Abstract
Polypyrimidine tract-binding protein (PTB) is a nuclear factor that binds to the polypyrimidine tract of pre-mRNA introns, where it is associated with negative regulation of RNA splicing and with exon silencing. We have previously demonstrated that PTB expression is increased during glial cell transformation and that this increase correlates with changes in the RNA splicing of the fibroblast growth factor receptor 1. In this paper we examine the specific cellular distribution of PTB expression in normal brain and in glial and neuronal tumors. Paraffin sections were stained by using a primary monoclonal antibody against PTB. Tissues that were analyzed included normal brain (n = 2) and tumors of various types (low-grade astrocytoma, n = 2; anaplastic astrocytoma, n = 2; glioblastoma, n = 4; medulloblastoma, n = 4; central neurocytoma, n = 2; dysplastic gangliocytoma, n = 1; ganglioglioma, n = 1; paraganglioma, n = 1). In glial cell populations the majority of astrocytes and oligodendrocytes were negative, but occasional positively staining cells were observed. Strongly positive PTB staining was observed in ependymocytes, choroid plexus epithelium, microglia, arachnoid membrane, and adenohypophysis, and weak staining was found in the neurohypophysis. In all cases vascular endothelium and smooth muscle stained strongly. In tumor samples, intense positive nuclear staining was observed in transformed cells of low-grade astrocytoma, anaplastic astrocytoma, glioblastoma multiforme, medulloblastoma, paraganglioma, and the glial population of both ganglioglioma and dysplastic gangliocytoma (the neuronal cells of both were negative). In medulloblastoma, neoplastic neuronal cells were positive, as were other cell lineages. In normal brain, all neuron populations and pineocytes were negative for PTB. We conclude that although glial cells show derepression of PTB expression, a similar mechanism is absent in both nonneoplastic neurons and in most neuronally derived tumor cells. Strong upregulation of PTB expression in tumor cells of glial or primitive neuroectodermal origin suggests involvement of this protein in cellular transformation. Whether PTB affects splicing of RNAs critical to cellular transformation or proliferation is an important question for future research.
With the complete sequencing of the human genome, it has become clear that the alternative processing of RNA transcripts plays a role in the creation of genetic diversity. Along with this realization is a newfound appreciation for the potential role that aberrant RNA processing may play in the development or progression of human disease. Current estimates suggest that approximately 10% to 15% of disease-causing mutations are associated specifically with RNA splice sites (Maniatis and Tasic, 2002; Nissim-Rafinia and Keren, 2002). More recent studies also suggest that mutation of other cis-regulatory elements and perturbation of the trans-acting regulators involved in alternative splicing serve as mechanisms for disease-causing aberrant RNA processing (Cartegni et al., 2002; Faustino and Cooper, 2003; Nissim-Rafinia and Keren, 2002).
Polypyrimidine tract-binding protein (PTB)2 is a member of the hnRNP family of RNA-binding proteins, which appear to have the generalized function of binding precursor mRNA to help differentiate intronic from exonic sequence and protect the RNAs from nonspecific degradation prior to splicing (Maniatis and Tasic, 2002; Wagner and Garcia-Blanco, 2001). Many members of this class of proteins also appear to function to specifically inhibit exon recognition during splicing by blocking access of the spliceosome (Cartegni et al., 2002; Maniatis and Tasic, 2002; Wagner and Garcia-Blanco, 2001). PTB specifically recognizes UCUU when present in pyrimidine-rich sequences; because this sequence is common to many 3′ splice sites, one mechanism of PTB action is to block recognition of the 3′ splice site (Garcia-Blanco et al., 1989; Wagner and Garcia-Blanco, 2001; Wang and Pederson, 1990). In addition, PTB has also been found to bind sequences distal from the RNA splice sites to regulate exon inclusion of several genes (Wagner and Garcia-Blanco, 2001).
