Abstract
In individuals with brain tumors, pharmacodynamic and pharmacokinetic studies of therapeutic agents have historically used analyses of drug concentrations in serum or cerebrospinal fluid, which unfortunately do not necessarily reflect concentrations within the tumor and adjacent brain. This review article introduces to neurological and medical oncologists, as well as pharmacologists, the application of microdialysis in monitoring drug metabolism and delivery within the fluid of the interstitial space of brain tumor and its surroundings. Microdialysis samples soluble molecules from the extracellular fluid via a semipermeable membrane at the tip of a probe. In the past decade, it has been used predominantly in neurointensive care in the setting of brain trauma, vasospasm, epilepsy, and intracerebral hemorrhage. At the first Carolyn Frye-Halloran Symposium held at Massachusetts General Hospital in March 2002, the concept of microdialysis was extended to specifically address its possible use in treating brain tumor patients. In doing so we provide a rationale for the use of this technology by a National Cancer Institute consortium, New Approaches to Brain Tumor Therapy, to measure levels of drugs in brain tissue as part of phase 1 trials.
Therapeutic approaches to malignant brain tumors impose significant constraints upon the clinicians and basic scientists designing therapy for brain tumor patients. Although surgical resection and radiation therapy are paramount to local control of the disease, microscopic foci of infiltrating tumor cells appear resistant to both interventions, which spur the use of adjuvant regimens of chemotherapy. Despite the logical selection of these drugs using preclinical screens of molecular and enzymatic targets, the past 3 decades have offered no improvement in the survival for patients with malignant brain tumors (DeAngelis, 2001; Dropcho, 2001). The lack of efficacy of therapies is all the more surprising in an era that has contributed to “rational drug design” for brain tumor therapies, administration and drug schedules to maximize brain penetration, and awareness of the powerful role of cytochrome P450 induction on the activation of brain tumor-specific agents.
Irrespective of location or histology, drug delivery is a critical determinant of successful chemotherapy. The blood-brain barrier (BBB)2 raises obstacles to drug delivery to brain tumors. Most drugs administered systemically for the treatment of brain tumors have properties that promote passage across the barrier such as low molecular weight (<200 Da), lipophilicity, and low protein-binding capability (Rall and Zubrod, 2000). It is likely that the barrier is disrupted in a nonhomogeneous fashion, permitting the passage of some agents (bleomycin, nitrosoureas, and platinum compounds) into tumors (Hargrave et al., 2002; Korppi-Tommola et al., 1999; Roche et al., 2000; Ryynanen et al., 1998; Tokunaga et al., 2000). The techniques used to show brain delivery of these compounds involve tissue analyses from biopsies, which provide minimal data concerning distribution kinetics or activated drug levels within tumor and/or its distant infiltrates. These issues are crucial to our understanding of the anticancer agents within brain tumors after administration by various routes, including systemic delivery (Cairncross et al., 2000; Graham et al., 1997; Kraemer et al., 2002), direct bolus injection into tumor (Boiardi et al., 1999; Disabato et al., 1999), intrathecal or intraventricular convection-enhanced parenchymal delivery (Bobo et al., 1994), or local implants (Lesniak et al., 2001). The last 2 techniques provide local therapies that emphasize drug diffusion driven by gradients in drug concentration or hydrostatic pressure. These techniques underscore the need for novel analytic strategies to measure drug concentrations within selected areas of brain.
Preclinical and phase 1 studies of anticancer drugs rely on animal models and surrogate measures of drug activity. When clinicians relate drug dose to drug effect, dose is the surrogate for concentration of activated drug assumed at the effector tumor site. Akin to this is the assumption that drug concentration within tumor is proportional to dose (Kellie et al., 2002; Koopmans, 2002). In pharmacokinetic (pK) studies, drug effects either are related to their plasma concentrations over time or are expressed as integrated effects of drug exposure (area under the concentration × time curve [AUC]), clearance, volume of distribution, and half-life (Gibaldi and Perrier, 1982). This assumes a measurable relation between plasma levels and concentration at the effector site (Lowe and Balis, 2001). Uncertain is whether tissue homogenates provide accurate measurement of integrated drug concentrations. These studies provide data at a single time point, from both vascularized and necrotic tumor tissue. Most important for clinical phase 1 trials are the differences in tumor exposure due to the intra- and interpatient variability observed in pK studies.
Recent advances in anticancer drug development have shifted from cytotoxic antimitotic agents in favor of agents that target specific points in the cell cycle or interfere with pathways of signal transduction. These therapies are cytostatic rather than cytotoxic. This shift in therapies has introduced novel end points to replace “cell kill” or tumor volume reduction on magnetic resonance scans. Since serial tumor biopsies are not possible, surrogate markers are obtained by other means, including functional imaging studies, PET, dynamic enhanced MRI, permeability maps, and MR spectroscopy. Validation of these markers will require that traditional pK analyses and imaging end points be coupled to the development of in situ pK and pharmacodynamic analyses in future trials (Chabner et al., 1998; Fox et al., 2002a). Implementation of these analyses in clinical trials will require minimally invasive techniques. Microdialysis, an evolving sampling technique suited for in vivo pK and pharmacodynamic studies in tumor tissue, was introduced in 1974 to monitor neurochemical events in awake animals (Ungerstedt and Pycock, 1974). The first human application was published over a decade later, in 1987, measuring interstitial glucose levels in adipose tissue (Lonnroth et al., 1987).
Since its introduction, the methodology has provided quantitative end points in the clinical evaluation of head trauma (Nilsson et al. 1996), stroke (Meixensberger et al., 2001), epilepsy (During and Spencer, 1993), Parkinson’s disease (Meyerson et al., 1990), and brain tumors (De Micheli et al., 2000; Roslin et al., 2003). Microdialysis has also been proposed as a method of intratumoral drug delivery.
The application of microdialysis to neuro-oncology was the subject of the First Annual Carolyn Frye-Halloran Symposium held by New Approaches to Brain Tumor Therapy consortium participants at the Massachusetts General Hospital in March 2002. This article summarizes the concepts and the preclinical and clinical applications of microdialysis presented at this conference.
Principles of Microdialysis
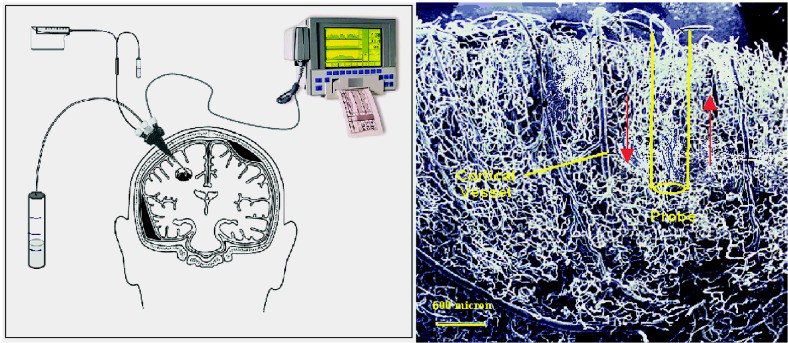
The technique of microdialysis makes use of an implanted probe communicating by diffusion with the brain’s extracellular space. A microsyringe pumps a solution (perfusate) into the proximal end of the cannula of a cylindrical probe. The perfusate flows distally to the semipermeable polymeric membrane (typically 5 to 20 kDa in pore size). There, solutes within the perfusate diffuse from the perfusion solution to the interstitium. At the same time, molecules from the extracellular fluid diffuse inward. In essence, an artificial capillary is created, albeit one that is more than 10 times the width of a cortical vessel (Fig. 1) but one that has predictable diffusion characteristics (Elmquist and Sawchuk, 1997).
