Abstract
The primary objective of this study was to determine the proportion of patients exhibiting a radiographic response in a cohort of patients with recurrent malignant glioma who were treated with irinotecan. Secondary objectives were to determine progression-free survival, overall survival, and toxicity. The trial was terminated after the first 18 patients were enrolled in this multicenter, 2-stage, phase 2 study. Twelve patients received concurrent enzyme-inducing antiepileptic drugs, and 6 did not. Each cycle consisted of a 90-min i.v. infusion of irinotecan every week for 4 consecutive weeks, followed by 2 weeks off. One patient had a complete response, 5 patients had stable disease, 5 patients had radiographic progression, 6 patients were removed from the study because of toxicity, and 1 patient refused further therapy and was removed from the study. The response rate in this study was 6% (1/18), and 28% (5/18) of these patients progressed while receiving irinotecan. Dose-limiting toxicities consisted of diarrhea in 5 patients, neutropenia in 1 patient, infection in 1 patient, and respiratory failure in 1 patient. Irinotecan had minimal efficacy in this cohort of 18 patients with recurrent malignant glioma. Toxicity was significant but similar to that reported in other patient populations.
Glioblastoma accounted for 51.9% of all gliomas diagnosed in the United States from 1995 to 1999. Although primary brain tumors are uncommon, one-fifth as common as lung cancer, these tumors are associated with a disproportionate amount of morbidity and mortality. There were an estimated 13,000 deaths attributed to malignant brain tumors in 2000, and the 5-year survival rates for these types of tumors are 32.7% for males and 31.6% for females (CBTRUS, 2002).
The standard treatment for patients with newly diagnosed malignant gliomas is maximal resection followed by external beam radiation therapy. The role of adjuvant chemotherapy remains controversial despite nearly 20 prospective randomized studies that have addressed this issue (Conrad et al., 1995). A meta-analysis of the results of 8 randomized clinical trials from 1975 to 1989 concluded that the addition of a nitrosourea agent to radiation therapy added a statistically significant, albeit marginal benefit, increasing 1-year survival by 10.1% and 2-year survival by 8.6% (Fine et al., 1993). Patients less than 60 years of age appear to benefit most from adjuvant chemotherapy, while malignant gliomas in elderly patients are resistant to chemotherapy. The most commonly used agents include carmustine, temozolomide, or the PCV (procarbazine, CCNU [lomustine], and vincristine) regimen. There is no convincing evidence that one conventional chemotherapy agent is superior to others in the management of malignant gliomas. It also remains questionable whether adjuvant chemotherapy confers any benefit over resection and radiation therapy.
There is no compelling evidence that any systemically administered chemotherapeutic agent confers a substantial benefit in patients with recurrent malignant gliomas. Nevertheless, there is an urgent need to identify effective antineoplastic therapies for this patient population. The camptothecin derivative irinotecan (CPT-11, 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyl-20-S-camptothecin) has demonstrated antitumor activity in animal models against human xenografts from glioma and medulloblastoma (Hare et al., 1997; Savaraj et al., 1995; Thompson et al., 1996; Vassal et al., 1994). Irinotecan is a prodrug that undergoes enzymatic hydrolysis to the potent topoisomerase I inhibitor SN-38. SN-38 is subsequently conjugated by uridine diphosphate glucuronyl transferase to the noncytotoxic metabolite SN-38 glucuronide (Bleiberg and Cvitkovic, 1996; Gupta et al., 1997a; Rivory, 1996). Both irinotecan and SN-38 enter the central nervous system following systemic administration (Savaraj et al., 1995). In a study utilizing glioma cell lines, a positive correlation between the activity of the target enzyme, topoisomerase I, and sensitivity to irinotecan was reported (Matsumoto et al., 1993). Based on these preclinical data and promising results from an early study in patients with recurrent malignant glioma (Friedman et al., 1999), a phase 1/2 trial of irinotecan was conducted by the New Approaches to Brain Tumor Therapy (NABTT)3 CNS consortium. The dose escalation and pharmacology portions of this trial are reported elsewhere (Gilbert et al., 2003). The results of the phase 2 component of this study are the subject of this report.
