Abstract
In preclinical studies, BCNU, or 1,3-bis(2-chloroethyl)-1-nitrosourea, plus CPT-11 (irinotecan) exhibits schedule-dependent, synergistic activity against malignant glioma (MG). We previously established the maximum tolerated dose of CPT-11 when administered for 4 consecutive weeks in combination with BCNU administered on the first day of each 6-week cycle. We now report a phase 2 trial of BCNU plus CPT-11 for patients with MG. In the current study, BCNU (100 mg/m2) was administered on day 1 of each 6-week cycle. CPT-11 was administered on days 1, 8, 15, and 22 at 225 mg/m2 for patients receiving CYP3A1- or CYP3A4-inducing anticonvulsants and at 125 mg/m2 for those not on these medications. Newly diagnosed patients received up to 3 cycles before radiotherapy, while recurrent patients received up to 8 cycles. The primary end point of this study was radiographic response, while time to progression and overall survival were also assessed. Seventy-six patients were treated, including 37 with newly diagnosed tumors and 39 with recurrent disease. Fifty-six had glioblastoma multiforme, 18 had anaplastic astrocytoma, and 2 had anaplastic oligodendroglioma. Toxicities (grade ⩾3) included infections (13%), thromboses (12%), diarrhea (10%), and neutropenia (7%). Interstitial pneumonitis developed in 4 patients. Five newly diagnosed patients (14%; 95% CI, 5%–29%) achieved a radiographic response (1 complete response and 4 partial responses). Five patients with recurrent MG also achieved a response (1 complete response and 4 partial responses; 13%; 95% CI, 4%–27%). More than 40% of both newly diagnosed and recurrent patients achieved stable disease. Median time to progression was 11.3 weeks for recurrent glioblastoma multiforme patients and 16.9 weeks for recurrent anaplastic astrocytoma/anaplastic oligodendroglioma patients. We conclude that the activity of BCNU plus CPT-11 for patients with MG appears comparable to that of CPT-11 alone and may be more toxic.
The outcome associated with current therapy for patients with glioblastoma multiforme(GBM)3 remains inadequate. Recurrence is nearly universal and is typically ccompanied by progressive physical and mental debilitation culminating in death 40 to 50 weeks from diagnosis. Available salvage therapies following progression are ineffective, with a median progression-free survival of only 9 weeks for patients with WHO grade IV GBM and 12 weeks for those with WHO grade III tumors, including anaplastic astrocytoma (AA), anaplastic oligodendroglioma (AO), and anaplastic oligoastrocytoma (Wong et al., 1999). The role of chemotherapy for patients with malignant glioma (MG) remains controversial. Regimens such as PCV (procarbazine, CCNU [lomustine], and vincristine) improve outcome for some patients with WHO grade III tumors (Cairncross et al., 1998; Ino et al., 2001; Kristof et al., 2002; Smith et al., 2000) but fail to increase survival of patients with GBM beyond that of radiation therapy alone (Medical Research Council Brain Tumour Working Party, 2001). Other studies ascribe a modest survival advantage to chemotherapy (Fine et al., 1993; Stenning et al., 1987; Stewart et al., 2002; Stupp et al., 2002). Our center and others have attempted to improve outcome for patients with MG by investigating rationally designed chemotherapy regimens.
Nitrosoureas including CCNU (lomustine) and BCNU (1,3-bis(2-chloroethyl)-1-nitrosourea; carmustine), administered as either monotherapy or as the principal component of the PCV regimen, are historically among the most widely used chemotherapeutics for patients with MG (Chang et al., 1983; Deutsch et al., 1989; Green et al., 1983; Levin et al., 1990; Shapiro et al., 1989; Walker et al., 1978). Irinotecan (CPT-11; Camptosar; Pharmacia & Upjohn, Kalamazoo, Mich.), a water soluble derivative of camptothecin extracted from the Chinese tree Camptotheca acuminata, inhibits topoisomerase I, an essential enzyme for DNA transcription, replication, and repair (Slichenmyer et al., 1994). CPT-11 has substantial activity against a broad panel of CNS xenografts (Hare et al., 1997; Houghton et al., 1995). In a phase 2 trial for patients with recurrent MG, 15% (95% CI, 6%– 24%) of the patients achieved a partial response (PR), while 55% achieved stable disease (SD) for more than 12 weeks following administration of CPT-11 (Friedman et al., 1999). Additional preclinical studies demonstrated that the efficacy of CPT-11 against CNS tumors is enhanced when combined with alkylators such as BCNU in a schedule-dependent manner (Coggins et al., 1998). We therefore sought to determine whether the antitumor activity of CPT-11 could be enhanced by BCNU in patients with MG. We first determined that the maximum tolerated dose (MTD) of CPT-11 administered weekly for 4 consecutive weeks of each 6-week cycle in combination with a fixed dose of BCNU (100 mg/m2 administered on day 1 of each cycle) is 225 mg/m2 for patients on CYP3A1- or CYP3A4-enzyme-inducing anticonvulsants (EIACs; phenytoin, phenobarbital, and carbamazepine) and 125 mg/m2 for patients not on EIAC (Quinn et al., 2004). We now report a phase 2 study designed to determine the efficacy of BCNU plus CPT-11 for patients with MG.
Patients and Methods
Protocol Objectives
The objectives of the study were to define the activity of BCNU plus CPT-11 in the treatment of adults with newly diagnosed or recurrent MG and to further define the toxicity of this regimen.