Fibroblast growth factor receptor-1 (FGFR-1) is a tyrosine kinase receptor that plays many roles in cellular growth and differentiation, including induction and inhibition of cellular differentiation, and it is also important during early development (Johnson and Williams, 1993). The specificity and affinity of the FGFR-1 receptor can be altered by alternative recognition of the α-exon during pre-mRNA processing (Galzie et al., 1997). Regulation of this splicing event is dependent on 2 surrounding intronic RNA sequences (ISS-1 and ISS-2) in the FGFR-1 gene, the presence of which results in exclusion of the α -exon from the RNA transcript and enhanced affinity of the protein product for fibroblast growth factor (FGF) (Jin et al., 1999a,b). It has been found that PTB interacts with ISS-1, an element required for exon exclusion, and that PTB levels are dramatically elevated during the malignant transformation of glial cells (Jin et al., 2000). This process of specifically excluding the α-exon may promote cellular growth and contribute to glial cell malignancy (Yamaguchi et al., 1994). The purpose of this study was to examine the distribution of PTB expression in various cellular populations in normal brain and in different tumors occurring in the central nervous system.
Materials and Methods
Patient samples were obtained from residual archival tissue blocks that were prepared by standard formalin fixation and paraffin embedding. Specimens included tissue samples from normal brain (n = 2), low-grade astrocytoma (n = 2), anaplastic astrocytoma (n = 2), glioblastoma (n = 4), medulloblastoma (n = 4), dysplastic gangliocytoma of the cerebellum (n = 1), ganglioglioma (n = 1), paraganglioma of the filum terminale (n = 1), and central neurocytoma (n = 2). For immunohistochemistry analysis, 4-μm-thick sections were cut, and staining was performed by the avidin-biotin-peroxidase complex method with a Vectorstain ABC kit (Vector Laboratories, Burlingame, Calif.). Slides were incubated with a 1:5 dilution of anti-PTB monoclonal antibody (DH3) (Grossman et al., 1998). The antigen-antibody reaction was visualized by using 3,3′-diaminobenzidine as the chromogen. Sections were counterstained with hematoxylin, and the degree of positive staining was assessed qualitatively for each cell type by one of the investigators (GNF). Normal brain was obtained at autopsy within 24 h of death. The positive control tissues demonstrated a functional reaction.
Results
In normal brain, all CNS neuronal populations and pineocytes were negative for PTB staining. While cells of the adenohypophysis were strongly positive, those in the neurohypophysis were only occasionally weakly positive. Astrocytic and oligodendrocytic populations demonstrated only occasional scattered positivity; however, ependymocytes and choroid plexus epithelium were strongly positive for PTB staining (Table 1). In contrast, neoplastic cells in the samples from low-grade astrocy-toma, anaplastic astrocytoma, glioblastoma, ganglioglioma, medulloblastoma, paraganglioma, and dysplastic gangliocytoma were all strongly positive, while those of central neurocytoma were weakly positive (Table 2). The neuronal populations found in the dysplastic gangliocytoma and ganglioglioma specimens were negative. Figures 1 and 2 illustrate some examples of PTB staining in selected normal and neoplastic tissues, respectively. Note that in all cases staining was predominantly localized to the nucleus.
Table 1.
Staining characteristics of normal tissues obtained at autopsy
| Tissue | Staining characteristics |
|---|---|
| Neurons (all CNS populations) | – |
| Astrocytes (including Bergmann glia) | Majority –, occasional scattered + |
| Oligodendrocytes | Majority –, occasional scattered + |
| Ependymocytes | ++ |
| Choroid plexus epithelium | ++ |
| Microglia and macrophages | ++ |
| Arachnoid membrane | ++ |
| Adenohypophysis | ++ |
| Neurohypophysis | Weak + |
| Pineocytes | – |
| Fibroblasts (choroid plexus stroma) | ++ |
| Vascular endothelium | ++ |
| Vascular smooth muscle | ++ |
Symbols used: –, negative for PTB; +, positive for PTB; ++, strongly positive for PTB.
Table 2.
