993). Likewise, other covariates including age and gender did not influence CPT-11 or SN-38 AUCs. We also observed that the non-EIAEDs, especially gabapentin, appeared to have a small but statistically significant effect on CPT-11 clearance. Preclinical data (Gupta et al., 1997) indicated that we might observe an increase in SN-38 exposure in those patients receiving valproic acid due to the inhibition of UGT1A1 conjugation of SN-38. We actually observed the opposite effect. For the small number of patients receiving valproic acid in combination with another non-EIAED, SN-38 AUCs were comparatively lower. Additionally, the grade of diarrhea and the percent change in neutrophil counts in the valproic acid group were similar to those of the other group A patients. The same observation has been recently reported by other investigators (Raymond et al., 2003). Neither SN-38 exposure nor toxicity was increased in patients with glioblastoma who were treated with 350 mg/m2 of CPT-11 while receiving concomitant valproic acid.
The relative metabolism ratios observed in our trial are compared with those reported in 3 recent clinical trials in Table 7. The analytical procedures and sampling schedules were similar with a few noted exceptions. Investigators at the Mayo Clinic (Pitot et al., 2000) and the Rotterdam Cancer Institute (de Jonge et al., 2000) conducted their phase 1 dose-escalating trials in patients with non-CNS malignancies, in which CPT-11 was administered on an every-3-week schedule. Within comparative patient groups, the ratios are remarkably similar. Changes in the metabolic ratios are subject to metabolism as well as elimination and therefore are not a direct measure of conversions. However, the metabolic ratios viewed within the context of the metabolism and elimination of CPT-11 and SN-38 can provide speculative mechanisms by which EIAEDs influence the disposition of CPT-11 and its metabolites. EIAEDs increased the clearance of CPT-11. Increased conversion of CPT-11 to its predominant metabolite, APC, by induction of CYP3A4 is suggested by the APC-to-CPT-11 ratio of 48.8 in the EIAED groups versus 28.8 in the non-EIAED group. Enhanced elimination of CPT-11 via biliary transport mechanisms could have also occurred. The low SN-38-to-CPT-11 ratio of 1.5 for the EIAED group versus 3.1 for the non-EIAED group suggests a decreased conversion of CPT-11 to SN-38, consistent with the 1.9-fold decrease in CPT-11’s AUC in the EIAED group. Saturation of the high-affinity, low-Km carboxylesterase with the higher doses of CPT-11 could have also accounted for the lower SN-38 AUCs. Additionally, enhanced elimination of SN-38 by one or more pathways, such as biliary elimination (MRP2, MXR) or glucuronidation, is also a plausible explanation. The comparatively low ratio of SN-38 to SN-38G of 18.0 in the EIAED group versus 35.1 in the non-EIAED group is suggestive of increased glucuronidation of SN-38 by the inducible UGT1A1. In conjunction, we observed approximately the same metabolic ratio for both the EIAED group and the non-EIAED group for the combined SN-38 + SN-38G-to-CPT-11 ratio, consistent with a decreased SN-38, increased SN-38G, and decreased CPT-11 in the EIAED group. EIAEDs appear to influence multiple pathways relevant to CPT-11 and SN-38 disposition.
Table 7.
Relative metabolic ratios of current trial compared with those of 3 recent trials
| Study Population (reference) Groups and Dose (mg/m2) | ||||||
|---|---|---|---|---|---|---|
| Gliomas (current study) | ||||||
| Ratio | Gliomas1 EIAEDs (n = 32) 125 q week | EIAEDs (n = 34) 350–800 q 3 weeks | Non-EIAEDs (n = 22) 350 q 3 weeks | Phase 12 (n = 34) 240–340 q 3 weeks | Colorectal Cancer1 (n = 99) 125 q 3 weeks | Phase 13 (n = 45) 175–300 q 3 weeks |
| SN-38/CPT-11 | 1.6 | 1.5 | 3.1 | 3.3 | 3.3 | 3.8 |
| SN-38/SN-38G | 17.9 | 18.0 | 35.1 | 31.9 | 27.9 | * |
| (SN-38 + SN-38G)/CPT-11 | 11.6 | 9.8 | 11.8 | 13.9 | 17.9 | * |
| APC/CPT-11 | Not Done | 48.8 | 28.8 | Not Done | Not Done | 30.2 |
Different analytical procedure for determination of SN-38G.