Materials and Methods
Patients
A multicenter, phase 1/2 protocol of irinotecan for patients with recurrent malignant gliomas was conducted in the NABTT CNS Consortium from 1998 to 2001. All 9 participating centers received local institutional review board approval prior to enrollment of subjects. Each participating patient signed an informed consent document prior to enrollment. To be eligible for this study all patients had to be ≥ 18 years old and have documented progressive or recurrent malignant glioma (glioblastoma, anaplastic astrocytoma, anaplastic oligodendroglioma, or anaplastic oligoastrocytoma), measurable (>1 cm in at least 1 diameter) contrast-enhancing disease on CT or MRI, and a Karnofsky performance status of 60 or greater. All patients had to have been on a stable or decreasing dose of corticosteroids for the week prior to entry into the trial and received no more than 1 prior chemotherapy regimen. In addition, the patients were required to have adequate baseline hematologic cell counts (absolute neutrophil count ≥ 1500/mm3; platelet count ≥ 100,000/mm3); minimum renal function, defined as serum creatinine ≤ 1.7 mg/dl; and normal hepatic function, defined as bilirubin ≤ 1.5 mg/dl and alanine transaminase (ALT) and aspartate transaminase (AST) ≤ 4 times the upper limit of institutional normal. Because of the potential increased risk of toxicity associated with concurrent administration of valproic acid and irinotecan, patients were restricted from taking valproic acid within 14 days from the start of irinotecan.
Treatment
Irinotecan was administered as a 90-min i.v. infusion in the outpatient department 1 day each week for 4 consecutive weeks followed by a 2-week rest. This 6-week treatment period was considered 1 cycle. Each patient was maintained on the same dose of irinotecan throughout the study unless toxicity mandated dose reduction. Patients were stratified into 2 groups: those in group A were concurrently using enzyme-inducing antiepileptic drugs (EIAEDs); those in group B were using antiepileptic drugs that were not enzyme-inducing or were using no antiepileptic drugs. The dose of irinotecan to be administered was the maximum tolerated dose (MTD) determined from the phase 1 portion of this trial. For group A patients, the MTD was 411 mg/m2, and for group B, it was 117 mg/m2 (Gilbert et al., 2003). All patients were premedicated with a seratonin-agonist antiemetic (most commonly ondansetron or granisetron). After irinotecan infusion, patients were observed for a minimum of 60 min. Acute diarrhea was treated with i.v. atropine (usually 1 mg). Patients were instructed to use loperamide at home at the earliest onset of diarrhea. The loperamide regimen consisted of an initial oral dose of 4 mg followed by 2 mg every 2 h until the diarrhea had resolved for a minimum of 12 h. Patients were followed with weekly complete blood count with differential. Cranial MRI studies were obtained within 2 weeks of study enrollment (baseline) and after every 2 cycles of therapy (every 12 weeks).
Statistical Methods
The primary end point of this phase 2 study was radiographic complete response (CR) or partial response (PR). Tumor response was assessed by measurement of contrast-enhancing volume in 3 dimensions. All responses were confirmed by centralized neuroradiology review. A CR was defined as complete disappearance of all tumor on MRI or CT scan in a patient who was off all corticosteroids, and a PR was defined as at least a 50% reduction in tumor on MRI or CT scan in a patient while on a stable or decreasing dose of corticosteroids. In order to qualify for either CR or PR, the patient must have had a stable or improving neurological examination for a minimum of 6 weeks. This study employed a 2-stage optimal design to assess a 20% improvement in response rate over a background rate of 10% (Simon, 1989). The type I error rate was 0.05, and the type II error rate was 0.10. Eighteen patients were to be enrolled in the first stage, and if more than 2 patients exhibited a response, then an additional 17 patients would be enrolled. If 2 or fewer responses were observed in the first stage, then the study would be stopped. Secondary end points included progression-free and overall survival as well as toxicity. Survival was measured from the time of entry into the study and was estimated by the product-limit method (Kaplan and Meier, 1958) with SAS version 8.2 (SAS Institute, Cary, N.C.). Failure rate was calculated by dividing the number of deaths by the total person-years of follow-up. Toxicity was reported with the National Cancer Institute Common Toxicity Criteria version 2.0 (NCI 1999). Exact binomial confidence intervals of the percentages of toxicity were estimated by using Stata 7.0 (Stata Corporation, College Station, TX).