Fig. 1.
Microdialysis probe. Left panel. The probe inserted into the brain and its laboratory components. Right panel. Probe width with respect to a cortical blood vessel. The length approximates 10 mm in actuality. The bar at lower left of the panel indicates 500 μm.
The analyte of interest is collected at regular intervals and analyzed at the bedside or in the laboratory. When microdialysis is used to sample the analyte from the extracellular fluid (ECF), the concentration of analyte in the dialysate is usually less than the concentration surrounding the probe membrane. Thus, the dialysate concentration relates to a fraction of that in the ECF. The ratio between the analyte in the tube and that in the extracellular space, or fractional recovery, provides an indirect measure of the concentration within the extra-cellular space. The movement by diffusion of analyte is from areas of high concentration to those of low concentration across a gradient created by the high concentrations within tissue ECF and lower concentrations within the perfusing solution of the microdialysis probe (Fig. 2).
Fig. 2.
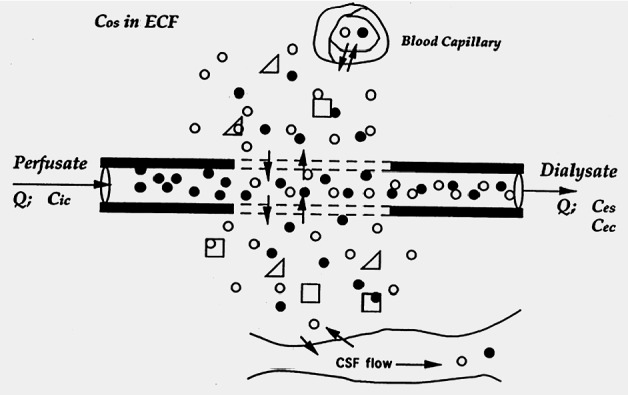
Diffusion pattern. An analyte (white spheres) present in the extracellular fluid (ECF) diffuses across the dialysis membrane (dashed lines) to be collected in the dialysate, while a retrodialysis calibrator solute (black spheres) entering the probe in the perfusate is delivered to the ECF by diffusing in the opposite direction.
Microdialysis sampling differs from that in conventional pK studies, which produce data at discrete time points. The dialysate is continuously collected. In pK transients, continuous collection produces an analyte concentration that is averaged over the sampling interval, thus reflecting an AUC integration. For linear systems, the dialysate AUC can be corrected by steady-state calibration to yield the AUC in the ECF (Bungay et al., 2001; Elmquist and Sawchuk, 1997).
Numerous factors influence the relationship between dialysate and ECF concentrations. Raising the perfusate flow rate lowers the concentration of analyte in the dialysate samples. Thus, fractional recovery is decreased as the perfusate flow rate is increased. This favors the use of slow perfusate flows, but then longer sampling intervals tend to be necessary to generate sufficient dialysate to assay. Alternatively, higher dialysate analyte concentrations can be promoted by lengthening the dialysis membrane. This factor is constrained by the dimensions of the tissue volume to be probed. Stated differently, slow perfusion and long probes promote equilibrium between outflow dialysate and surrounding extracellular fluid, as illustrated in the following formula for analyte recovery (Ed):
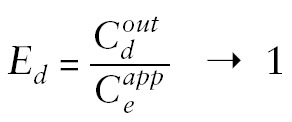 |
Drug concentrations:
Cdout = in outflow dialysate
Ceapp= in sampling volume of extracellular fluid
When equilibrium is not achieved, Ed is a direct function of the rate of analyte clearance from the ECF (Sun et al., 2001), along with many other parameters, such as the perfusate flow rate, diffusion coefficients, geometry of the probes, and the extracellular volume. Many of these parameters are not well known. Determining the ECF concentration then requires calibration to evaluate the fractional recovery. Empirical approaches have been developed for probe calibration when the ECF concentration does not vary over time. The “no-net-” or “zero-net-flux” technique involves perfusing the probe successively with solutions containing the analyte at different, known concentrations. “No-net flux” refers to the situation in which the outflow dialysate concentration is the same as the inflow perfusate concentration (Lonnroth et al., 1987). This concentration is presumed to equal the ECF concentration and can be calculated by interpolation (Fig. 3).
Fig. 3.
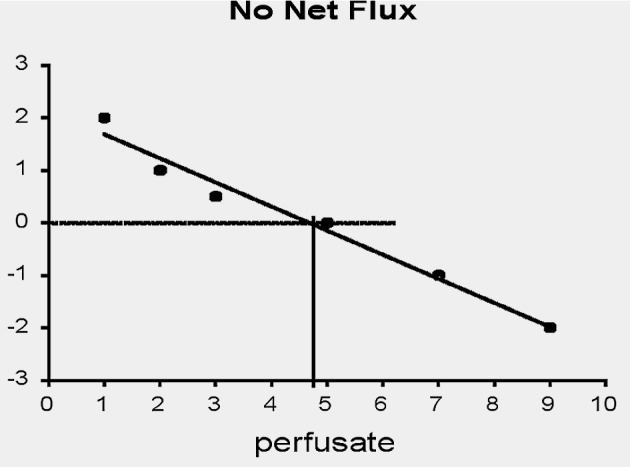
Probe calibration by the no-net-flux technique involving multiple perfusions of the probe with solutions of varying analyte concentration. The fractional recovery is provided as the absolute value of the slope. The extracellular concentration is estimated by interpolation to the point indicated by the intersection of the perpendicular lines at which the effluent dialysate concentration of the analyte is the same as in the inflowing perfusate.
Retrodialysis is an alternative steady-state calibration technique that makes use of a calibrator (an analog of the analyte of interest), which is added to the perfusate (Wang et al., 1993). Probe calibration under conditions of time-varying ECF concentrations is more problematic (Bungay et al., 2001). Where feasible, maintaining the recovery close to 100% at all times by slow perfusion is an approach to avoiding the difficulties of calibration.
Application of Microdialysis
Animal Studies
Studies with rodents have been used to demonstrate the utility of microdialysis techniques for the measurement of active metabolites of drugs in brain tissue. These studies have made use of platinum compounds, methotrexate (MTX), angiogenesis inhibitors, and cytokines. One such example is the measurement of platinum and its DNA adducts in mice bearing flank B16 murine melanoma and non-small cell lung cancers, after the delivery of either cisplatin or liposomal (SPI-077 Stealth [Sequus Pharmaceuticals, Menlo Park, Calif.]) cisplatin (Zamboni et al., 2002). In this study, a 4-mm-long, 0.5-mm-wide probe was inserted into the tumor bed, and dialysate samples were obtained every 12 min. The plasma concentration of unbound cisplatin decayed rapidly, having a first-phase half-life of 12 to 15 min and peak plasma concentration of 15 min. Within both types of tumors tested, drug decay was delayed. There was 10-fold intratumoral concentration variability between mice. The liposomal formulations provided 2.2- to 5-fold increase in the total platinum within tumor compared with cisplatin alone, but no platinum reached detectable concentrations in ECF after liposomal delivery. The platinum adducts of DNA were 3.5-fold higher after cisplatin than after liposomal formulation. The researchers thus concluded that liposomal formulation reached the tumor site at higher doses than cisplatin, but it did not release the drug itself.