Our pharmacokinetic results are somewhat in contrast with the recently reported phase 1 escalation trial in glioma patients treated with CPT-11 using the weekly schedule (Gilbert et al., 2003). Dose escalations ranged from 125 to 444 mg/m2 for patients receiving EIAEDs. CPT-11 clearance values were found to be dose dependent. At the reported MTD of 411 mg/m2, however, the mean clearance value for CPT-11 (29.7 l/h/m2) was virtually identical to our observed mean CPT-11 clearance value (29.3 l/h/m2) and to the clearance value that Friedman et al. (1999) reported (30.4 l/h/m2). Similarly to the find-ing in our study, no relationship was found between CPT-11 dose and SN-38G AUC values. We observed no relationship between CPT-11 dose and SN-38 AUCs. In contrast, a fair relationship (r2 = 0.46) between CPT-11 dose and SN-38 AUCs was reported by Gilbert et al. (2003). This latter observation again raises the issue of the saturability of the human carboxylesterase responsible for the conversion of CPT-11 to the active metabolite SN-38 (Slatter et al., 1997). Preliminary results from the pooled analysis of data from the 4 NCI-sponsored trials in glioma patients with CPT-11 doses ranging from 100 to 800 mg/m2 indicated that SN-38 AUCs did not increase at doses greater than 350 mg/m2 (Reid et al., 2001).
For patients receiving stable (2 weeks) doses of EIAEDs, the recommended phase 2 dose of CPT-11 is 750 mg/m2 repeated every 3 weeks. The efficacy of CPT-11 is currently being evaluated in an NABTC phase 2 trial for glioma patients receiving EIAEDs. As a translational message, our NABTC experiences strongly suggest that the newer second-generation non-EIAEDs with little or no enzyme-inducing potential should be used when clinically indicated for patients with gliomas.
Acknowledgement
The NABTC members express their gratitude to the Pharmacia Corporation, especially Drs. Larry Schaaf, J. Patrick McGovern, and Barbara Duncan, for the analytical support provided in this study.
Appendix
The North American Brain Tumor Consortium investigators and grant numbers for this study are presented in the table below.
Appendix Table.
North American Brain Tumor Consortium investigators and funding under Prime Award CA 62399
| Institution | Investigators | NABTC Grant | GCRC Grant |
|---|---|---|---|
| University of California at San Francisco | Michael Prados, M.D.*
Susan Chang, M.D. M. Kelly Nicholas, M.D., Ph.D. |
CA 62422 | M01-RR00079 |
| The University of Texas M.D. | W.K.A. Yung, M.D.* | CA 62412 | None |
| Anderson Cancer Center | Kurt Jaeckle, M.D. | ||
| University of Texas Southwestern Medical Center | Karen Fink, M.D., Ph.D.* | CA 62455 | M01-RR00633 |
| The Dana Farber Cancer Center | Howard Fine, M.D.*
Patrick Wen, M.D. |
CA 62407 | None |
| University of Pittsburgh | David Schiff, M.D.* | CA 62404 | M01-RR00056 |
| University of Texas Health Science Center at San Antonio | John Kuhn, Pharm.D.* | CA 62426 | M01-RR0134 |
| University of California, Los Angeles | Timothy Cloughesy, M.D.* | CA 62399 | M01-RR0865 |
| University of Michigan | Harry Greenberg, M.D.*
Larry Junck, M.D. |
CA 62399 | M01-RR00042 |
| University of Wisconsin | Minesh Mehta, M.D.*
I.H. Robbins, M.D., Ph.D. |
CA 62421 | M01-RR03186 |
Abbreviations used are as follows: GCRC 5 General Clinical Research Center, NABTC, North American Brain Tumor Consortium.
Principal Investigator. Dr. Fine is currently at the National Institutes of Health, and Dr. Schiff is at the University of Virginia.
Footnotes
The North American Brain Tumor Consortium Investigators and grant numbers are listed in the Appendix.
Abbreviations used are as follows: APC, 7-ethyl-10[4-N-(5 aminopentanoic acid)-1-piperidine]-carbonyloxycamptothecin; AUC, area under the concentration-time curve; CPT-11, irinotecan; DLT, dose-limiting toxicity; EIAED, enzyme-inducing antiepileptic drug; GBM, glioblastoma multiforme; HPLC, high-performance liquid chromatography; IS, internal standard; MRP2, multidrug resistance protein 2; MTD, maximum tolerated dose; NABTC, North American Brain Tumor Consortium; NCI, National Cancer Institute; UGT1A1, uridine-diphosphoglucuronosyl transferase.