Results
Patient Characteristics
Eighteen subjects (6 in group A, 12 in group B) were enrolled on the first stage of this phase 2 study to assess the efficacy of irinotecan administered at the MTD. Twelve patients had recurrent glioblastoma, 5 patients had recurrent anaplastic astrocytoma, and 1 patient had recurrent gliosarcoma. Baseline demographic and clinical characteristics are presented in Table 1. Prior surgery in this cohort included biopsy in 10 patients, craniotomy in 6, and biopsy plus craniotomy in 2. Sixteen of the 18 patients (89%) had received prior chemotherapy: 15 had one prior chemotherapy regimen, and 1 patient had received 2 prior chemotherapy regimens. Nine of the 16 (56%) patients treated with chemotherapy had received temozolomide, 2/16 (13%) carmustine, and 5/16 (31%) other drugs. All patients had received prior radiation therapy. The patient who received 2 prior chemotherapy regimens represented a protocol violation.
Table 1.
Baseline demographic and clinical characteristics
| Characteristic | Median (range) or N (%) |
|---|---|
| Age, years | 55.6 (30.9–62.7) |
| Gender, male | 9 (50) |
| Race, white | 16 (89) |
| Karnofsky performance status | 90 (60–100) |
Response
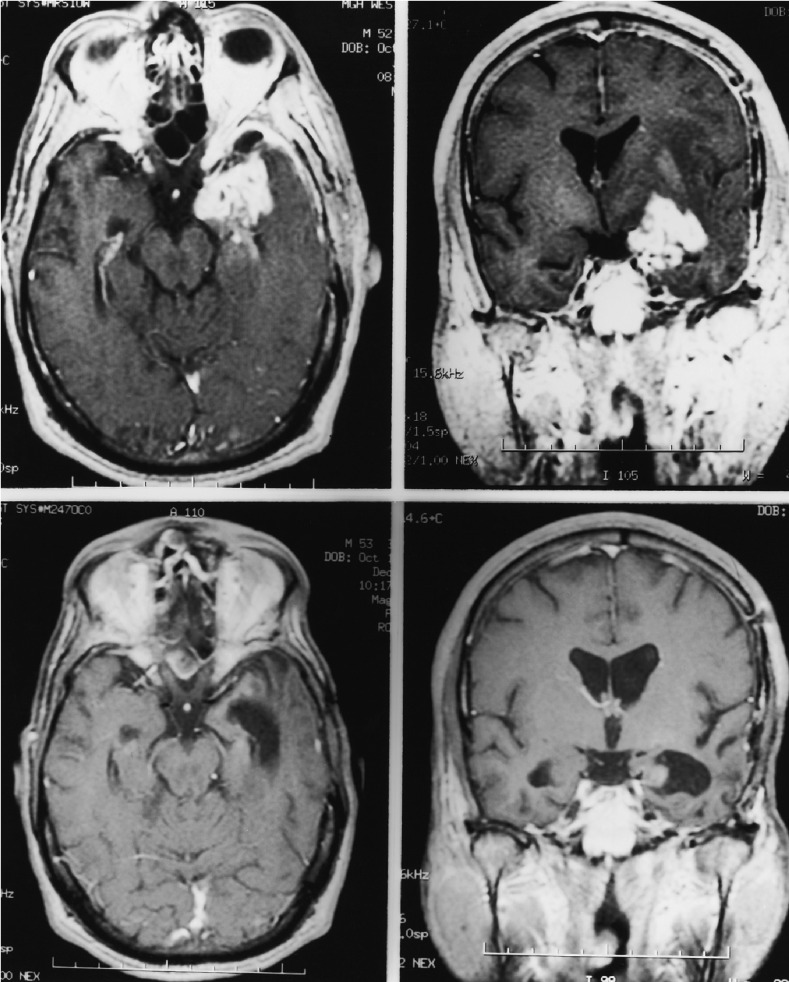
Eighteen patients were enrolled in the first stage of the trial. In this cohort, 1 patient (6%) had a complete response (Fig. 1), and no patients had a partial response; 5 (28%) had stable disease (SD), and 5 (28%) progressed during treatment with irinotecan. In addition, 6 patients (33%) were removed from treatment because of toxicity, and 1 patient (6%) refused further treatment. Because we observed only one radiographic response in the first stage of this trial, the study was closed.