In a similar vein, the interaction between angiogenesis inhibitors and cytotoxic drugs has been shown by Gallo and colleagues (Devineni et al., 1996a; Ma et al., 2001, 2003). The argument is that with successful antiangiogenic treatment, the hyperpermeable tumor vasculature can revert to normal, thus reducing the uptake of a co-administered cytotoxic agent like temozolomide (TMZ). In both syngeneic and xenograft brain-tumor models that overexpressed vascular endothelial growth factor, TMZ tumor concentrations were reduced following a therapeutic course of TNP-470. The reduced tumor concentrations of TMZ were ascribed to pharmacodynamic-mediated changes of reduced microvessel density and permeability by the antiangiogenic drug. By using another, more specific vascular endothelial growth factor inhibitor, SU5416, a contradictory effect occurred: TMZ concentrations increased in brain tumors by 50%. It appears that capillary density and permeability of the tumor are not the only pharmacodynamic factors of antiangiogenic effects, but blood flow, interstitial fluid pressure, pH, and hypoxia may also play a vital role.
Of potential interest to clinicians treating patients who have brain lymphoma, subarachnoid cancer, and glioblastoma, studies have made use of the hydrophilic agent MTX (de Lang et al., 1995a; Devineni et al., 1996b; Dukic et al., 1998). In prior studies cerebrospinal fluid (CSF) concentrations reached 2% to 3% of injected parenteral MTX concentrations, and administration into the subarachnoid space or ventricles was fraught with difficulties of uneven flow and drug sequestration.
De Groot (unpublished data) has used male Fisher rats and CMA-12 microdialysis probes (CMA Microdialysis, North Chelmsford, Mass.). Microdialysis probes were placed in the ventricular system, interstitial space, and the thoracic drainage to identify the entry point of MTX. The perfusate flow rate at 3 μl/min provided sufficient sample collections every 20 min. Drug concentration plots from the 3 sites were superimposed without time lag (Fig. 4), suggesting that intravenous MTX becomes homogeneously distributed between CSF and brain ECF via capillaries, rather than from the choroid plexus.
Fig. 4.
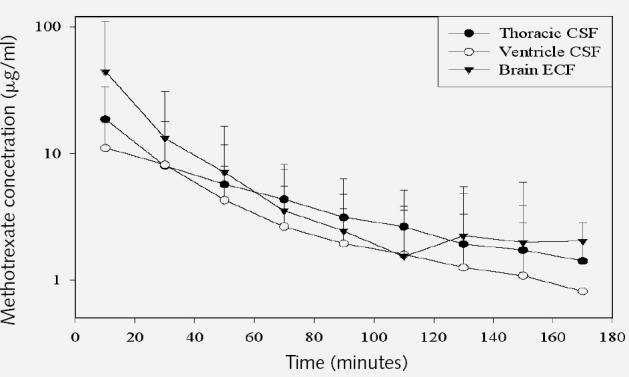
Methotrexate concentration in the three CSF chambers.
The role of CSF in drug distribution appears to be even more complicated. Although it has been assumed that drug levels within CSF reflect brain concentrations, this hypothesis has not been clearly tested. To determine CSF penetration as a surrogate for brain penetration, Fox and colleagues (2002b) at the National Cancer Institute have studied concentrations of zidovudine in nonhuman primates under steady-state (continuous infusion, n = 5) and transient (bolus dosing, n = 3) conditions. Drug levels were obtained from serum, CSF (Ommaya reservoir), or subarachnoid space over the cortex, or by lumbar puncture. Microdialysis probes (with a 10-mm-length × 0.5-mm-width probe membrane) were positioned in a peripheral vein, temporalis muscle, and cerebral cortex. CSF penetration was calculated as the ratio of free zidovudine in CSF to that in serum for standard sampling. CNS penetration was calculated as the brain ECF/blood ECF ratio for microdialysis samples. Under steady-state conditions (continuous infusion), the penetration of zidovudine was similar in CSF and CNS, 17% and 13%, respectively. The CSF penetration was comparable to prior studies of zidovudine CSF penetration with standard sampling. However, under transient conditions (bolus dosing), the CSF penetration by standard sampling methods was 28% compared to CNS penetration of 18% using microdialysis. These data suggest that zidovudine penetration of brain ECF and CSF is limited by partitioning differences under transient conditions, hypothetically related to saturation of active transport mechanisms. This study included an analysis of micro-dialysis recovery of analyte (Table 1). This was determined in vivo for each tissue using the zero-net flux method (steady-state experiments) and by retrodialysis (bolus experiments). Additionally, in vitro recovery was determined for each probe at the conclusion of the in vivo experiment to verify probe integrity.
Table 1.
Comparison of zidovudine recovery by microdialysis*
| Recovery | ||||||||
|---|---|---|---|---|---|---|---|---|
| Blood | Muscle | Brain | ||||||
| Method (Flow Rate) | N | In Vitro | In Vivo | In Vitro | In Vivo | In Vitro | In Vivo | |
| Zero Net Flux (1μl/min) | 5 | Mean: | 0.79 | 0.40 | 0.66 | 0.26 | 0.67 | 0.15 |
| SD: | 0.11 | 0.29 | 0.12 | 0.11 | 0.06 | 0.07 | ||
| CV (%): | 14 | 72 | 19 | 42 | 9 | 46 | ||
| Retrodialysis (0.5 μl/min) | 3 | Mean: | 0.90 | 0.69 | 0.92 | 0.50 | 0.90 | 0.29 |
| SD: | 0.07 | 0.06 | 0.06 | 0.10 | 0.05 | 0.10 | ||
| CV (%): | 8 | 8 | 7 | 20 | 6 | 32 | ||
Abbreviations used are as follows: CV, coefficient of variation; SD, standard deviation.
From Fox et al. (2002b).
In summary, a few intriguing but unsettling issues can be illustrated from the above animal studies. One is the relationship between drug delivery and drug activity. As shown in cisplatin studies, drug concentrations in the extracellular space do not necessarily correlate with drug uptake by tumor cells. The most that could be inferred is that the drug has reached the vicinity of the tumor, in the extracellular space; but it provides no information regarding the intercellular physiology. The second issue lies in parameters other than interstitial hydrostatic pressure that may influence permeability through the micro-dialysis membrane, such as the pH and level of oxygenation within the microenvironment being studied. Perhaps of greater concern is an issue that arises from the MTX study, whereby for those drugs that require capillary distribution, any disruption to the capillaries can lead to falsely elevated drug levels, which includes the insertion of the microcatheter itself.
Human Studie
Animal studies have demonstrated the safe use of dialysis catheters for sampling from multiple tissues over time points approaching several weeks. Cerebral monitoring (having recently received FDA 510 (k) clearance) in humans has extended these observations and demonstrated both safety and ease of use of these systems. Microdialysis catheters for sampling are routinely used in centers from around the world, including Uppsala (Sweden), Stockholm, London, Berlin, Richmond (Va.), Houston (Tex.), Los Angeles (Calif.), Pittsburgh (Pa.), Minneapolis (Minn.), and New Haven (Conn.), to name a few. The microcatheters are visible on CT scans and pose minimal invasive risks at implantation, sampling, or removal (Fig. 5). Complications of bleeding or infection equal the morbidity seen in stereotactic biopsy, craniotomy, or intracerebral monitoring.