References
- Brangi M, Litman T, Ciotti M, Nishiyama K, Kohlagen G, Takimoto C, Robey R, Pommier Y, Fojo T, Bates SE. Camptothecin resistance: Role of the ATP-binding cassette (ABC), mitoxantrone-resistance half-transporter (MXR), and potential for glucuronidation in MXR-expressing cells. Cancer Res. 1999;59:5938–5946. [PubMed] [Google Scholar]
- Chu XY, Kato Y, Ueda K, Suzuki H, Niinuma K, Tyson CA, Weizer V, Dabbs JE, Froehlich R, Green CE, Sugiyama Y. Biliary excretion mechanism of CPT-11 and its metabolites in humans: Involvement of primary active transporters. Cancer Res. 1998;58:5137–5143. [PubMed] [Google Scholar]
- de Jonge MJ, Verweij J, de Bruijn P, Brouwer E, Mathijssen RH, van Alphen RJ, de Boer-Dennert MM, Vernillet L, Jacques C, Sparreboom A. Pharmacokinetic, metabolic, and pharmacodynamic profiles in a dose-escalating study of irinotecan and cisplatin. J Clin Oncol. 2000;18:195–203. doi: 10.1200/JCO.2000.18.1.195. [DOI] [PubMed] [Google Scholar]
- Friedman HS, Petros WP, Friedman AH, Schaaf LJ, Kerby T, Lawyer J, Parry M, Houghton PJ, Lovell S, Rasheed K, Cloughesy T, Stewart ES, Colvin OM, Provenzale JM, McLendon RE, Bigner DD, Cokgor I, Haglund M, Rich J, Ashley D, Malczyn J, Elfring GL, Miller LL. Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol. 1999;17:1516–1525. doi: 10.1200/JCO.1999.17.5.1516. [DOI] [PubMed] [Google Scholar]
- Gilbert MR, Supko JG, Batchelor T, Lesser G, Fisher JD, Piantadosi S, Grossman S. Phase I clinical and pharmacokinetic study of irinotecan in adults with recurrent malignant glioma. Clin Cancer Res. 2003;9:2940–2949. [PubMed] [Google Scholar]
- Gupta E, Wang X, Ramirez J, Ratain MJ. Modulation of glucuronidation of SN-38, the active metabolite of irinotecan, by valproic acid and phenobarbital. Cancer Chemother Pharmacol. 1997;39:440–444. doi: 10.1007/s002800050595. [DOI] [PubMed] [Google Scholar]
- Haaz MC, Riche C, Rivory L.P, Robert J. Biosynthesis of an aminopiperidino metabolite of irinotecan [7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecine] by human hepatic microsomes. Drug Metab Dispos. 1998;26:769–774. [PubMed] [Google Scholar]
- Hagenbuch N, Reichel C, Stieger B, Cattori V, Fattinger KE, Landmann L, Meier PJ, Kullak-Ublick GA. Effect of phenobarbital on the expression of bile salt and organic anion transporters of rat liver. J Hepatol. 2001;34:881–887. doi: 10.1016/s0168-8278(01)00097-6. [DOI] [PubMed] [Google Scholar]
- Hare CB, Elion GB, Houghton PJ, Houghton JA, Keir S, Marcelli SL, Bigner DD, Friedman HS. Therapeutic efficacy of the topoisomerase I inhibitor 7-ethyl-10-(4-[1-piperidino]-1-piperidino)-carbonyloxy-camptothecin against pediatric and adult central nervous system tumor xenografts. Cancer Chemother Pharmacol. 1997;39:187–191. doi: 10.1007/s002800050558. [DOI] [PubMed] [Google Scholar]
- Hess KR, Wong ET, Jaeckle KA, Kyritsis AP, Levin VA, Prados MD, Yung WKA. Response and progression in recurrent malignant glioma. Neuro-Oncol. 1999;1:282–288. doi: 10.1215/15228517-1-4-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humerickhouse R, Lohrbach K, Li L, Bosron WF, Dolan ME. Characterization of CPT-11 hydrolysis by human liver carboxylesterase isoforms hCE-1 and hCE-2. Cancer Res. 2000;60:1189–1192. [PubMed] [Google Scholar]