Staining characteristics of various central nervous system tumors
| Tumor | Staining characteristics |
|---|---|
| Low-grade astrocytoma (2 cases) | ++ |
| Anaplastic astrocytoma (2 cases) | ++ |
| Glioblastoma multiforme (4 cases) | ++ |
| Medulloblastoma (4 cases) | ++ |
| Dysplastic gangliocytoma of cerebellum (1 case) | ++ (glial population) – (neuronal population) |
| Ganglioglioma (1 case) | ++ (glial population) – (neuronal population) |
| Paraganglioma of the filum terminale | ++ |
| Central neurocytoma | + |
Symbols used: –, negative for PTB; +, positive for PTB; ++, strongly positive for PTB.
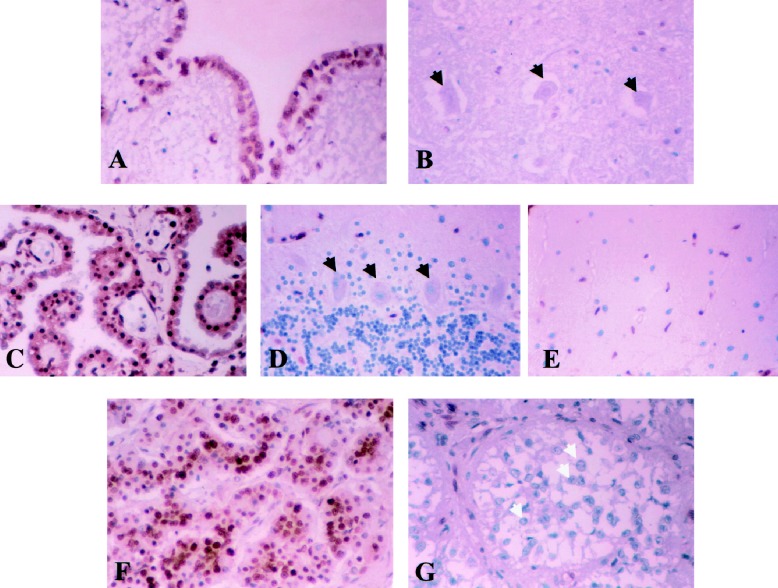
Fig. 1.
Normal tissues stained with PTB monoclonal antibody: (A) ependyma, (B) neuron in hypoglossal nucleus, (C) choroid plexus, (D) cerebellum, (E) microglia, (F) adenohypophysis, and (G) pineal gland. Ependyma, choroid plexus, microglia, and adenohypophysis are densely positive, while neurons (black arrows) in the medulla and cerebellum are negative, as are the other cellular populations in the cerebellum, that is, the granule and molecular cells. Pineocytes (white arrows) are the only cell population other than neurons that are consistently negative for PTB.
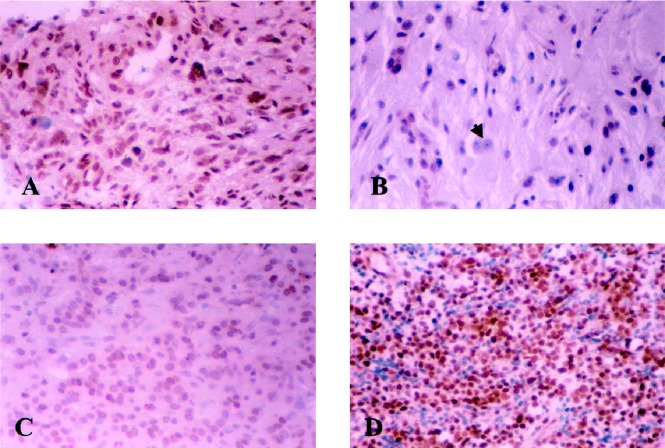
Fig. 2.
Tumor tissue stained with PTB monoclonal antibody. (A) glioblastoma, (B) ganglioglioma, (C) paraganglioma, and (D) medulloblastoma. All neoplastic cellular populations are positive. The neuronal population (black arrow) in ganglioglioma (B) is negative.