Fig. 5.
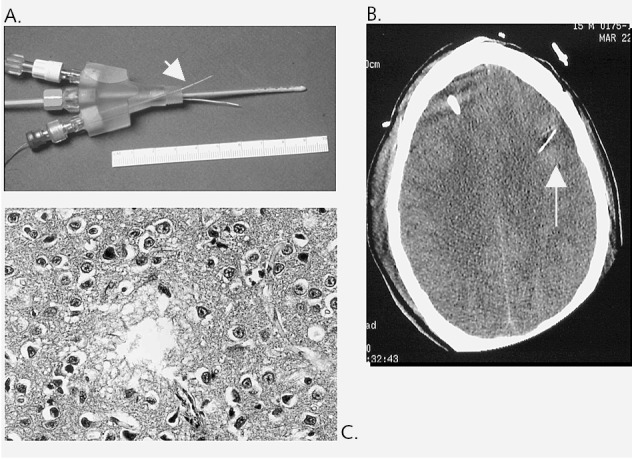
Microdialysis probe. A. Probe (arrow) in relation to bolt, intracranial pressure monitor, brain O2 sensor; B. Probe as seen on CT scan (arrow). C. Gliosis at the site of probe implant.
The utility of the technique is most apparent in the evaluation of changes after head trauma or subarachnoid hemorrhage. In many institutions microdialysis catheters, which could remain in place for up to 3 weeks, can provide continuous measures of brain biochemical changes. In humans the basic rules apply: longer membranes and lower flow rates yield greater relative recovery of analytes into the dialysate. Tissue tortuosity shortens the diffusion pathway and leads to decreased analyte recovery. Unlike animal studies, however, some human studies have indicated erratic results after day 7 of analyte recovery in humans. The results may be secondary to in situ gliosis.
The extended experience from Uppsala of catheter use in several hundred patients has been complemented by the efforts of Goodman and co-workers at Baylor College of Medicine. In the latter setting, probes were implanted in 147 head-trauma patients for up to 5 consecutive days without complication. The microdialysis catheters appear safer than intracranial pressure bolts (Gopinath et al., 2002; Hlatky et al., 2002). (Some centers routinely administer prophylactic antibiotics to retain a meningitis rate of 2%, which is similar to ventriculostomy.) Patients with poor outcome exhibit elevated levels of excitatory amino acids, particularly aspartate and glutamate (Vespa et al., 2003). Those with gross tissue disruption typically have had the highest levels of these amino acids. Similar data have been provided for nitric oxide breakdown products of nitrate and nitrite, which according to dialysate levels are lowest in patients who expire shortly after head injury. Arguably, understanding prognostic factors after head trauma has proven difficult, and in certain circumstances entirely paradoxical (Alessandri et al., 1999a,b). These include death or vegetative state in the setting of normal intracerebral pressure or unremarkable CT scans of the brain, or lack of correlation between brain energy demands and decreased cerebral blood flow (Oertal et al., 2002). With the aid of microdialysis, however, it has been shown that favorable outcomes in traumatic brain are correlated with lower levels of potassium and glutamate. Hyperventilation therapy increases both extracellular lactate and glutamate levels, accounting for its dire outcome (Marion et al., 2002). Furthermore, such data underscore the poor prognosis associated with low glucose levels and elevated lactate, changes that are duplicated by PET studies (Vespa et al., 1998, 2003; Hutchinson et al., 2002).
Few data are available from studies of human tumors. Epileptic patients with tumors often exhibit elevated levels of excitatory amino acids during ictus, along with an increase in lactate, pyruvate, and glycerol (reflective of lipolysis). In fact, the seizure focus has markedly elevated glutamate levels, by up to 10-fold. Since 1990, Spencer and co-workers at Yale University have simultaneously implanted 2 to 3 probes in more than 100 patients with epilepsy as a result of brain tumor or cortical dysplasia. Complications have not been observed during the 3-week use of implanted probes, with flow rates at the time of ictal events at 2.5 μl/min. No evidence of bleeding or infection has ever occurred in their cohort; the 5-kDa membrane appeared to act as a barrier to pathogens and microorganisms. The studies have demonstrated the heterogeneity of brain tumors and resulting seizures. For instance, elevated glutamate levels were centered in seizure focus in some, whereas GABA was more prominent in others (Abi-Saab et al., 2002; During and Spencer, 1993).
A recent publication confirms the higher energy demand by the tumor (Roslin et al. 2003). Microdialysis analyses have indirectly shown the relatively lower glucose (and higher lactate) levels within the interstitial space fluid. Moreover, the necrotic tissue contains significantly higher levels of glutamate.
The conclusions that can be drawn from human studies involving microdialysis are twofold: (1) The procedure poses minimal risk in a mixture of neurological conditions in awake and mobilized patients, but (2) there probably exists interpatient variability, which complicates the application of generalized results to any individual patient. In brain tumor patients, the latter setback may perhaps be downplayed since the major use of microdialysis would be to analyze drug penetration or perform direct drug infusion into the tumor bed. Nonetheless, a few significant limitations cannot be ignored, and they are addressed below.
Limitations and Benefits of Microdialysis
Animal and human studies raise several issues. As indicated, the analyte recovery reflects the perfusate flow rate. Lower flow rate yields higher recovery, but longer sampling intervals. Even at high flow rates of 2 to 3 μl/min, it will require half an hour to collect 60-μl samples for analysis. Recovery, even in trapping buffers, can be sensitive to changes of temperature, minor movements of the probe, and sample handling. Probe membrane permeability has not been tested for many drugs and their active metabolites. Adsorption of interstitial macromolecules to the probe membrane and gliosis can further reduce permeability and diminish recovery.
Diffusional exchange of solutes between the probe and surrounding tissue (i.e., sampling or delivery of analyte) creates spatial disturbances in the ECF concentration. This disturbance occurs over radial distances ranging from micrometers to millimeters, depending upon the avidity of clearance mechanisms for removing the solute from the ECF (Bungay et al., 2001; Dykstra et al., 1992). This distance corresponds at most to the width of a few pixels on MRI; hence the disturbance would be difficult to observe except by autoradiography. Stated differently, the region of tissue affected by analyte sampling or delivery is variable depending upon the analyte, but rarely extends greater than a couple of millimeters into the surrounding tissue. The probe membrane length is a consideration when compared to the size of anatomic structures such as tumor vasculature or tumor center versus infiltrative margin. Accurate MRI-guided implantation of the device by a skilled neurosurgeon is therefore necessary.
Although dynamic microscopic interactions may alter sample recovery, the system should still provide an order of magnitude better resolution than currently provided by MR spectroscopy, radiolabeled drugs for PET imaging, or 3H-labeled drugs. The insertion of the device into the brain tumor could be confirmed by any imaging studies. Multiple probes can be provided to sample microscopic zones designated by MR spectroscopy, MR vascular, or MR permeability studies. As the probe produces minimal gliosis at the parenchymal site within 2 weeks of use, study designs should provide drug levels for lipophilic agents with long half-lives. The risk of complicating hemorrhage, infection, or epilepsy is no greater than the current intracerebral monitoring units. Studies could be performed in freely moving animals or humans and then during therapies that include combinations of radiation and various cytotoxic and cytostatic agents. A potential boon is the monitoring of transgene expression rates for gene therapy trials in which proto-oncogenes like TP53 and immunomodulators (interferon-α) are provided. Recently, interleukin-1β, interleukin-6, and nerve growth factor have been sampled in the interstitial space fluid of patients with severe head trauma (Winter et al., 2002). For all studies, drug concentrations from brain tumor and surrounding tissue can be compared with levels obtained from CSF and plasma.
