Abstract
This study reports the initial experience at the University of California San Francisco (UCSF) with tumor resection and permanent, low-activity iodine 125 (125I) brachytherapy in patients with progressive or recurrent glioblastoma multiforme (GM) and compares these results to those of similar patients treated previously at UCSF with temporary brachytherapy without tumor resection. Thirty-eight patients with progressive or recurrent GM were treated at UCSF with repeat craniotomy, tumor resection, and permanent, low-activity 125I brachytherapy between June 1997 and May 1998. Selection criteria were Karnofsky performance score ⩾60, unifocal, contrast-enhancing, well-circumscribed progressive or recurrent GM that was judged to be completely resectable, and no evidence of leptomeningeal or subependymal spread. The median brachytherapy dose 5 mm exterior to the resection cavity was 300 Gy (range, 150–500 Gy). One patient was excluded from analysis. Median survival was 52 weeks from the date of brachytherapy. Age, Karnofsky performance score, and preimplant tumor volume were all statistically significant on univariate analyses. Multivariate analysis for survival showed only age to be significant. Median time to progression was 16 weeks. Both univariate and multivariate analysis of freedom from progression showed only preoperative tumor volume to be significant. Comparison to temporary brachytherapy patients showed no apparent difference in survival time. Chronic steroid requirements were low in patients with minimal postoperative residual tumor. We conclude that permanent 125I brachytherapy for recurrent or progressive GM is well tolerated. Survival time was comparable to that of a similar group of patients treated with temporary brachytherapy.
Durable control of glioblastoma multiforme (GM)2 remains elusive. Although GMs are diffusely infiltrative, most recur in a localized fashion (Agbi et al., 1992; Garden et al., 1991; Gaspar et al., 1992; Hochberg and Pruitt, 1980; Lee et al., 1999; Liang et al., 1991; Oppitz et al., 1999; Schupak et al., 1995; Sneed et al, 1994a; Wallner et al., 1989). Local progression occurs despite various forms of aggressive local therapy and despite imaging advances that better delineate local tumor. Achieving local control should be a necessary step in improving survival. Fractionated external beam radiation therapy is effective in prolonging survival of patients with primary malignant gliomas, in a dose-dependent manner (Walker et al., 1979). For patients with progressive or recurrent GM, additional external beam radiation therapy is often not a safe and effective option. However, repeat resection is often an option, and some studies suggest that it provides a quality of life and/or survival benefit (Ammirati et al., 1987a, b; Dirks et al., 1993; Harsh et al., 1987; Vick et al., 1989). Likewise, temporary or permanent brachytherapy may be an option, and some studies suggest a quality of life and/or survival benefit as well (Halligan et al., 1996; Scharfen et al., 1992; Sneed et al., 1994a, 1998; Videtic et al., 1999).
Investigators at the University of Washington hypothesized that the results of temporary brachytherapy, in which brachytherapy sources are placed in catheters without simultaneous tumor resection, might be improved by performing both resection and brachytherapy (Halligan et al., 1996). Because the tumor would be resected, temporary brachytherapy using flexible catheters, otherwise anchored in tumor, was not considered suitable. Instead, iodine 125 (125I) seeds could be permanently affixed to the surface of the resection cavity to provide a conformal radiation dose distribution in the tissue immediately surrounding the cavity. This brachytherapy technique achieves a highly conformal dose pattern.
Initial results using this technique in patients with recurrent GM demonstrated significantly improved survival compared to that of internal historical controls treated with resection and chemotherapy (Halligan et al., 1996). Here we report the initial University of California San Francisco (UCSF) experience in a study of patients with recurrent GM treated with the University of Washington technique of repeat resection and permanent 125I brachytherapy, and we compare survival with that obtained in similar patients previously treated at UCSF with temporary brachytherapy, without resection.
Materials and Methods
Patient Selection
From June 1997 through May 1998, a total of 38 patients with progressive or recurrent GM were treated at UCSF with repeat craniotomy, tumor resection, and placement of permanent, low-activity 125I seeds. This report is a retrospective review of those patients. Cases were reviewed prior to surgery at a multidisciplinary neuro-oncology tumor board. Although these patients were not treated as part of a prospective clinical trial, the UCSF practice pattern required that patients selected for tumor resection and brachytherapy have a Karnofsky performance score (KPS) ⩾60; unifocal, contrast-enhancing, well-circumscribed progressive or recurrent GM that was thought to be completely resectable; and no evidence of leptomeningeal or subependymal spread (Fig. 1a). Whether or not the tumor was thought to be totally resectable was dependent mainly on the anatomic site of the contrast-enhancing portion of the tumor rather than on the volume of contrast enhancement. Candidate tumors must have increased in linear dimension by at least 25% following prior therapy. There was no maximum tumor size limitation. Tumors located such that resection would enter the ventricular space were initially accepted for brachytherapy, but after an instance of seed migration (without apparent clinical consequences) was observed on follow-up scans, periventricular tumors were no longer included because of the uncertain consequence of seed migration. The diagnosis of GM was based on initial pathologic diagnosis. Review of patient records was approved by UCSF’s Committee on Human Research.
Fig. 1a.