Fig. 1.
Axial (L) and coronal (R) MRI images of the 1 patient who had a complete response. Top Panels. Prechemotherapy, contrast-enhanced, T1-weighted axial and coronal magnetic resonance images demonstrate heterogeneous enhancement of the tumor in the left temporal lobe. Bottom Panels. The enhancement has resolved after CPT-11 therapy, consistent with a complete response.
Survival
Thirteen patients (72%) have died, and 16 (89%) have progressed or died. The 5 living patients have been followed for more than 17 months. Total follow-up time for the cohort was 16.98 person-years. The failure rate was 0.77 (95% confidence interval [CI], 0.44–1.32) deaths per person-year. Kaplan-Meier estimates of overall survival and progression-free survival are presented in Fig. 2. Median progression-free survival was 7.3 months, and median overall survival was 10.4 months. Six-month progression-free survival was 56% (95% CI, 31%–79%).
Fig. 2.

Kaplan-Meier curve of overall survival (solid line) and progression-free survival (broken line) of the 18 patients with recurrent glioma.
Toxicity
Patients in this study received 0.25 to 10 cycles of irinotecan (median = 2 cycles). Twelve of the patients (67%, 95% CI, 41%–87%) experienced grade 3 or grade 4 toxicity during this study. Six of these 12 patients (3 in group A, 3 in group B) were removed from the study because of toxicity, and 2 of these patients (both in group B) had dose reductions due to toxicity. The dose-limiting toxicities that necessitated removal from the study or dose reduction included grade 3 or 4 diarrhea in 5 patients, grade 5 infection in 1 patient, grade 4 neutropenia in 1 patient, and grade 3 respiratory failure and increased intracranial pressure in 1 patient.
Discussion
There was little efficacy in this phase 2 study of irinotecan in adults with recurrent malignant glioma. Only 1 patient (6%) had a CR, and there were no PRs. In addition, when treated at the MTD that was defined in the phase 1 part of this trial, 8 (44%) patients had dose-limiting toxicity.
Preclinical studies have indicated that irinotecan is an attractive candidate drug for use in patients with malignant gliomas (Hare et al., 1997; Savaraj et al., 1995; Thompson et al., 1996; Vassal et al., 1994). Previous phase 1 trials of irinotecan have used a wide variety of schedules and doses, but the drug is commonly given as a 30- or 90-min i.v. infusion. The MTD of irinotecan identified in these studies has ranged from 145 mg/m2 to 350 mg/m2, depending upon the schedule, with delayed onset diarrhea and neutropenia being the most common dose-limiting toxicities (Armand et al., 1996; Masuda et al., 1996; O’Reilly and Rowinsky, 1996). The 2 dosing schedules used most commonly in phase 2 trials are (1) intermittent dosing with 250 to 350 mg/m2 every 3 to 4 weeks (non–small cell lung cancer) and (2) doses in the 100- to 200-mg/m2 range given on a weekly or biweekly basis (breast, colon, gastric, and lung cancer). Preclinical studies using glioma cell lines suggest that frequent exposure is important in augmenting responses (Savaraj et al., 1995).