Other more serious limitations of microdialysis should not be overlooked. One concern raised in the animal studies is that BBB and its transcapillary transport system can be disrupted by the simple insertion of the micro-catheter, which may cause an overestimation of drug uptake into the brain. In some studies the disruption may persist for nearly a month (Groothuis et al., 1998; Morgan et al., 1996; de Lang et al., 1995b). The disturbance could result not only from direct tissue injury, but also indirectly. It has been observed that matrix metalloproteinases are activated and released during probe insertion, which leads to neutrophil infiltration and subsequent tissue remodeling (Planas et al., 2002). The greatest disruption appears after repeated microcatheter insertions, however (de Lang et al., 1995b). Also, the function of BBB is not significantly affected if surgical conditions have been controlled (de Lang et al., 1997). In brain tumors, blood-tumor barrier may already be compromised, as contrast-enhanced imaging studies attest. More interesting, perhaps, is the recovery of the drug as an analyte by the probe, which depends on the concentration of the drug at that site and its metabolism, as well as its diffusion. If the first process of interstitial-microvascular exchange could be estimated by micro-dialysis, it would suggest nothing with respect to the metabolism or diffusion of the drug. It is hoped that the preclinical experiments have already elucidated the metabolism of the drug in hand. The major concern of early clinical trials would therefore be to demonstrate drug penetration.
Conclusion
Evaluating the response of a particular therapy in patients with malignant gliomas poses many challenges and difficulties. In phase 2 studies, outcome measurements are usually based on progression-free survival at 6 months, and the measurements usually correlate with neurological status or functional impairment. However, most randomized trials of chemotherapy have fallen short in showing a substantial benefit, and the new agents that provide a slight improvement in survival in phase 2 trials fail when tested in large randomized trials (Brada and Shape, 1996; Brada and Yung, 2000; Haines, 2002). For example, microdialysis studies in individuals with melanoma and breast cancer have shown no relation between serum concentrations of anticancer drugs and tumor exposure to the drugs (Muller, 2000; Muller et al., 1997).
The current trend by many neuro-oncologists is to follow the stability or reduction in size of an enhancing glioma on imaging studies after treatment. Ideally, this may represent loss of tumor cells if there is a reduction, which should be a reliable surrogate for cytotoxic agents. However, the decrease in enhancement may also reflect a change in properties of blood-brain or blood-tumor barrier, undifferentiated by conventional MRI or CT scans that do not rely on perfusion measurements or 3-D reconstruction analyses. These limitations are compounded when therapy is provided in the setting of steroids, radiation, or antiseizure medications. Furthermore, a large percentage of high-grade gliomas display drug resistance to numerous chemotherapeutic agents, and chemoresistance profiles have been proposed to assist in treating patients with such tumors appropriately, either in or out of experimental trials (Haroun et al., 2002). Such drug profiles can be performed in vitro easily, but are not as practical because the specific tumor bioavailability, or the fraction of the dose that gets to the tumor site, is not known. Plasma or CSF concentrations of drugs and compounds are typically used as substitutes of what actually occurs within the tumor and the surrounding extracellular fluid. In particular, active transport processes at the level of blood-brain, blood-CSF, and blood-tumor barriers lead to intricate relationships among concentrations in plasma, CSF, and the tumor compartment.
Microdialysis could sidestep such indirect measurements obtained by conventional methods. It has been used to effectively detect short-term changes in excitatory and inhibitory amino acids, fatty acid amides, and other endogenous substances within the interstitial fluid in a variety of clinical settings, including epilepsy, stroke, traumatic brain injury, and intracerebral hemorrhage. The microcatheter can be easily implanted anywhere within the cerebral cortex, hippocampus, pineal gland, and even nucleus accumbens. The semipermeable membrane of the probe measures only 10 mm in length and 500 μm in width, but it is large enough to be visualized on imaging studies. In addition to the feasibility of micro-dialysis, it has other attractive advantages, including only rare complications, “invasiveness” that is comparable to other monitoring methods, and feasible bedside monitoring, as well as the capabilities to select specific analytes to answer the questions at hand, monitor effects of treatment, and study drug pKs and pharmacodynamics within the extracellular space.
The last advantage leads to many promising and potential uses for microdialysis. It may redefine phase 1 studies by detecting the presence of drug within the tumor and thus guiding the continuation or discontinuation of the drug for future phase 2/3 studies. This will save the health care industry a great financial expenditure and spare the patients from unnecessary exposure to ineffectual drugs. Finally, the technique can be used not only to continuously monitor the ECF concentration of analytes, but also to deliver drugs to a specific tissue region (retrodialysis), even though such a method could face the same limitations that are faced by other interventions such as brachytherapy and chemotherapy using impregnated wafers. The major difference, however, would be the length of treatment, which could be extended by another week by continuous microdialysis infusion. More importantly, drug-drug interactions could be avoided, especially in the setting of P450-inducing antiepileptics, and systemic complications and side effects could be minimized.
The trend in neuromonitoring until recently has been to follow brain pO2, pCO2, pH, and temperature (see Table 2). With the current microdialysis, amino acids and ischemic markers could be monitored and might aid in prognostication (Persson and Hillered, 1992).
Table 2.
Trend in neuromonitoring
| Neuromonitoring 10 to 15 years ago | Neuromonitoring in 2002 | |||
|---|---|---|---|---|
| Pressure | Ventriculostomy | Ventriculostomy, miniature transducer-tipped catheters | ||
| Flow | CPP | CPP | Transcranial Doppler | |
| Global CBF | Global CBF | Regional CBF | Continuous local CBF | |
| Kety-Schmidt | Kety-Schmidt | Stable Xe CBF | TD-CBF | |
| 133Xe CBF | 133Xe CBF | SPECT | Laser Doppler | |
| PET | ||||
| fMRI | ||||
| Oxygenation | SjvO2 | Global O2 | Regional O2 | Local O2 |
| CVP catheter | SjvO2 | NIRSO2 | PbtO2 | |
| Fiberoptic catheter | ||||
| Metabolism | CSF | Global | Regional | Local |
| CSF | PET | Microdialysis | ||
| CMRO2,-G,-L | pCO2, pH | |||
Abbreviations used are as follows: CBF, cerebral blood flow; CMRO2,-G,-L, cerebral metabolic rate of oxygen, glutamate, lactate consumption; CPP, cerebral perfusion pressure; CVP, central venous pressure; TD-CBF, transcranial Doppler – CBF; NIRSO2, near-infrared spectroscopy of oxygen saturation; PbtO2, brain tissue oxygen pressure; SjvO2, internal jugular venous oxygen hemoglobin saturation.