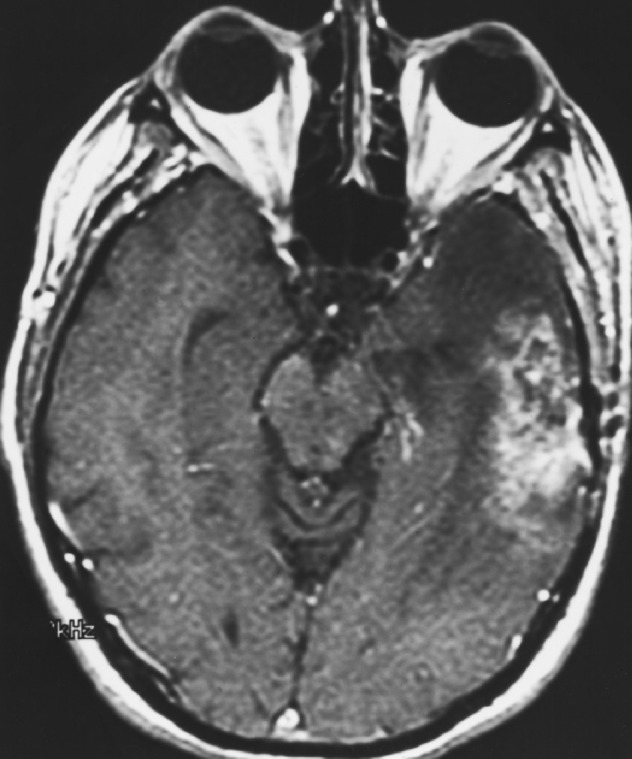
Preimplant MRI showing recurrent left temporal lobe enhancing glioblastoma multiforme.
Study group characteristics are shown in Table 1. All patients had previously completed prior standard therapy, consisting of biopsy or resection of the tumor followed by conventional external beam radiotherapy, with the exception of 2 patients who received hyperfractionated radiotherapy (72 Gy, 70.4 Gy) and a third whose initial treatment was with boron neutron capture therapy.
Table 1.
Patient and treatment characteristics of 38 patients treated with tumor resection and permanent brachytherapy for progressive or recurrent glioblastoma multiforme
| Parameter | Median | Range |
|---|---|---|
| Age | 47 | 6–70 |
| Karnofsky performance score | 90 | 60–100 |
| Prior fractionated radiotherapy dose (Gy) | 60 | 59.4–72 |
| Time from end of radiotherapy to brachytherapy (weeks) | 39 | 2–301 |
| Preimplant tumor volume (cm3) | 21 | 1–245 |
| Number of seeds implanted | 84 | 28–171 |
| Initial seed activity (mCi) | 0.67 | 0.40–0.93 |
| Brachytherapy dose at 5-mm depth (Gy) | 300 | 150–500 |
Five patients underwent prior radiosurgery. Two patients underwent prior temporary brachytherapy with hyperthermia. Carmustine wafers (Gliadel, Rhone-Poulenc Rorer Pharmaceuticals, Inc., Collegeville, Penn.) were used at the time of initial surgery in 2 patients, and 3 patients had received hydroxyurea concurrently with external beam radiotherapy. Subsequent chemotherapy treatments received by our study group, prior to referral for brachytherapy, consisted of marimastat (1 patient), carmustine (6 patients), PCV (procarbazine-CCNU [lomustine]-vincristine; 9 patients), temozolomide (1 patient), cisplatin (1 patient), and tamoxifen (1 patient). Some patients received a combination of the above therapies.
Brachytherapy Procedure
At the time of screening and selection of the patient for permanent 125I brachytherapy, the number of s7eeds was estimated by an assessment of the tumor surface area and potential resection cavity area, based on review of preoperative MRI scans. Seed number and activity were selected to achieve a desired dose, at a depth of 5 mm exterior to the resection cavity, of ⩾250 Gy at infinite decay (seed model #6711, Nycomed-Amersham, Buckinghamshire, U.K.). Because the postoperative cavity configuration only approximates the preoperative tumor configuration, formal dose preplanning was not possible. As a result, the achieved prescription dose varied. At the time of surgery and immediately following resection of the tumor, the seeds were sterilized and brought to the operating suite. Seeds were individually placed on the walls of the subcortical resection cavity with a surface density of approximately one seed per square centimeter, depending on source strength, and affixed with surgical adhesive. Seeds were not affixed to the dural surface directly underlying the surgical approach, given the technical difficulties of placing seeds on and then closing dura. The resultant conformal spatial distribution of seeds always resulted in a highly conformal dose distribution to the wall of the subcortical resection cavity, but not necessarily to the dural surface. The median number of seeds implanted per patient was 84 (range, 28–171), and the median initial seed activity was 0.67 mCi (range, 0.40–0.93 mCi).
Radiation Safety
Operating room personnel were instructed to take appropriate radiation safety precautions, including the use of a lead vest, thyroid shield, and shielded rubber gloves, consistent with maintaining radiation at a level determined to be as low as reasonably achievable. Area radiation surveys were performed before the patient left the operating room, and hospital personnel were instructed to observe standard radiation safety precautions prior to patient discharge. The median initial radiation exposure rate measured at 1 m from the patient’s head was 1.2 mR/h (range, 0.3–2.0 mR/h; 1 R = 2.58 × 10−4 C/kg air), and no patient’s discharge was delayed because of radiation safety considerations. Specific counseling was given to each patient regarding radiation safety, the temporary use of a radiation protection cap containing a thin lead foil, and the significance of the measured exposure rate.