Recent studies have demonstrated that patients receiving EIAEDs show decreased plasma levels of certain chemotherapeutic drugs that are administered at conventional doses (Fetell et al., 1997; Grossman et al., 1998). Failure to achieve adequate plasma levels of these drugs may have contributed to the lack of efficacy in past brain tumor trials. The effects of EIAED on irinotecan and SN-38 metabolism are uncertain, although preclinical studies have suggested complicated pharmacokinetic effects. One such study has shown that phenobarbital markedly increased the extent of glucuronidation of SN-38 in rodents, as demonstrated by a decrease in the area under the curve (AUC) of SN-38 by 31% and irinotecan by 59% (Rivory, 1996). Conversely, valproic acid competitively inhibits the glucuronidation of SN-38. A study performed with Wistar rats demonstrated that concurrent administration of valproic acid resulted in 99% inhibition of glucuronidation, which resulted in a 270% increase in the AUC of SN-38 (Gupta et al., 1997b). As a result of these preclinical data, patients receiving valproic acid as an antiepileptic drug have been excluded from most studies of irinotecan in brain tumors.
In the phase 1 part of this phase 1/2 study of irinotecan for recurrent malignant glioma (Gilbert et al., 2003), it was determined that the MTD was 411 mg/m2 in patients taking EIAEDs, a dose 3.5-fold greater than the MTD in patients not on EIAEDs (117 mg/m2). This finding correlated with concurrent studies of irinotecan and SN-38 plasma pharmacokinetics. The lack of toxicity at lower doses in patients taking EIAEDs correlated with the enhanced systemic clearance of irinotecan. In addition, mean values of the AUC of SN-38 and SN-38 glucuronide were comparable at the MTDs of irinotecan in patients who were receiving EIAEDs and those who were not. Based on the results of the phase 1 study, it was not possible to determine whether the local tumor concentrations of irinotecan and SN-38 mirrored the changes seen in the plasma compartment. Although this study identified the significant impact that EIAEDs have on irinotecan pharmacokinetics, the optimal dose of irinotecan that maximizes the production and tumor delivery of SN-38 has not been defined.
In a phase 2 trial of 60 adult patients with progressive or recurrent malignant gliomas, irinotecan was administered at a dose of 125 mg/m2 as a 90-min i.v. infusion every week for 4 weeks followed by a 2-week rest period. Partial responses were observed in 9 patients (15%), and 33 patients achieved SD (55%) lasting more than 3 months. The toxicity seen in this trial was less than expected when compared to that observed with a similar treatment regimen in patients with colon cancer. Furthermore, the pharmacokinetic data obtained during this trial suggested that the AUC for irinotecan and SN-38 were significantly lower than those noted in the treatment of systemic cancers (Friedman et al., 1999). This strongly implicated the induction of hepatic enzymes involved in the elimination of both compounds by anti-epileptic drugs, an effect analogous to that described with paclitaxel and 9-aminocamptothecin (Fetell et al., 1997; Grossman et al., 1998).
In another phase 1 study of irinotecan for recurrent malignant glioma, irinotecan was administered at escalating doses once every 3 weeks. In this study, no dose-limiting toxicities were encountered in 48 patients treated at doses up to 650 mg/m2 every 21 days (Prados et al., 2000). Investigators in another phase 1 study of 35 patients treated subjects at doses up to 1200 mg/m2 every 21 days and reported dose-limiting toxicity consisting of diarrhea, abdominal cramping, and fatigue or asthenia. In this phase 1 study, 35 patients were enrolled at various doses, and partial responses were achieved in 3 patients, with a median overall survival of 7.5 months (Cloughesy et al., 2000). Since neither of these reports included complete data from the phase 2 components of the studies, no definitive conclusions can be reached with respect to efficacy of irinotecan administered on this schedule to patients with recurrent malignant glioma. However, in a study of 52 patients with glioblastoma treated with irinotecan at 350 mg/m2 every 21 days either prior to radiation (group A = 25 patients) or at the time of recurrence (group B = 27 patients), only 1 objective radiographic response (partial response) was documented, for an overall response proportion of 2.2% (Raymond et al., 2003).