Discussion
The observation that PTB is overexpressed in a wide variety of glia-derived tumors suggests that aberrant RNA splicing may play an important role either in glial cell transformation or in progression of lower grade astrocytic neoplasms to glioblastoma. PTB has been shown to have a major influence on preventing exon inclusion during RNA splicing (Jin et al., 2000; Wagner and Garcia-Blanco, 2001). Although it is generally accepted that “exon silencing” by PTB is likely to be a generalized mechanism, to date relatively few genes (including c-src and the genes that code for α-actinin, calcitonin/CGRP, caspase 2, FGFR-1, FGFR-2, GABAA receptor γ2, and α- and β-tropomyosin) have been clearly established to have such mechanisms (Cote et al., 2001; Jin et al., 2000; Valcarcel and Gebauer, 1997; Wagner and Garcia-Blanco, 2001). Of this list of genes, only the splicing of FGFR-1 has been examined in association with glioblastoma. We have previously demonstrated that two intronic sequences (ISS-1 and ISS-2) flank the α-exon in FGFR-1 RNA transcripts and that PTB binds to the upstream sequence, ISS-1 (Fig. 3) (Jin et al., 1999a,b; 2000). Mutation or deletion of ISS-1 increases inclusion of the α-exon from 29% (no mutation/deletion) to 70% in the glioblastoma cell line SNB-19 (Jin et al., 1999b). The level of PTB was also found to correlate with α-exon exclusion in human glioblastoma tumor samples, with decreased levels of PTB frequently found in adjacent normal tissue correlating with increased α-exon inclusion (Jin et al., 1999a). This partial discrepancy was felt to reflect either differences in cell composition in adjacent normal tissues or the involvement of additional factors in the process of malignant transformation.
Fig. 3.
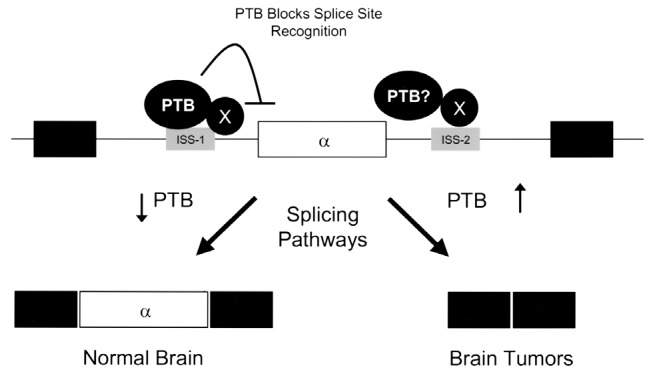
Alternative splicing mechanism for FGFR-1. PTB binds to ISS-1, which results in exclusion of the α-exon from the RNA transcript. This form of exon silencing may result in neoplastic transformation or progression.
In this study, the finding of absent PTB staining in all neuronal populations in both the normal and tumor specimens implies differential processing of FGFR-1 transcripts in neurons and glial cells, which is in part supported by previous observations (Jin et al., 2000; Yamaguchi et al., 1994). The finding of very low levels of PTB staining in nonneoplastic astrocytes and oligodendrocytes, but high levels in neoplastic astrocytes (of all grades), may indicate either direct activation via upregulation of PTB expression or indirect activation by another related pathway.