Implant Evaluation
Patients underwent postoperative brain MRI and CT scanning prior to hospital discharge to evaluate the extent of tumor resection, to inspect seed placement, and to document radiation isodose surfaces with respect to the resection cavity. Because dose falls off rapidly beyond a depth of about 5 mm from the seeds, resections were categorized as having maximum residual tumor thickness at any point of either “<5 mm” or “⩾5 mm,” based on postoperative MRI or CT scans. The selected prescription dose was the isodose surface that enclosed the walls of the subcortical resection cavity plus a margin of approximately 5 mm. Isodose curves were not required to enclose the dura that was opened to perform the resection, given the technical difficulties of placing seeds on and then closing dura. Figures 1b and 1c show a postoperative MRI with seeds affixed to the resection cavity surface and a postoperative CT image demonstrating the radiation isodose lines around the resection cavity.
Fig. 1b.
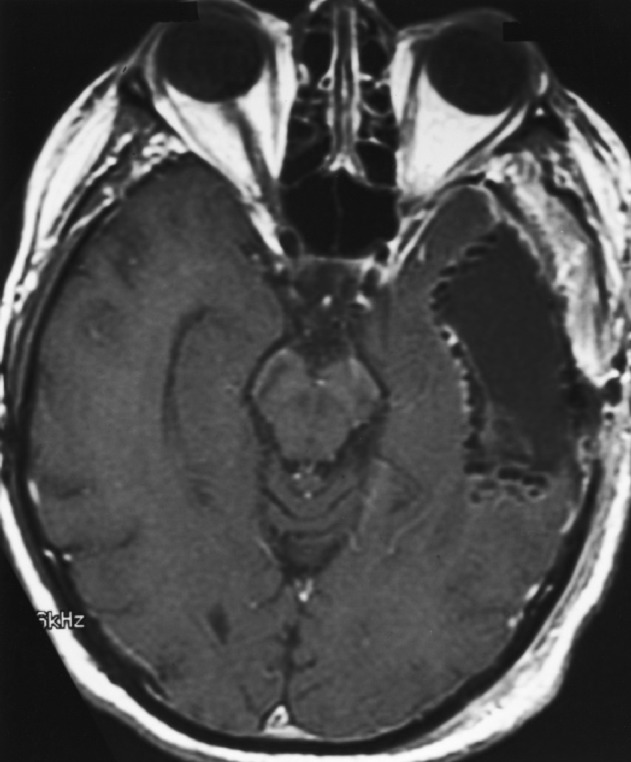
Postimplant MRI showing resection cavity lined with permanent 125I brachytherapy seeds.
Fig. 1c.
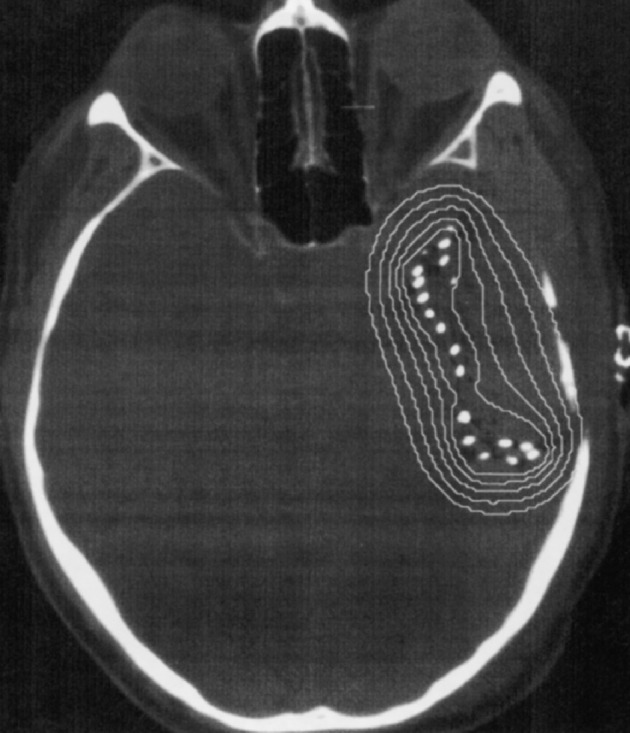
Postimplant CT image with superimposed radiation isodose curves for permanent brachytherapy. A total of 76 125I seeds of strength 0.8 mCi each were placed on the subcortical wall of the resection cavity, which resulted in a prescribed dose at average 5-mm depth of 28,000 cGy. The isodose lines displayed are 10,000 (36%), 14,000 (50%), 20,000 (71%), 28,000 (100%), and 40,000 cGy (143%) at infinite decay. The exposure rate immediately following surgery was 1.6 mR/h at 1 m (1 R = 2.58 × 10−4 C/kg air).
Statistical Methods
The end points analyzed for the study group were survival and freedom from progression (FFP). Survival and FFP were calculated with the Kaplan-Meier actuarial method. Survival was measured from the date of brachytherapy to the date of death or last follow-up. Freedom from progression was measured from the date of brachytherapy to the date of follow-up imaging study (MRI and/or magnetic resonance spectroscopy and/or PET) showing progression. Lesions without progression were censored on the date of last follow-up imaging. A combination of neurological examination and imaging information was used to define progression. In one patient, no post-implant follow-up scans were available, and that patient was not included in FFP analysis. There was one death (unrelated trauma) without evidence of tumor progression. This patient was censored for FFP (i.e., this patient was included in FFP analysis but censored at the time of the last follow-up exam).
Factors associated with FFP or survival were determined by performing univariate analysis using the Cox proportional hazards model. Five variables were studied: age, KPS, preimplant tumor volume, maximum residual tumor thickness (<5mm or ⩾5 mm), and brachytherapy prescription dose. Because changes in risk are more likely related to proportional changes in volume than absolute changes, loge of the volume was used in the modeling. Backwards stepwise multivariate regression was performed using the Cox proportional hazards model. For multivariate analyses, all variables were retained in the model if P ⩽ 0.1. Although multivariate analyses can be difficult to interpret for relatively small numbers of patients, their purpose was to ensure that no factor was determined to be nonpredictive because of confounding with another factor.