- Iyer L, King CD, Whitington PF, Green MD, Roy SK, Tephly TR, Coffman BL, Ratain MJ. Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J Clin Invest. 1998;101:847–854. doi: 10.1172/JCI915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R, Morton CL, Danks MK, Potter PM. Proficient metabolism of irinotecan by a human intestinal carboxylesterase. Cancer Res. 2000;60:4725–4728. [PubMed] [Google Scholar]
- Maliepaard M, van Gastelen MA, Tohgo A, Hausheer FH, van Waardenburg RC, de Jong LA, Pluim D, Beijnen JH, Schellens JH. Circumvention of breast cancer resistance protein (BCRP)-mediated resistance to camptothecins in vitro using non-substrate drugs or the BCRP inhibitor GF120918. Clin Cancer Res. 2001;7:935–941. [PubMed] [Google Scholar]
- McCune JS, Hawke RL, LeCluyse EL, Gillenwater HH, Hamilton G, Ritchie J, Lindley C. In vivo and in vitro induction of human cytochrome P4503A4 by dexamethasone. Clin Pharmacol Ther. 2000;68:356–366. doi: 10.1067/mcp.2000.110215. [DOI] [PubMed] [Google Scholar]
- NCI. National Cancer Institute (1999) Common Toxicity Criteria version 2.0. Available at https://webapps.ctep.nci.nih.gov/ctcv2/plsql/ctc000w$.startup. [PubMed]
- Pitot HC, Goldberg RM, Reid JM, Sloan JA, Skaff PA, Erlichman C, Rubin J, Burch PA, Adjei AA, Alberts SA, Schaaf LJ, Elfring G, Miller LL. Phase I dose-finding and pharmacokinetic trial of irinotecan hydrochloride (CPT-11) using a once-every-three-week dosing schedule for patients with advanced solid tumor malignancy. Clin Cancer Res. 2000;6:2236–2244. [PubMed] [Google Scholar]
- Raymond E, Fabbro M, Boige V, Rixe O, Frenay M, Vassal G, Faivre S, Sicard E, Germa C, Rodier JM, Vernillet L, Armand JP. Multicentre phase II study and pharmacokinetic analysis of irinotecan in chemotherapynaïve patients with glioblastoma. Ann Oncol. 2003;14:603–614. doi: 10.1093/annonc/mdg159. [DOI] [PubMed] [Google Scholar]
- Reid JM, Cha S, Buckner JC, Petros WP, Friedman HS, Tourt-Uhlig S, Walker A, Kuhn JG, Prados M, Supko JG, Gilbert MR, Batchelor TT, Schaaf L, Gaylor SK, McGovren JP, Gallo JM. Pharmacokinetics of CPT-11 in glioma patients: Pooled analysis of data from 4 NCI-sponsored trials (Duke, NABTC, NABTT, NCCTG) Clin Cancer Res. 2001;7:3737S. (abstract 415) [Google Scholar]
- Saltz LB, Kanowitz J, Kemeny NE, Schaaf L, Spriggs D, Staton BA, Berkery R, Steger C, Eng M, Dietz A, Locker P, Kelsen DP. Phase I clinical and pharmacokinetic study of irinotecan, fluorouracil, and leucovorin in patients with advanced solid tumors. J Clin Oncol. 1996;14:2959–2967. doi: 10.1200/JCO.1996.14.11.2959. [DOI] [PubMed] [Google Scholar]
- Santos A, Zanetta S, Cresteil T, Deroussent A, Pein F, Raymond E, Vernillet L, Risse ML, Boige V, Gouyette A, Vassal G. Metabolism of irinotecan (CPT-11) by CYP3A4 and CYP3A5 in humans. Clin Cancer Res. 2000;6:2012–2020. [PubMed] [Google Scholar]
- Slatter JG, Su P, Sams JP, Schaaf LJ, Wienkers LC. Bioactivation of the anticancer agent CPT-11 to SN-38 by human hepatic microsomal carboxylesterases and the in vitro assessment of potential drug interactions. Drug Metab Dispos. 1997;25:1157–1164. [PubMed] [Google Scholar]
- Sutherland L, Ebner T, Burchell B. The expression of UDP-glucuronosyltransferases of the UGT1 family in human liver and kidney and in response to drugs. Biochem Pharmacol. 1993;45:295–301. doi: 10.1016/0006-2952(93)90064-4. [DOI] [PubMed] [Google Scholar]