In addition to studies of irinotecan monotherapy for malignant gliomas, a number of phase 1 studies have been initiated that combine irinotecan with other potentially active agents for the treatment of these tumors. Treatment of human glioma xenografts in nude mice with a combination of carmustine and irinotecan has resulted in enhanced activity, as compared to activity with either agent alone (Coggins et al., 1998). A supraadditive effect of irinotecan and temozolomide has also been reported (Patel et al., 2000). A phase 1 study of carmustine 100 mg/m2 every 6 weeks, followed by irinotecan at escalating doses every week for 4 weeks, followed by a 2-week break, has enrolled 72 patients, with an MTD of 125 mg/m2 in patients not on EIAED and an MTD of 225 mg/m2 in patients on EIAED (Friedman et al., 2001). A phase 1 study of irinotecan combined with Gliadel has also been initiated (Colvin et al., 2000).
In the combined cohort of 58 patients treated in the phase 1 (40 patients) and phase 2 (18 patients) parts of this study, 1/58 patients had a CR (2%), 4/58 had a PR (7%), 11/58 had SD (19%), and 31/58 (53%) patients had progressive disease. There were 8/58 (14%) patients removed from the study because of toxicity, and 3/58 (5%) patients refused further therapy. The overall proportion of objective response was 9% (5/58), and the overall proportion of patients with SD or better was 28% (16/58). All 5 patients with objective radiographic responses (CR, PR) were in group A, and responses occurred at 4 different doses of irinotecan (189 mg/m2, 238 mg/m2, 411 mg/m2, and 419 mg/m2). One of the 5 patients with an objective radiographic response remains alive without evidence of progression at 753+ days. After receiving 10 cycles of irinotecan, he refused further therapy but remains under follow-up without treatment or evidence of progression. For the entire cohort of patients treated on the phase 1 and phase 2 components of this study, the 6-month progression-free survival was 36% (95% CI, 24%–50%) and the median survival was 9.3 months (95% CI, 6.3–12.3 months).
These data are comparable to the results reported with other cytotoxic drugs currently used in the treatment of malignant gliomas. The radiographic responses observed in this phase 1/2 study occurred at 4 different dose levels, with only a single response reported at the MTD. Although there were only a few responses in this study, this observation suggests that the propensity of a recurrent malignant glioma to respond to irinotecan may be independent of dose level. The overall survival and 6-month progression-free survival achieved in this study were slightly better than the survival observed in other recent studies of agents for recurrent malignant glioma. Irinotecan was associated with significant toxicity in this phase 1/2 trial, as 14% of the patients were removed from the study because of adverse effects. The low response rate and toxicity associated with irinotecan observed in this study do not support an important role for this drug as a single agent in the management of patients with recurrent malignant gliomas. Ongoing combination studies will determine if irinotecan will assume any role in the management of malignant gliomas.
Footnotes
Financial support was received through National Cancer Institute grant UO1 CA62475.
Abbreviations used are as follows: AUC, area under the curve; CI, confidence interval; CR, complete response; EIAED, enzyme-inducing anti-epileptic drugs; MTD, maximum tolerated dose; NABTT, New Approaches to Brain Tumor Therapy; PR, partial response; SD, stable disease.
References
- Armand JP, Extra YM, Catimel G, Abigerges D, Marty M, Clavel M. Rationale for the dosage and schedule of CPT-11 (irinotecan) selected for phase II studies, as determined by European phase I studies. Ann Oncol. 1996;7:837–842. doi: 10.1093/oxfordjournals.annonc.a010763. [DOI] [PubMed] [Google Scholar]
- Bleiberg H, Cvitkovic E. Characterisation and clinical management of CPT-11 (irinotecan)-induced adverse events: The European perspective. Eur J Cancer. 1996;32A:S18–S23. doi: 10.1016/0959-8049(96)00293-6. [DOI] [PubMed] [Google Scholar]
- CBTRUS. Central Brain Tumor Registry of the United States (2002). Statistical Report: Primary Brain Tumors in the United States, 1995 –1999. Chicago: Central Brain Tumor Registry of the United States (available at http://www.cbtrus.org/2002/2002report.pdf).