We compared the survival of patients treated with resection and permanent brachytherapy (study group) to that of a group of similar patients who were treated with temporary brachytherapy without resection (historical group). For this comparison, we used a database containing historical information on UCSF patients treated with temporary brachytherapy for recurrent GM included in previous reports (Sneed et al., 1997, 1998). In selecting patients for this comparison, we identified those cases that might reasonably have been considered for either treatment, in order to limit selection bias. Specifically, we limited the comparison to patients in both groups with KPS ⩾70, age ⩾16, and pretreatment tumor volume ⩽75 cm3. This latter volume was not known in some patients in the historical group, though the brachytherapy dose prescription volume was always known. If pretreatment volume was not available in the historical group, but the brachytherapy dose prescription volume was ⩽75 cm3, the patient was included since in patients where both were known, the latter was larger than the former in 97% of cases.
The analysis was conducted by using a multivariate Cox proportional hazards model to adjust for the effects of known prognostic factors. The analysis was performed including only patients for whom we had preimplant tumor volume information, and it was repeated with the larger group without including tumor volume in the model. These analyses did not attempt to test for other variables. Other variables were included only to adjust for possible differences in the 2 patient groups with regard to patient characteristics. Freedom from progression was not compared because it was not accurately known for all patients in the historical database.
Results
At 5-mm depth, the median prescription brachytherapy dose, the dose achieved at infinite decay time, was 300 Gy (range, 150–500 Gy), and the median initial dose rate was 15 cGy/h (range, 7–24 cGy/h). The dose and dose rate at 10-mm depth were approximately half those at 5 mm, for all patients. The median dose absorbed during the follow-up period was 281 Gy (range, 112–499 Gy). Twenty-three patients had residual tumor thickness ⩾5 mm; 15 had residual thickness <5 mm. The median preimplant tumor volume was 21 cm3 (range, 1–245 cm3). However, one patient’s preimplant tumor volume (245 cm3) was far outside the range of the others; the next largest preimplant volume was 68 cm3. Because the pre-resection tumor volume for this patient was substantially different from all others, this patient was excluded from subsequent analyses. Of the remaining 37 patients, 32 had died at the time of analysis. Follow-up for the 5 living patients ranged from 65 to 272 weeks, with a median of 140 weeks.
The median survival was 52 weeks after brachytherapy (95% CI, 40–76 weeks) for 37 patients, and it was 98 weeks from the date of initial diagnosis. The median time to progression for 36 patients was 16 weeks (95% CI, 10–21 weeks). Of the 37 tumors, 34 progressed, and 2 were censored at the time of last follow-up or MRI. In one additional case, a patient was known to have progressed, but information was not adequate to identify time interval to progression, as this patient did not have postoperative follow-up imaging. This patient was excluded from analysis of FFP. Freedom from progression is displayed graphically in Fig. 2a.
Fig. 2a.
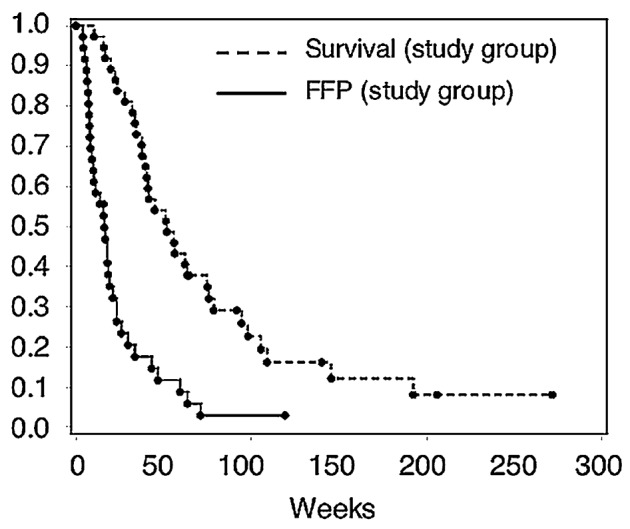
Kaplan-Meier curves for freedom from progression (FFP) and survival in weeks after brachytherapy following tumor resection and permanent brachytherapy
Univariate analysis results are shown in Table 2. For survival, univariate analysis showed that KPS, age, and preimplant volume were significant. In a stepwise multivariate analysis for survival, with criterion of P ⩽ 0.1 to remain in the model, age was statistically significant (P ⩽ 0.05), and loge of pretreatment volume met the criteria to stay in the model (P = 0.07). The hazard ratios and 95% confidence intervals were 1.03 (CI, 1.00–1.07) and 1.41 (CI, 0.97–2.06) for age and loge of pretreatment volume, respectively. However, no variable met the stepwise criteria when we excluded the 2 patients who were less than 18 years of age. For FFP, univariate analysis involving KPS, age, dose at 5 mm, residual tumor thickness (<5 mm or ⩾5 mm), and log of volume showed that KPS and preimplant tumor volume were significant (P ⩽ 0.05). In stepwise multivariate analysis with criterion for retention in the model of P ⩽ 0.1, only KPS was retained in the model.
Table 2.