Another interesting observation from this study is the densely positive staining for PTB in medulloblastoma, often considered to be a “neuronal” neoplasm. This finding may reflect the ambiguous ontogeny of this particular neoplasm. The cell of origin for medulloblastomas is controversial, with some authors describing undifferentiated cells from the roof of the fourth ventricle (which eventually form the external granular layer and the granule cells) as the cells of origin (VandenBerg et al., 1987), while others believe the source to be the subependymal matrix (which gives rise to the deep cerebellar neurons, Purkinje cells, molecular layer, glia, and ependyma) (Trojanowski et al., 1992), and still others consider both as potential sources to account for the different tissue types present in medulloblastoma (Katsetos and Burger, 1994). Thus, the neoplastic cells of medulloblastoma are not typical neurons and are not derived from neuroblasts, which are the precursors to neurons (Moore and Persaud, 1998). However, the Purkinje cells, which are neurons, were negative for PTB staining. The neurons seen in the ganglioglioma tissue sample also were negative for PTB staining in this study. Because the neuronal element in these tumors may not be neoplastic, the conclusion cannot be drawn, based on the results of this study alone, that PTB is not activated in the neoplastic neuron. It would be informative to examine samples from a neuroblastoma, a tumor with definite neoplastic neurons. A similar situation applies to paragangliomas and central neurocytomas, which although classified as “neuronal” tumors, actually contain neoplastic cells derived from neural crest and not from neural tube (where neuronal precursors arise) (Moore and Persaud, 1998).
The process of glial cell malignant transformation is complex and involves changes in the expression of multiple genes. Involved genetic elements include p53, the retinoblastoma gene, cyclic AMP-dependent kinase number 2 (CDKN2A), mutated in multiple advanced cancers 1 (MMAC1) or phosphatase and tensin homology (PTEN), platelet-derived growth factor (PDGF), MDM2, vascular endothelial growth factor (VEGF), neuroglial cell adhesion molecule (NCAM L1), cathepsins, matrix metalloproteases, integrins, interleukin-13 receptor, and heat shock proteins (Maher et al., 2001; Sehgal, 1998). It is certain that no one pathway is the primary regulator of neoplasia and that all these pathways are interactive in some way. Therefore, the identification of factors that broadly mediate cellular function is of critical importance. In this report, we have identified an association of PTB overexpression specifically with tumors of glial cell origin. This finding was primarily driven by the discovery that alternative RNA splicing of FGFR-1, which produces receptor isoforms with increased affinity for FGF, appears to be regulated by PTB. While FGF clearly plays an important role in glioblastoma progression, the effects of PTB overexpression may be even more far-reaching. As a regulator of both RNA splicing and gene transcription, PTB certainly has the potential to alter the function of genes that may be critical to either the initial transformation or the progression of the malignant phenotype. As such, PTB may serve as another potentially modifiable factor in the complex process of neoplasia with particular relevance to gliomas.
Acknowledgment
The anti-PTB monoclonal antibody DH3 was the generous gift of David Helfman, Cold Spring Harbor Laboratories.
Footnotes
Abbreviations used are as follows: FGF, fibroblast growth factor; FGFR-1, fibroblast growth factor receptor-1; PTB, polypyrimidine tract-binding protein.
References
- Cartegni L, Chew SL, Krainer AR. Listening to silence and understanding nonsense: Exonic mutations that affect splicing. Nat Rev Genet. 2002;3:285–298. doi: 10.1038/nrg775. [DOI] [PubMed] [Google Scholar]
- Cote J, Dupuis S, Wu JY. Polypyrimidine track-binding protein binding downstream of the caspase-2 alternative exon 9 represses its inclusion. J Biol Chem. 2001;276:8535–8543. doi: 10.1074/jbc.M008924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustino NA, Cooper TA. Pre-mRNA splicing and human disease. Genes Dev. 2003;17:419–437. doi: 10.1101/gad.1048803. [DOI] [PubMed] [Google Scholar]