- Cloughesy T, Filka E, Friedman H, Kabbinavar F, Kuhn J, Selch M, Miller L. A phase I (intrapatient dose escalation) open-label study of irinotecan (CPT-11) in patients with recurrent or progressive malignant glioma. 19. 2000;Proc Am Soc Clin Oncol:161a. (abstract) [Google Scholar]
- Coggins CA, Elion GB, Houghton PJ, Hare CB, Keir S, Colvin OM, Bigner DD, Friedman HS. Enhancement of irinotecan (CPT-11) activity against central nervous system tumor xenografts by alkylating agents. Cancer Chemother Pharmacol. 1998;41:485–490. doi: 10.1007/s002800050771. [DOI] [PubMed] [Google Scholar]
- Colvin OM, Cokgor I, Edwards S, Albergo G, Tourt-Uhlig S, Rich JN, Quinn JA, Stewart B, McLendon RE, Provenzale JM, Sampson JH, Haglund MM, Beason R, Miller L, Zaknoen S, Fraass U, Dugan M, Bigner D, Stafford-Fox V, Cohen L, Friedman A, Friedman H. Phase I trials of Gliadel plus CPT-11 or Temodar. Proc Am Soc Clin Oncol. 2000;19:172a. (Abstract) [Google Scholar]
- Conrad CA, Milosavljevic VP, Yung WK. Advances in chemotherapy for brain tumors. Neurol Clin. 1995;13:795–812. [PubMed] [Google Scholar]
- Fetell MR, Grossman SA, Fisher JD, Erlanger B, Rowinsky E, Stockel J, Piantadosi S. Preirradiation paclitaxel in glioblastoma multiforme: Efficacy, pharmacology and drug interactions. New Approaches to Brain Tumor Therapy Central Nervous System Consortium. J Clin Oncol. 1997;15:3121–3128. doi: 10.1200/JCO.1997.15.9.3121. [DOI] [PubMed] [Google Scholar]
- Fine HA, Dear KB, Loeffler JS, Black PM, Canellos GP. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71:2585–2597. doi: 10.1002/1097-0142(19930415)71:8<2585::aid-cncr2820710825>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Friedman AH, Quinn JA, Tourt-Uhlig S, Rich JN, Stewart ES, Affronti ML, Provenzale J, McLendon R, Gururangan S, Buchanan W, Colvin OM, Bigner DD, Beason R, Sampson J, Haglund M, Miller L, Friedman HS. Phase I trial of CPT-11 plus BCNU in malignant glioma. Proc Am Soc Clin Oncol. 2001;20:64a. (abstract) [Google Scholar]
- Friedman HS, Petros WP, Friedman AH, Schaaf LJ, Kerby T, Lawyer J, Parry M, Houghton PJ, Lovell S, Rasheed K, Cloughesy T, Stewart ES, Colvin OM, Provenzale JM, McLendon RE, Bigner DD, Cokgor I, Haglund M, Rich J, Ashley D, Malczyn J, Elfring GL, Miller LL. Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol. 1999;17:1516–1525. doi: 10.1200/JCO.1999.17.5.1516. [DOI] [PubMed] [Google Scholar]
- Gilbert MR, Supko JG, Batchelor T, Lesser G, Fisher JD, Piantadosi S, Grossman S. Phase I clinical trial and pharmacokinetic study of irinotecan in adults with recurrent malignant glioma. Clin Cancer Res. 2003;9:2940–2949. [PubMed] [Google Scholar]
- Grossman SA, Hochberg F, Fisher J, Chen T.-L, Kim L, Gregory R, Grochow LB, Piantadosi S. Increased 9-aminocamp-tothecin dose requirements in patients on anticonvulsants. Cancer Chemother Pharmacol. 1998;42:118–126. doi: 10.1007/s002800050794. [DOI] [PubMed] [Google Scholar]
- Gupta E, Mick R, Ramirez J, Wang X, Lestingi TM, Vokes EE, Ratain MJ. Pharmacokinetic and pharmacodynamic evaluation of the topoisomerase inhibitor irinotecan in cancer patients. J Clin Oncol. 1997a;15:1502–1510. doi: 10.1200/JCO.1997.15.4.1502. [DOI] [PubMed] [Google Scholar]