Univariate analysis results for patients with progressive or recurrent glioblastoma multiforme after tumor resection and permanent brachytherapy (study group)
| Survival (n=37) | FFP (n=36) | |||
|---|---|---|---|---|
| Variable | Hazard ratio (CI) | P | Hazard ratio (CI) | P |
| KPS | 0.96 (0.926–0.996) | 0.03 | 0.95 (0.902, 0.991) | 0.02 |
| Age | 1.03 (1.01–1.07) | 0.02 | 1.00 (0.98–1.03) | 0.77 |
| Dose at 5 mm | 0.999 (0.995–1.003) | 0.56 | 1.001 (0.997–1.006) | 0.55 |
| Residual tumor thickness (<5 mm vs. ⩾5 mm) | 0.65 (0.32–1.34) | 0.24 | 0.61 (0.30–1.25) | 0.18 |
| Loge(preimplant volume) | 1.47 (1.02–2.12) | 0.04 | 1.48 (1.03–2.12) | 0.03 |
Abbreviations: FFP, freedom from progression; KPS, Karnofsky performance score.
Thirty-five permanent implant patients and 54 temporary implant patients met criteria for inclusion in the planned survival comparison. The patient characteristics for these 2 groups are summarized in Table 3, demonstrating the similarity of the 2 groups with regard to key prognostic variables. A multivariate Cox proportional hazards model was used to test whether there was any difference in outcome between the 2 groups after adjusting for age, KPS at implant, and loge of tumor volume. There was no significant difference in survival (P = 0.73, hazard ratio = 0.91, 95% CI, 0.54 – 1.54). When the analysis was repeated without loge of tumor volume, to increase the number of historical control patients that could be included, the results were similar (P = 0.62, hazard ratio 1.12, 95% CI, 0.71–1.77). The Kaplan-Meier survival curves (Fig. 2b) show similar survival patterns.
Table 3.
Comparative information for patients treated with tumor resection and permanent 125I implants (study group) or temporary implants (historical group) for recurrent/progressive glioblastoma multiforme
| Parameter | Permanent 125I (study group) | Temporary 125I (historical group) |
|---|---|---|
| KPS | 90 (70–100) | 90 (70–100) |
| Number of patients | 35 | 54 |
| Median age | 47 (19–69) | 52 (16–78) |
| Tumor volume (cm3) | 25.0 (3–68) | 15.9 (2–52)a |
| Median survival (weeks) | 51 (CI 38–75) | 54 (CI 40–62) |
Abbreviations: 125I, iodine 125; KPS, Karnofsky performance status.
See text for information on pretreatment tumor volume.
Fig. 2b.
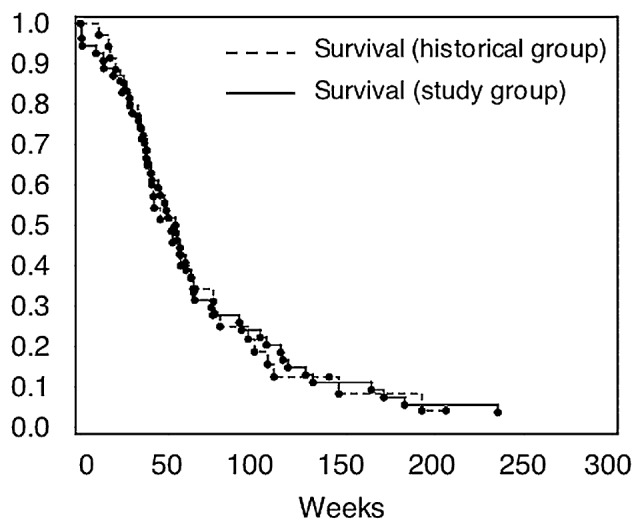
Kaplan-Meier survival curves in weeks after brachytherapy for tumor resection and permanent brachytherapy (study group, n = 35) and for temporary brachytherapy alone (historical group, n = 54)
A total of 24 patients (63%) in the study group underwent some form of salvage treatment at the time of subsequent progression or recurrence. Four patients underwent additional surgery. Three patients underwent gamma knife radiosurgery, and one underwent proton radiosurgery. Additional salvage treatments included chemotherapy with one or more of the following drugs or drug combinations: PCV, temozolomide, carboplatin, thymidine, thalidomide, isotretinoin, tamoxifen, or lomustine. In addition, one patient underwent a course of hyperbaric oxygen treatment for presumed radionecrosis.
Toxicity
Detailed follow-up information for steroid use was available for 20 patients who elected to continue their routine follow-up at UCSF. In the 20 patients analyzed for steroid use, 11 had residual tumor thickness <5 mm, and 9 had residual thickness ⩾5 mm. In the 11 patients with thickness <5 mm, 9 were off steroids by 2 months. Of the 9 patients with ⩾5-mm residual tumor thickness, only 2 were able to discontinue steroids. The remaining 7 remained on steroids until death. Four patients underwent at least 1 subsequent craniotomy following seed implant, with pathology demonstrating recurrent tumor in 3 patients and gliosis without evidence of recurrent tumor in 1 patient. All 4 patients were asymptomatic but had imaging changes thought to represent recurrent tumor. One of the 4 patients underwent 2 additional postbrachytherapy craniotomies for recurrent tumor, with tumor growing through the bone flap and into epidermis. Two other patients had recurrent tumor at the time of resection, and the fourth was found to have gliosis and necrosis with no evidence of tumor at 12 months. Postoperatively, this patient developed a CSF leak and wound infection, and at 19 months following brachytherapy developed diffuse bony metastatic involvement of the spine.