The First Annual Carolyn Frye-Halloran Symposium in March 2002 provided the platform for the use of microdialysis in brain tumor patients. The symposium illustrated the methodological concept of microdialysis and its diverse uses in both animal and human studies. To further advance this technology in the field of neuro-oncology, a few points must be first clarified. Multicenter trials using microdialysis monitors in the future should consider a universal perfusion rate and perfusate, along with the membrane permeability and the length of time a probe can be inserted. Normal saline or a solution that closely resembles CSF could be standardized to flow at 0.3 μl/min for a fixed period of time before running into interference and misinterpretation from the probe itself as a result of gliosis or faulty calibration techniques. The 0.3-μl/min rate is also ideal for application with the CMA 600 bedside analyzer (CMA Microdialysis). However, the unique flow rate should be determined by the recovery of the analyte of interest, the needed sample volume, and the expected concentration of analyte in the ECF sampled. Other methods of analysis can be combined with microdialysis to boost its sensitivity, such as capillary electrophoresis and mass spectroscopy (Kennedy et al., 2002). What must be emphasized is that the results obtained with microdialysis should be compared with results obtained with other in vivo techniques. For example, perhaps microdialysis could be used to measure choline:creatine ratio within a known malignant glioma and to compare the results with those obtained concurrently from magnetic resonance spectroscopy. This will minimize the natural tendency for errors to be accepted as part of the methodology.
Footnotes
Abbreviations used are as follows: AUC, area under the concentration x time curve; BBB, blood-brain barrier; CSF, cerebrospinal fluid; ECF, extracellular fluid; MTX, methotrexate; pK, pharmacokinetic; TMZ, temozolomide.
References
- Abi-Saab WM, Maggs DG, Jones T, Jacob R, Srihari V, Thompson J, Kerr D, Leone P, Krystal JH, Spencer DD, During MJ, Sherwin RS. Striking differences in glucose and lactate levels between brain extracellular fluid and plasma in conscious human subjects: Effects of hyperglycemia and hypoglycemia. J Cereb Blood Flow Metab. 2002;22:271–279. doi: 10.1097/00004647-200203000-00004. [DOI] [PubMed] [Google Scholar]
- Alessandri B, Doppenberg E, Bullock R, Woodward J, Choi S, Koura S, Young HF. Glucose and lactate metabolism after severe human head injury: Influence of excitatory neurotransmitters and injury type. Acta Neurochir Suppl (Wien) 1999a;75:21–24. doi: 10.1007/978-3-7091-6415-0_5. [DOI] [PubMed] [Google Scholar]
- Alessandri B, Doppenberg E, Zauner A, Woodward J, Choi S, Bullock R. Evidence for time-dependent glutamate-mediated glycolysis in head-injured patients: A microdialysis study. Acta Neurochir Suppl (Wien) 1999b;75:25–28. doi: 10.1007/978-3-7091-6415-0_6. [DOI] [PubMed] [Google Scholar]
- Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Nat Acad Sci USA. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiardi A, Eoli M, Pozzi A, Salmaggi A, Broggi G, Silvani A. Locally delivered chemotherapy and repeated surgery can improve survival in glioblastoma patients. Ital J Neurol Sci. 1999;20:43–48. doi: 10.1007/s100720050009. [DOI] [PubMed] [Google Scholar]
- Brada M, Shape G. Chemotherapy of high grade gliomas: Beginning of a new era or the end of the old? Eur J Cancer. 1996;32A:2193–2194. doi: 10.1016/s0959-8049(96)00400-5. (editorial) [DOI] [PubMed] [Google Scholar]
- Brada M, Yung WK. Clinical trial end points in malignant glioma: Need for effective trial design strategy. Semin Oncol. 2000;27:11–19. (suppl) [PubMed] [Google Scholar]
- Bungay PM, Dedrick RL, Fox E, Balis FM. Probe calibration in transient microdialysis in vivo. Pharm Res. 2001;18:361–366. doi: 10.1023/a:1011015316327. [DOI] [PubMed] [Google Scholar]
- Cairncross G, Swinnen L, Bayer R, Rosenfeld S, Salzman D, Paleologos N, Kaminer L, Forsyth P, Stewart D, Peterson K, Hu W, Macdonald D, Ramsay D, Smith A for the Oligodendroglioma Study Group. Myeloablative chemotherapy for recurrent aggressive oligodendroglioma. Neuro-Oncol. 2000;2:114–119. doi: 10.1093/neuonc/2.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabner BA, Boral AL, Multani P. Translational research: Walking the bridge between idea and cure—Seventeenth Bruce F. Cain Memorial Award Lecture. Cancer Res. 1998;58:4211–4216. [PubMed] [Google Scholar]
- DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- de Lang EC, de Vries JD, Zurcher C, Danhof M, de Boer AG, Breimer DD. The use of intracerebral microdialysis for the determination of pharmacokinetic profiles of anticancer drugs in tumor-bearing rat brain. Pharm Res. 1995a;12:1924–1931. doi: 10.1023/a:1016239822287. [DOI] [PubMed] [Google Scholar]
- de Lang EC, Danhof M, Zurcher C, de Boer AG, Breimer DD. Repeated microdialysis perfusions: Periprobe tissue reactions and BBB permeability. Brain Res. 1995b;702:261–265. doi: 10.1016/0006-8993(95)01184-x. [DOI] [PubMed] [Google Scholar]
- de Lang EC, Danhof M, de Boer AG, Breimer DD. Methodological considerations of intracerebral microdialysis in pharmacokinetic studies on drug transport across the blood-brain barrier. Brain Res Rev. 1997;25:27–49. doi: 10.1016/s0165-0173(97)00014-3. [DOI] [PubMed] [Google Scholar]
- De Micheli E, Alfieri A, Pinna G, Bianchi L, Colivicchi MA, Melani A, Pedata F, Della Corte L, Bricolo A. Extracellular levels of taurine in tumoral, peritumoral, and normal brain tissue in patients with malignant glioma: An intraoperative microdialysis study. Adv Exp Med Biol. 2000;483:621–625. [PubMed] [Google Scholar]
- Devineni D, Klein-Szanto A, Gallo JM. Uptake of temozolomide in a rat glioma model in the presence and absence of the angiogenesis inhibitor TNP-470. Cancer Res. 1996a;56:1983–1987. [PubMed] [Google Scholar]
- Devineni D, Klein-Szanto A, Gallo JM. In vivo microdialysis to characterize drug transport in brain tumors: Analysis of methotrexate uptake in rat glioma-2 (RG-2)-bearing rats. Cancer Chemother Pharmacol. 1996b;38:499–507. doi: 10.1007/s002800050518. [DOI] [PubMed] [Google Scholar]
- Disabato JA, Handler MH, Strain JD, Fleitz JM, Foreman NK. Successful use of intracavitary bleomycin for low-grade astrocytoma tumor cyst. Ped Neurosurg. 1999;31:246–250. doi: 10.1159/000028871. [DOI] [PubMed] [Google Scholar]
- Dropcho EJ. Novel chemotherapeutic approaches to brain tumors. Hematol Oncol Clin North Am. 2001;15:1027–1052. doi: 10.1016/s0889-8588(05)70266-5. [DOI] [PubMed] [Google Scholar]
- Dukic S, Kaltenbach ML, Gourdier B, Marty H, Vistelle R. Determination of free extracellular levels of methotrexate by microdialysis in muscle and solid tumor of the rabbit. Pharm Res. 1998;15:133–138. doi: 10.1023/a:1011973409022. [DOI] [PubMed] [Google Scholar]
- During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341:1607–1610. doi: 10.1016/0140-6736(93)90754-5. [DOI] [PubMed] [Google Scholar]
- Dykstra KH, Hsiao JK, Morrison PF, Bungay PM, Mefford IN, Scully MM, Dedrick RL. Quantitative examination of tissue concentration profiles associated with microdialysis. J Neurochem. 1992;58:931–940. doi: 10.1111/j.1471-4159.1992.tb09346.x. [DOI] [PubMed] [Google Scholar]
- Elmquist WF, Sawchuk RJ. Application of microdialysis in pharmacokinetic studies. Pharm Res. 1997;14:267–288. doi: 10.1023/a:1012081501464. [DOI] [PubMed] [Google Scholar]
- Fox E, Curt GA, Balis FM. Clinical trial design for target-based therapy. Oncologist. 2002a;7:401–409. doi: 10.1634/theoncologist.7-5-401. [DOI] [PubMed] [Google Scholar]
- Fox E, Bungay PM, Bacher J, McCully CL, Dedrick RL, Balis FM. Zidovudine concentration in brain extracellular fluid measured by microdialysis: Steady-state and transient results in rhesus monkey. J Pharmacol Exp Ther. 2002b;301:1003–1011. doi: 10.1124/jpet.301.3.1003. [DOI] [PubMed] [Google Scholar]
- Gibaldi, M., and Perrier, D. (1982) Pharmacokinetics, 2nd ed. New York: Marcel Dekker, Inc.