- Gupta E, Wang X, Ramirez J, Rattain MJ. Modulation of glucuronidation of SN-38, the active metabolite of irinotecan, by valproic acid and phenobarbital. Cancer Chemother Pharmacol. 1997b;39:440–444. doi: 10.1007/s002800050595. [DOI] [PubMed] [Google Scholar]
- Hare CB, Elion GB, Houghton PJ, Houghton JA, Keir S, Marcelli SL, Bigner DD, Friedman HS. Therapeutic efficacy of the topoisomerase I inhibitor 7-ethyl-10-(4-[1-piperidino]-1-piperidino)-carbonyloxy-camptothecin against pediatric and adult central nervous system tumor xenografts. Cancer Chemother Pharmacol. 1997;39:187–191. doi: 10.1007/s002800050558. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- Masuda N, Kudoh S, Fukuoka M. Irinotecan (CPT-11): Pharmacology and clinical applications. Crit Rev Oncol Hematol. 1996;24:3–26. doi: 10.1016/1040-8428(96)00201-6. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Fujiwara T, Honjo Y, Sasaoka N, Tsuchida T, Nagao S. Quantitative analysis of DNA topoisomerase I activity in human and rat glioma: Characterization and mechanism of resistance to antitopoisomerase chemical, camptothecin 11. J Surg Oncol. 1993;53:97–103. doi: 10.1002/jso.2930530210. [DOI] [PubMed] [Google Scholar]
- NCI. National Cancer Institute (1999) Common Toxicity Criteria version 2.0. Available at https://webapps.ctep.nci.nih.gov/ctcv2/plsql/ctc000w$.startup. [PubMed]
- O’Reilly S, Rowinsky EK. The clinical status of irinotecan (CPT-11), a novel water soluble camptothecin analogue: 1996. Crit Rev Oncol Hematol. 1996;24:47–70. doi: 10.1016/1040-8428(96)00211-9. [DOI] [PubMed] [Google Scholar]
- Patel VJ, Elion GB, Houghton PJ, Keir S, Pegg AE, Johnson SP, Dolan ME, Bigner DD, Friedman HS. Schedule-dependent activity of temozolomide plus CPT-11 against a human central nervous system tumor-derived xenograft. Clin Cancer Res. 2000;6:4154–4157. [PubMed] [Google Scholar]
- Prados M, Kuhn J, Yung WA, Robbins HI, Fink K, Greenberg H, Junck L, Cloughesy T, Chang S, Fine H, Schiff D, Nicholas MK. A phase-1 study of CPT-11 given every 3 weeks to patients with recurrent malignant glioma. A North American Brain Tumor Consortium (NABTC) study. Proc Am Soc Clin Oncol. 2000;19:162a. (abstract) [Google Scholar]
- Raymond E, Fabbro M, Boige V, Rixe O, Frenay M, Vassal G, Faivre S, Sicard E, Germa C, Rodier JM, Vernillet L, Armand JP. Multicentre phase II study and pharmacokinetic analysis of irinotecan in chemotherapynaïve patients with glioblastoma. Ann Oncol. 2003;14:603–614. doi: 10.1093/annonc/mdg159. [DOI] [PubMed] [Google Scholar]
- Rivory LP. Irinotecan (CPT-11): A brief overview. Clin Exp Pharmacol Physiol. 1996;23:1000–1004. doi: 10.1111/j.1440-1681.1996.tb01158.x. [DOI] [PubMed] [Google Scholar]
- Savaraj N, Xu R, Wu CJ, Landy H, Chua L, Solomon J, Feun L. Correlation of in vitro antitumor activity of irinotecan and topoisomerase I activity and levels in brain tumors. 14. 1995;Proc Am Soc Clin Oncol:492. (abstract) [Google Scholar]
- Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- Thompson J, Houghton JA, Houghton PJ. Responses of neuroblastoma (Nb) xenografts to systemic administration of irinotecan. Proc Am Assoc Cancer Res. 1996;37:436. (abstract) [Google Scholar]
- Vassal G, Morizet J, Bissery M.-C, Boland I, Ardouin P, Mathieu-Boué A, Gouyette A. Activity of the camptothecin analog CPT-11 (irinotecan) against medulloblastoma xenografts. Proc Am Assoc Cancer Res. 1994;35:366. (abstract) [Google Scholar]