Discussion
Our results continue to demonstrate the difficulty in controlling GM, even with the dose escalation possible with permanent 125I brachytherapy. A brachytherapy dose response has been suggested in primary GM patients undergoing temporary 125I implant boost (dose rate of 30–60 cGy/h over 4–6 days) after external beam radiotherapy (Sneed et al., 1996). In that study a quadratic relationship was found between total biological effective dose and survival. Although higher minimum brachytherapy tumor dose was associated with better local control, life-threatening necrosis limits the maximum dose that can be safely used with temporary afterloading brachytherapy. In the current report, the median dose achieved was 300 Gy (range, 100–500 Gy) at 5-mm depth. The median initial dose rate was 15 cGy/h at 5-mm depth. A dose response was not apparent in the range of doses achieved, and no patient required reoperation for symptomatic necrosis or edema. Most patients with residual tumor thickness <5 mm (9/11) were able to discontinue steroids, while few (2/9) patients with residual thickness ⩾5 mm were able to discontinue steroids. This suggests that the steroid dependence may be a function of gross residual tumor burden, rather than of direct or indirect sequelae of the brachytherapy. This suggestion is supported by our previous report that 67% of 18-month survivors of temporary brachytherapy without surgery for malignant glioma are steroid dependent (Leibel et al., 1989). The reoperation rate in our study was 10% (4/38), in contrast to a 40% reoperation rate for high-activity temporary interstitial implants (Scharfen et al., 1992). The 4 reoperations in patients in this study were done electively for suspected tumor recurrence.
Median survival in the study group was 52 weeks measured from the date of brachytherapy, similar to survival rates reported for other brachytherapy techniques (Table 4).
Table 4.
Summary of results of iodine 125 brachytherapy for progressive or recurrent glioblastoma multiforme
| Reference | Implant type | Number of patients | Median. survival (weeks) | Time to progression (weeks) |
|---|---|---|---|---|
| Sneed et al., 1994a | Temporary | 66 | 51 | — |
| Sneed et al., 1994b | Temporary + hyperthermia | 25 | 49 | 26 |
| Zamorano et al., 1992 | Permanent (catheter-based) | 11 | 39.9 | — |
| Gaspar et al., 1999 | Permanent (catheter-based) | 37 | 46.8 | — |
| Halligan et al., 1996 | Permanent | 18 | 64 | 29 |
| Patel et al., 2000 | Permanent | 40 | 59 | 25 |
| Current study | Permanent | 37 | 52 | 17 |
Outcome data from previously reported patients treated at UCSF with temporary 125I implants were compared with that of patients treated at UCSF with permanent 125I. Using a proportional hazards model and adjusting for age and KPS, we found no indication of any survival difference for the 2 groups. Zamorano et al. (1992) reported a Wayne State University permanent 125I brachytherapy study using stereotactically placed catheters and low-activity 125I seeds in patients with newly diagnosed or recurrent malignant brain tumors following prior surgery, radiotherapy, and chemotherapy. In the subgroup of patients with recurrent tumors (11 patients), a median survival of 40 weeks was achieved. Gaspar et al. (1999) subsequently reported a Wayne State University series of patients treated with permanent 125I brachytherapy for recurrent malignant gliomas using a stereotactically placed permanent catheter technique. In that study, the prescription dose at the tumor periphery was 103.68 Gy, and the median survival for the glioblastoma subgroup was 47 weeks (0.9 years). The 2 series using a technique most similar to that used in our current study are those of Halligan et al. (1996) and Patel et al. (2000). For 18 patients with recurrent GM, Halligan et al. reported a median survival of 64 weeks from the time of brachytherapy with doses of 150 to 230 Gy at 5-mm depth. Patel et al. reported on 40 patients with recurrent GM treated with resection plus permanent 125I brachytherapy. Brachytherapy doses of 120 to 160 Gy at a prescription depth of 5 mm were given, and an actuarial survival of 47 weeks from the date of brachytherapy was reported.
Three groups (Halligan et al., 1996; Patel et al., 2000; Sneed et al., 1994b) have reported times to progression following brachytherapy for recurrent GM, with times in the range of 25 to 29 weeks, apparently longer than the 17 weeks found in the current study. Whether that apparent difference is real or simply reflects follow-up MRI technique and frequency is unclear. In any case, the range of survivals in those 3 studies (49–64 weeks) may not differ from that found in the current study (52 weeks).
It might seem that tumor resection plus brachytherapy ought to provide improved outcome compared to brachytherapy alone. However, this study shows that overall survival for patients with progressive or recurrent GM undergoing tumor resection and permanent brachytherapy is similar to that of patients undergoing temporary brachytherapy alone, at least based on the selection factors, doses, and techniques used to date. It is possible that the dose rates we obtained at 5-mm depth or deeper with permanent brachytherapy were too low to have a significant impact on rapidly dividing GM, even though some glioblastoma cell lines appear to be vulnerable to low-dose-rate in vitro irradiation (Marin et al., 1991). Koot et al. (2000) used widely varying dose rates to perform brachytherapy in patients with primary glioblastoma and concluded that dose rate does not play a major role in determining survival. Likewise, it is possible that the doses at 5-mm depth and deeper were too low, and that higher permanent brachytherapy doses are needed, especially since permanent brachytherapy toxicity appears to be low. With temporary brachytherapy, further dose escalation is unlikely to lead to increased survival, as indicated by the dose-response data of Sneed et al. (1996), and, in any case, toxicity with that technique is already high. Whether further dose escalation with permanent 125I brachytherapy would lead to improved survival and FFP remains speculative. Dose escalation with permanent 125I brachytherapy appears not to be limited by toxicity (Halligan et al., 1996; Patel et al., 2000; Zamorano et al., 1992), though further escalation could well be limited by considerations of radiation exposure to caregivers and family members. A much larger study would be required to confidently determine the relationship between dose, necrosis, survival, and quality of life. Whether the results might be improved if it were technically feasible to place seeds on the dural surface and still effect surgical closure remains speculative, and our limited data on patterns of failure did not allow us to draw conclusions. Finally, 125I brachytherapy is only one radiation modality requiring further investigation, among others such as high-dose-rate brachytherapy, radiosurgery, and intensity-modulated radiotherapy.