- Gopinath SP, Valadka AB, Goodman JC, Robertson CS. Extracellular glutamate and aspartate in head injured patients. Acta Neurochir Suppl. 2002;76:437–438. doi: 10.1007/978-3-7091-6346-7_90. [DOI] [PubMed] [Google Scholar]
- Graham ML, Herndon JE, 2nd, Casey JR, Chaffee S, Ciocci GH, Krischer JP, Kurtzberg J, Laughlin MJ, Longee DC, Olson JF, Paleologus N, Pennington CN, Friedman HS. High-dose chemotherapy with autologous stem-cell rescue in patients with recurrent and high-risk pediatric brain tumors. J Clin Oncol. 1997;15:1814–1823. doi: 10.1200/JCO.1997.15.5.1814. [DOI] [PubMed] [Google Scholar]
- Groothuis DR, Ward S, Schlageter KE, Itskovich AC, Schwerin SC, Allen CV, Dills C, Levy RM. Changes in blood-brain barrier permeability associated with insertion of brain cannulas and micro-dialysis probes. Brain Res. 1998;803:218–230. doi: 10.1016/s0006-8993(98)00572-1. [DOI] [PubMed] [Google Scholar]
- Haines SJ. Moving targets and ghosts of the past: Outcome measurement in brain tumour therapy. J Clin Neurosci. 2002;9:109–112. doi: 10.1054/jocn.2001.1013. [DOI] [PubMed] [Google Scholar]
- Hargrave DR, Bouffet E, Gammon J, Tariq N, Grant RM, Baruchel S. Phase I study of fotemustine in pediatric patients with refractory brain tumors. Cancer. 2002;95:1294–1301. doi: 10.1002/cncr.10814. [DOI] [PubMed] [Google Scholar]
- Haroun RI, Clatterbuck RE, Gibbons MC, Burger PC, Parker R, Fruehauf JP, Brem H. Extreme drug resistance in primary brain tumors: In vitro analysis of 64 resection specimens. J Neurooncol. 2002;58:115–123. doi: 10.1023/a:1016049111941. [DOI] [PubMed] [Google Scholar]
- Hlatky R, Furuya Y, Valadka AB, Goodman JC, Robertson CS. Comparison of microdialysate arginine and glutamate levels in severely head-injured patient. Acta Neurochir Suppl. 2002;81:347–349. doi: 10.1007/978-3-7091-6738-0_88. [DOI] [PubMed] [Google Scholar]
- Hutchinson PJ, Gupta AK, Fryer TF, Al-Rawi PG, Chatfield DA, Coles JP, O’Connell MT, Kett-White R, Minhas PS, Aigbirhio FI, Clark JC, Kirkpatrick PJ, Menon DK, Pickard JD. Correlation between cerebral blood flow, substrate delivery, and metabolism in head injury: A combined microdialysis and triple oxygen positron emission tomography study. J Cereb Blood Flow Metab. 2002;22:735–745. doi: 10.1097/00004647-200206000-00012. [DOI] [PubMed] [Google Scholar]
- Kellie SJ, Barbaric D, Koopmans P, Earl J, Carr DJ, de Graaf SS. Cerebrospinal fluid concentrations of vincristine after bolus intravenous dosing: A surrogate marker of brain penetration. Cancer. 2002;94:1815–1820. doi: 10.1002/cncr.10397. [DOI] [PubMed] [Google Scholar]
- Kennedy RT, Watson CJ, Haskins WE, Powell DH, Strecker RE. In vivo neurochemical monitoring by microdialysis and capillary separations. Curr Opin Chem Biol. 2002;6:659–665. doi: 10.1016/s1367-5931(02)00373-3. [DOI] [PubMed] [Google Scholar]
- Koopmans PP. Clinical endpoints in trials of drugs for cancer: Time for a rethink? BMJ. 2002;324:1389–1391. doi: 10.1136/bmj.324.7350.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korppi-Tommola T, Huhmar H, Aronen HJ, Penttila P, Hiltunen J, Savolainen S, Kallio ME, Liewendahl K. 111In-labelled bleomycin complex for the differentiation of high- and low-grade gliomas. Nuclear Med Commun. 1999;20:145–152. doi: 10.1097/00006231-199902000-00006. [DOI] [PubMed] [Google Scholar]
- Kraemer DF, Fortin D, Neuwelt EA. Chemotherapeutic dose intensification for treatment of malignant brain tumors: Recent developments and future directions. Curr Neurol Neurosci Rep. 2002;2:216–224. doi: 10.1007/s11910-002-0080-8. [DOI] [PubMed] [Google Scholar]
- Lesniak MS, Langer R, Brem H. Drug delivery to tumors of the central nervous system. Curr Neurol Neurosci Rep. 2001;1:210–216. doi: 10.1007/s11910-001-0020-z. [DOI] [PubMed] [Google Scholar]
- Lonnroth P, Jansson PA, Smith U. A microdialysis method allowing characterization of intracellular water space in humans. Am J Physiol. 1987;253:E228–E231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- Lowe, E., and Balis, F.M. (2001) Dose-effect and concentration-effect analysis. In: Atkinson, A.J., Jr., Daniels, C.E., Deddrick, R.L., Grudzinskas, C.V., and Markey, S.P. (Eds.), Principles of Clinical Phamacology, San Diego, Calif.: Academic Press. pp. 235–244.