- Galzie Z, Kinsella AR, Smith JA. Fibroblast growth factors and their receptors. Biochem Cell Biol. 1997;75:669–685. [PubMed] [Google Scholar]
- Garcia-Blanco MA, Jamison SF, Sharp PA. Identification and purification of a 62,000-dalton protein that binds specifically to the polypyrimidine tract of introns. Genes Dev. 1989;3:1874–1886. doi: 10.1101/gad.3.12a.1874. [DOI] [PubMed] [Google Scholar]
- Grossman JS, Meyer MI, Wang YC, Mulligan GJ, Kobayashi R, Helfman DM. The use of antibodies to the polypyrimidine tract binding protein (PTB) to analyze the protein components that assemble on alternatively spliced pre-mRNAs that use distant branch points. RNA. 1998;4:613–625. doi: 10.1017/s1355838298971448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Bi W, Huang ES, Cote GJ. Glioblastoma cell-specific expression of fibroblast growth factor receptor-1b requires an intronic repressor of RNA splicing. Cancer Res. 1999a;59:316–319. [PubMed] [Google Scholar]
- Jin W, Huang ES, Bi W, Cote GJ. Redundant intronic repressors function to inhibit fibroblast growth factor receptor-1 α-exon recognition in glioblastoma cells. J Biol Chem. 1999b;274:28035–28041. doi: 10.1074/jbc.274.39.28035. [DOI] [PubMed] [Google Scholar]
- Jin W, McCutcheon IE, Fuller GN, Huang ES, Cote GJ. Fibroblast growth factor receptor-1 α-exon exclusion and polypyrimidine tract-binding protein in glioblastoma multiforme tumors. Cancer Res. 2000;60 :1221–1224. [PubMed] [Google Scholar]
- Johnson DE, Williams LT. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res. 1993;60:1–41. doi: 10.1016/s0065-230x(08)60821-0. [DOI] [PubMed] [Google Scholar]
- Katsetos CD, Burger PC. Medulloblastoma. Semin Diagn Pathol. 1994;11:85–97. [PubMed] [Google Scholar]
- Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA. Malignant glioma: Genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- Maniatis T, Tasic B. Alternative pre-mRNA splicing and proteome expansion in metazoans. Nature. 2002;418:236–243. doi: 10.1038/418236a. [DOI] [PubMed] [Google Scholar]
- Moore, K., and Persaud, T. (1998) The nervous system, in The Developing Human: Clinically Oriented Embryology, 6th ed. Philadelphia: W.B. Saunders Company, pp. 451–489.
- Nissim-Rafinia M, Keren B. Splicing regulation as a potential genetic modifier. Trends Genet. 2002;18:123–127. doi: 10.1016/s0168-9525(01)02619-1. [DOI] [PubMed] [Google Scholar]
- Sehgal A. Molecular changes during the genesis of human gliomas. Semin Surg Oncol. 1998;14:3–12. doi: 10.1002/(sici)1098-2388(199801/02)14:1<3::aid-ssu2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Tohyama T, Lee VM. Medulloblastomas and related primitive neuroectodermal brain tumors of childhood recapitulate molecular milestones in the maturation of neuroblasts. Mol Chem Neuropathol. 1992;17:121–135. doi: 10.1007/BF03159987. [DOI] [PubMed] [Google Scholar]
- Valcarcel J, Gebauer F. Post-transcriptional regulation: The dawn of PTB. Curr Biol. 1997;7:R705–R708. doi: 10.1016/s0960-9822(06)00361-7. [DOI] [PubMed] [Google Scholar]
- VandenBerg SR, Herman MM, Rubinstein LJ. Embryonal central neuroepithelial tumors: Current concepts and future challenges. Cancer Metastasis Rev. 1987;5:343–365. doi: 10.1007/BF00055377. [DOI] [PubMed] [Google Scholar]
- Wagner EJ, Garcia-Blanco MA. Polypyrimidine tract binding protein antagonizes exon definition. Mol Cell Biol. 2001;21:3281–3288. doi: 10.1128/MCB.21.10.3281-3288.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Pederson T. A 62,000 molecular weight spliceosome protein crosslinks to the intron polypyrimidine tract. Nucleic Acids Res. 1990;18:599–6001. doi: 10.1093/nar/18.20.5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi F, Saya H, Bruner JM, Morrison RS. Differential expression of two fibroblast growth factor-receptor genes is associated with malignant progression in human astrocytomas. Proc Natl Acad Sci USA. 1994;91:484–488. doi: 10.1073/pnas.91.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]