Conclusions
The combination of surgery and permanent low-activity 125I brachytherapy for progressive or recurrent GM is well tolerated. In the current series a median survival of 1 year following implant was observed. It appears that in patients with recurrent GM who have excellent performance status, younger age, aggressive surgical management, close follow-up, and varied chemotherapy use, overall survival after this combination therapy is similar to that of patients who are given temporary brachytherapy. The toxicity profile may be improved over that for temporary 125I brachytherapy, and permanent brachytherapy adds little time or complexity to the operative procedure. Based on these data, a prospective, phase 2 study combining tumor resection, permanent 125I brachytherapy, and adjuvant fractionated external radiotherapy in newly diagnosed GM cases was initiated at UCSF to examine toxicity and patterns of failure in detail.
Footnotes
Abbreviations used are as follows: FFP, freedom from progression; GM, glioblastoma multiforme; 125I, iodine 125; KPS, Karnofsky performance score; UCSF, University of California San Francisco.
References
- Agbi CB, Bernstein M, Laperriere N, Leung P, Lumley M. Patterns of recurrence of malignant astrocytoma following stereotactic interstitial brachytherapy with iodine-125 implants. Int J Radiat Oncol Biol Phys. 1992;23:321–326. doi: 10.1016/0360-3016(92)90748-7. [DOI] [PubMed] [Google Scholar]
- Ammirati M, Vick N, Liao YL, Ciric I, Mikhael M. Effect of the extent of surgical resection on survival and quality of life in patients with supratentorial glioblastomas and anaplastic astrocytomas. Neurosurgery. 1987a;21:201–206. doi: 10.1227/00006123-198708000-00012. [DOI] [PubMed] [Google Scholar]
- Ammirati M, Galicich JH, Arbit E, Liao Y. Reoperation in the treatment of recurrent intracranial malignant gliomas. Neurosurgery. 1987b;21:607–614. doi: 10.1227/00006123-198711000-00001. [DOI] [PubMed] [Google Scholar]
- Dirks P, Bernstein M, Muller PJ, Tucker WS. The value of reoperation for recurrent glioblastoma. Can J Surg. 1993;36:271–275. [PubMed] [Google Scholar]
- Garden AS, Maor MH, Yung WK, Bruner JM, Woo SY, Moser RP, Lee YY. Outcome and patterns of failure following limited-volume irradiation for malignant astrocytomas. Radiother Oncol. 1991;20:99–110. doi: 10.1016/0167-8140(91)90143-5. [DOI] [PubMed] [Google Scholar]
- Gaspar LE, Fisher BJ, Macdonald DR, LeBer DV, Halperin EC, Schold SC, Jr, Cairncross JG. Supratentorial malignant glioma: Patterns of recurrence and implications for external beam local treatment. Int J Radiat Oncol Biol Phys. 1992;24:55–57. doi: 10.1016/0360-3016(92)91021-e. [DOI] [PubMed] [Google Scholar]
- Gaspar LE, Zamorano LJ, Shamsa E, Fontanesi J, Ezzell GE, Yakar DA. Permanent 125iodine implants for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 1999;43:977–982. doi: 10.1016/s0360-3016(98)00494-5. [DOI] [PubMed] [Google Scholar]
- Halligan JB, Stelzer KJ, Rostomily RC, Spence AM, Griffin TW, Berger MS. Operation and permanent low activity 125I brachytherapy for recurrent high-grade astrocytomas. Int J Radiat Oncol Biol Phys. 1996;35:541–547. doi: 10.1016/s0360-3016(96)80017-4. [DOI] [PubMed] [Google Scholar]
- Harsh GR, 4th, Levin VA, Gutin PH, Seager M, Silver P, Wilson CB. Reoperation for recurrent glioblastoma and anaplastic astrocytoma. Neurosurgery. 1987;21:615–621. doi: 10.1227/00006123-198711000-00002. [DOI] [PubMed] [Google Scholar]
- Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30:907–911. doi: 10.1212/wnl.30.9.907. [DOI] [PubMed] [Google Scholar]
- Koot RW, Maarouf M, Hulshof MC, Voges J, Treuer H, Koedooder C, Sturm V, Bosch DA. Brachytherapy: Results of two different therapy strategies for patients with primary glioblastoma multiforme. Cancer. 2000;88:2796–2802. [PubMed] [Google Scholar]
- Lee SW, Fraass BA, Marsh LH, Herbort K, Gebarski SS, Martel MK, Radany EH, Lichter AS, Sandler HM. Patterns of failure following high-dose 3-D conformal radiotherapy for high-grade astrocytomas: A quantitative dosimetric study. Int J Radiat Oncol Biol Phys. 1999;43:79–88. doi: 10.1016/s0360-3016(98)00266-1. [DOI] [PubMed] [Google Scholar]