- Ma J, Pulfer S, Li S, Chu J, Reed K, Gallo JM. Pharmacodynamic-mediated reduction of temozolomide tumor concentrations by the angiogenesis inhibitor TNP-470. Cancer Res. 2001;61:5491–5498. [PubMed] [Google Scholar]
- Ma J, Li S, Reed K, Guo P, Gallo JM. Pharmacodynamic-mediated effects of the angiogenesis inhibitor SU5416 on the tumor disposition of temozolomide (TMZ) in subcutaneous and intracerebral human glioma xenograft models. J Pharmacol Exp Ther. 2003;305:833–839. doi: 10.1124/jpet.102.048587. [DOI] [PubMed] [Google Scholar]
- Marion DW, Puccio A, Wisniewski SR, Kochanek P, Dixon CE, Bullian L, Carlier P. Effect of hyperventilation on extracellular concentrations of glutamate, lactate, pyruvate, and local cerebral blood flow in patients with severe traumatic brain injury. Crit Care Med. 2002;30:2619–2625. doi: 10.1097/00003246-200212000-00001. [DOI] [PubMed] [Google Scholar]
- Meixensberger J, Kunze E, Barcsay E, Vaeth A, Roosen K. Clinical cerebral microdialysis: Brain metabolism and brain tissue oxygenation after acute brain injury. Neurol Res. 2001;23:801–806. doi: 10.1179/016164101101199379. [DOI] [PubMed] [Google Scholar]
- Meyerson BA, Linderoth B, Karlsson H, Ungerstedt U. Microdialysis in the human brain: Extracellular measurements in the thalamus of parkinsonian patients. Life Sci. 1990;46:301–308. doi: 10.1016/0024-3205(90)90037-r. [DOI] [PubMed] [Google Scholar]
- Morgan ME, Singhal D, Anderson BD. Quantitative assessment of blood-brain barrier damage during microdialysis. J Pharmacol Exp Ther. 1996;277:1167–1176. [PubMed] [Google Scholar]
- Muller M. Microdialysis in clinical drug delivery studies. Adv Drug Deliv Rev. 2000;45:255–269. doi: 10.1016/s0169-409x(00)00113-7. [DOI] [PubMed] [Google Scholar]
- Muller M, Mader RM, Steiner B, Steger GG, Jansen B, Gnant M, Helbich T, Jakesz R, Eichler HG, Blochl-Daum B. 5-Fluorouracil kinetics in the interstitial tumor space: Clinical response in breast cancer patients. Cancer Res. 1997;57:2598–2601. [PubMed] [Google Scholar]
- Nilsson OG, Saveland H, Boris-Moller F, Brandt L, Wieloch T. Increased levels of glutamate in patients with subarachnoid haemorrhage as measured by intracerebral microdialysis. Acta Neurochir Suppl (Wien) 1996;67:45–47. doi: 10.1007/978-3-7091-6894-3_10. [DOI] [PubMed] [Google Scholar]
- Oertel M, Kelly DF, Lee JH, McArthur DL, Glenn TC, Vespa P, Boscardin WJ, Hovda DA, Martin NA. Efficacy of hyperventilation, blood pressure elevation, and metabolic suppression therapy in controlling intracranial pressure after head injury. J Neurosurg. 2002;97:1045–1053. doi: 10.3171/jns.2002.97.5.1045. [DOI] [PubMed] [Google Scholar]
- Persson L, Hillered L. Chemical monitoring of neurosurgical intensive care patients using intracerebral microdialysis. J Neurosurg. 1992;76:72–80. doi: 10.3171/jns.1992.76.1.0072. [DOI] [PubMed] [Google Scholar]
- Planas AM, Justicia C, Sole S, Friguls B, Cervera A, Adell A, Chamorro A. Certain forms of matrix metalloproteinase-9 accumulate in the extracellular space after microdialysis probe implantation and middle cerebral artery occlusion/reperfusion. J Cereb Blood Flow Metab. 2002;22:918–925. doi: 10.1097/00004647-200208000-00003. [DOI] [PubMed] [Google Scholar]
- Rall, D.P., and Zubrod, C.G. (2000) Mechanisms of drug absorption and excretion: Passage of drugs in and out of the central nervous system. In: Abeloff, M.D. (Ed.), Clinical Oncology, 2nd ed., New York: Churchill Livingstone, pp. 109–128.
- Roche H, Cure H, Adenis A, Fargeot P, Terret C, Lentz MA, Madelmont JC, Fumoleau P, Hanausk A, Chollet P. Phase II trial of cystemustine, a new nitrosourea, as treatment of high-grade brain tumors in adults. J Neurooncol. 2000;49:141–145. doi: 10.1023/a:1026524825573. [DOI] [PubMed] [Google Scholar]
- Roslin M, Henriksson R, Bergström P, Ungerstedt U, Bergenheim TA. Baseline levels of glucose metabolites, glutamate and glycerol in malignant glioma assessed by stereotactic microdialysis. J Neurooncol. 2003;61:151–160. doi: 10.1023/a:1022106910017. [DOI] [PubMed] [Google Scholar]
- Ryynanen PM, Savolainen SE, Aronen HJ, Korppi-Tommola ET, Huhmar HM, Kallio ME, Hiltunen JV. Kinetics of 111In-labeled bleomycin in patients with brain tumors: Compartmental vs. non-compartmental models. Ann Nuclear Med. 1998;12:313–321. doi: 10.1007/BF03164920. [DOI] [PubMed] [Google Scholar]
- Sun H, Bungay PM, Elmquist WF. Effect of capillary efflux transport inhibition on the determination of probe recovery during in vivo microdialysis in the brain. J Pharmacol Exp Ther. 2001;297:991–1000. [PubMed] [Google Scholar]
- Tokunaga Y, Nakashima M, Sasaki H, Tomiyama N, Nakashima MN, Ichikawa M, Kaminogo M, Shibata S. Local distribution into brain tumor and pharmacokinetics of 4-pyridoxate diammine hydroxy platinum, a novel cisplatin derivative, after intracarotid administration in rats with 9L malignant glioma: Simultaneous brain microdialysis study. Biol Pharm Bull. 2000;23:1491–1496. doi: 10.1248/bpb.23.1491. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U, Pycock C. Functional correlates of dopamine neurotransmission. Bull Schweizerischen Akad Med Wiss. 1974;30:44–55. [PubMed] [Google Scholar]
- Vespa P, Prins M, Ronne-Engstrom E, Caron M, Shalmon E, Hovda DA, Martin NA, Becker DP. Increase in extracellular glutamate caused by reduced cerebral perfusion pressure and seizures after human traumatic brain injury: A microdialysis study. J Neurosurg. 1998;89:971–982. doi: 10.3171/jns.1998.89.6.0971. [DOI] [PubMed] [Google Scholar]
- Vespa PM, McArthur D, O’Phelan K, Glenn T, Etchepare M, Kelly D, Bergsneider M, Martin NA, Hovda DA. Persistently low extracellular glucose correlates with poor outcome 6 months after human traumatic brain injury despite a lack of increased lactate: A microdialysis study. J Cereb Blood Flow Metab. 2003;23:865–877. doi: 10.1097/01.WCB.0000076701.45782.EF. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wong SL, Sawchuk RJ. Microdialysis calibration using retrodialysis and zero-net flux: Application to a study of the distribution of zidovudine to rabbit cerebrospinal fluid and thalamus. Pharm Res. 1993;10:1411–1419. doi: 10.1023/a:1018906821725. [DOI] [PubMed] [Google Scholar]
- Winter CD, Iannotti F, Pringle AK, Trikkas C, Clough GF, Church MK. A microdialysis method for the recovery of IL-1beta, IL-6 and nerve growth factor from human brain in vivo. J Neurosci Methods. 2002;119:45–50. doi: 10.1016/s0165-0270(02)00153-x. [DOI] [PubMed] [Google Scholar]
- Zamboni WC, Gervais AC, Egorin MJ, Schellens JH, Hamburger DR, Delauter BJ, Grim A, Zuhowski EG, Joseph E, Pluim D, Potter DM, Eiseman JL. Inter- and intratumoral disposition of platinum in solid tumors after administration of cisplatin. Clin Cancer Res. 2002;8:2992–2999. [PubMed] [Google Scholar]