- Leibel SA, Gutin PH, Wara WM, Silver PS, Larson DA, Edwards MS, Lamb SA, Ham B, Weaver KA, Barnett C, Phillips TL. Survival and quality of life after interstitial implantation of removable high-activity iodine-125 sources for the treatment of patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 1989;17:1129–1139. doi: 10.1016/0360-3016(89)90518-x. [DOI] [PubMed] [Google Scholar]
- Liang BC, Thornton AF, Jr, Sandler HM, Greenberg HS. Malignant astrocytomas: Focal tumor recurrence after focal external beam radiation therapy. J Neurosurg. 1991;75:559–563. doi: 10.3171/jns.1991.75.4.0559. [DOI] [PubMed] [Google Scholar]
- Marin LA, Smith CE, Langston MY, Quashie D, Dillehay LE. Response of glioblastoma cell lines to low dose rate irradiation. Int J Radiat Oncol Biol Phys. 1991;21:397–402. doi: 10.1016/0360-3016(91)90788-6. [DOI] [PubMed] [Google Scholar]
- Oppitz U, Maessen D, Zunterer H, Richter S, Flentje M. 3D-recurrence-patterns of glioblastomas after CT-planned postoperative irradiation. Radiother Oncol. 1999;53:53–57. doi: 10.1016/s0167-8140(99)00117-6. [DOI] [PubMed] [Google Scholar]
- Patel S, Breneman JC, Warnik RE, Albright RE, Jr, Tobler WD, van Loveren HR, Tew JM. Permanent iodine-125 interstitial implants for the treatment of recurrent glioblastoma multiforme. Neuro-surgery. 2000;46:1123–1128. doi: 10.1097/00006123-200005000-00019. [DOI] [PubMed] [Google Scholar]
- Scharfen CO, Sneed PK, Wara WM, Larson DL, Phillips TL, Prados MD, Weaver KA, Malec M, Acord P, Lamborn KR, Lamb SA, Ham B, Gutin PH. High activity iodine-125 interstitial implant for gliomas. Int J Radiat Oncol Biol Phys. 1992;24:583–591. doi: 10.1016/0360-3016(92)90702-j. [DOI] [PubMed] [Google Scholar]
- Schupak K, Malkin M, Anderson L, Arbit E, Lindsley K, Leibel S. The relationship between the technical accuracy of stereotactic interstitial implantation for high grade gliomas and the pattern of tumor recurrence. Int J Radiat Oncol Biol Phys. 1995;32:1167–1176. doi: 10.1016/0360-3016(94)00652-2. [DOI] [PubMed] [Google Scholar]
- Sneed PK, Gutin PH, Larson DA, Malec MK, Phillips TL, Prados MD, Scharfen CO, Weaver KA, Wara WM. Patterns of recurrence of glioblastoma multiforme after external irradiation followed by implant boost. Int J Radiat Oncol Biol Phys. 1994a;29:719–727. doi: 10.1016/0360-3016(94)90559-2. [DOI] [PubMed] [Google Scholar]
- Sneed PK, Larson DA, Gutin PH. Brachytherapy and hyperthermia for malignant astrocytomas. Semin Oncol. 1994b;21:186–197. [PubMed] [Google Scholar]
- Sneed PK, Lamborn KR, Larson DA, Prados MD, Malec MK, McDermott MW, Weaver KA, Phillips TL, Wara WM, Gutin PH. Demonstration of brachytherapy boost dose-response relationships in glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1996;35:37–44. doi: 10.1016/s0360-3016(96)85009-7. [DOI] [PubMed] [Google Scholar]
- Sneed PK, McDermott MW, Gutin PH. Interstitial brachytherapy procedures for brain tumors. Semin Surg Oncol. 1997;13:157–166. doi: 10.1002/(sici)1098-2388(199705/06)13:3<157::aid-ssu2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Sneed PK, Stauffer PR, McDermott MW, Diederich CJ, Lamborn KR, Prados MD, Chang S, Weaver KA, Spry L, Malec MK, Lamb SA, Voss B, Davis RL, Wara WM, Larson DA, Phillips TL, Gutin PH. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost ± hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1998;40:287–295. doi: 10.1016/s0360-3016(97)00731-1. [DOI] [PubMed] [Google Scholar]
- Vick NA, Ciric IS, Eller TW, Cozzens JW, Walsh A. Reoperation for malignant astrocytoma. Neurology. 1989;39:430–432. doi: 10.1212/wnl.39.3.430. [DOI] [PubMed] [Google Scholar]
- Videtic GM, Gaspar LE, Zamorano L, Fontanesi J, Levin KJ, Kupsky WJ, Tekyi-Mensah S. Use of the RTOG recursive partitioning analysis to validate the benefit of iodine-125 implants in the primary treatment of malignant gliomas. Int J Radiat Oncol Biol Phys. 1999;45:687–692. doi: 10.1016/s0360-3016(99)00244-8. [DOI] [PubMed] [Google Scholar]
- Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5:1725–1731. doi: 10.1016/0360-3016(79)90553-4. [DOI] [PubMed] [Google Scholar]
- Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989;16:1405–1409. doi: 10.1016/0360-3016(89)90941-3. [DOI] [PubMed] [Google Scholar]
- Zamorano L, Yakar D, Dujovny M, Sheehan M, Kim J. Permanent iodine-125 implant and external beam radiation therapy for the treatment of malignant brain tumors. Stereotact Funct Neurosurg. 1992;59:183–192. doi: 10.1159/000098940. [DOI] [PubMed] [Google Scholar]