Patient Eligibility Criteria
Patients were required to have a histologically confirmed diagnosis of MG (GBM, AA, AO, or anaplastic oligoastrocytoma) and were either (a) newly diagnosed and previously untreated or (b) had recurrent disease defined by the presence of unequivocal progressive disease (PD) following prior radiation or chemotherapy. Eligible patients were also required to be at least 18 years of age, have measurable tumor on contrast-enhanced MRI obtained within 2 weeks of study initiation, have a Karnofsky performance status ⩾60%, and be on a stable corticosteroid dose for at least 1 week prior to therapy initiation. Additional enrollment criteria included hematocrit >29%, absolute neutrophil count >1500 cells/μl, platelet count >125,000 cells/μl, serum creatinine <1.5 mg/dl, blood urea nitrogen ⩽25 mg/dl, serum aspartate aminotransferase and bilirubin ⩽1.5 times the institutional upper limit of normal, and a diffusing capacity of the lung for carbon monoxide (DLCO) >60% of the predicted value. At least 3 weeks between prior surgical resection, or 6 weeks between prior radiotherapy or chemotherapy, and enrollment were required unless there was unequivocal progression of tumor. All patients were informed of the investigational nature of the study and provided informed consent as approved by the Duke University Medical Center Institutional Review Board.
The following patients were excluded: pregnant or nursing women; those with reproductive potential and not using an effective contraceptive method; patients with PD following prior treatment with either nitrosoureas (CCNU or BCNU) or CPT-11; patients who underwent prior stereotactic radiosurgery, radiation implants, or radiolabeled monoclonal antibody therapy unless there was unequivocal radiographic evidence of disease progression (such as a new or distant lesion) or biopsy-proven confirmation of recurrent tumor.
Treatment Design
Each 6-week treatment cycle began with BCNU (100 mg/m2) as a 1-h infusion approximately an hour before the first CPT-11 dose of each cycle. CPT-11 was given weekly for 4 weeks; this was followed by a 2-week rest at 225 mg/m2 for patients on EIAC and 125 mg/m2 for those not on EIAC. Newly diagnosed patients received up to 3 cycles and proceeded to radiotherapy thereafter or immediately upon either tumor progression or unacceptable toxicity. Patients with recurrent tumors received up to 8 cycles unless unacceptable toxicity or tumor progression occurred.
Dose Modification and Retreatment Criteria
Toxicity was graded according to NCI CTC version 2 (NCI 2004). CPT-11 was reduced by 20 mg/m2 for grade 3 or greater nonhematologic toxicity or grade 4 hematologic toxicity. The criteria for retreatment consisted of the following: absolute neutrophil count >1000 cells/μl; platelets >100,000 cells/μl; serum aspartate aminotransferase, total bilirubin, and creatinine ⩽1.5 times upper limit of normal. All other toxicities were required to resolve to grade ⩽1 for retreatment.
Patients were removed from study for evidence of PD after completion of at least 1 cycle, grade 4 nonhematopoietic toxicity, DLCO ⩽75% of baseline, more than 2 dose reductions due to toxicity, noncompliance, or voluntary withdrawal.
Supportive Care
Antiemetic therapy with ondansetron and dexamethasone was given before each weekly dose of chemotherapy. Atropine (1 mg i.v.) was administered for acute cholinergic symptoms, and loperamide was prescribed for late diarrhea as previously described (Quinn et al., 2004). Hematopoietic growth factors and blood products were administered for grade 4 hematologic toxicity.
Evaluations Prior to and During Therapy
Patients underwent physical and neurologic examinations and MRI scans within 3 weeks of enrollment and before every 6-week cycle. A complete blood count with differential was performed weekly, and a serum biochemistry profile was assessed every 6 weeks. A urinalysis and a pulmonary function test were performed prior to the first cycle, along with a beta human chorionic gonadotropin test in women with reproductive potential. Pulmonary function tests were repeated before odd-numbered cycles.
Response Evaluation
Response determination, performed by the study investigators, was based on neurologic examination and comparison of the baseline contrast-enhanced MRI scan with the scans performed before each cycle. A complete response (CR) was defined as the disappearance of all enhancing tumor from baseline on consecutive MRI at least 6 weeks apart, combined with discontinuation of corticosteroids and achievement of neurologic stability or improvement. A PR was defined as ⩾50% reduction from baseline in the size (measured as the product of largest perpendicular diameters) of enhancing tumor maintained for at least 6 weeks with a stable or improved neurologic exam and a stable or reduced corticosteroid requirement. A minor response (MR) was defined as more than 25% but less than 50% reduction from baseline in the size of enhancing tumor on MRI scan, neurologic stability, and a stable or reduced corticosteroid dose. Progressive disease was defined as more than 25% increase in size of enhancing tumor from baseline or any new tumor on MRI. Stable disease was defined as any clinical status not meeting the criteria for CR, PR, or PD for more than 1 course of therapy. Patient response was defined on the basis of the best response achieved at any point while enrolled on the clinical trial.
Statistical Considerations
The primary goal of this study was to evaluate the response rate of BCNU plus CPT-11 in the treatment of 2 patient populations: those with newly diagnosed MG, and those with recurrent or progressive MG. Each patient population was evaluated separately. Given a 15% response rate for CPT-11 alone (Friedman et al., 1999), we employed a 2-stage “minimax” phase 2 design to differentiate between a 5% and 20% response rate in the current study among both newly diagnosed and recurrent/progressive MG patient populations.
Eighteen patients were treated in the first stage with a plan to terminate the study if none of the patients responded to treatment. If any of the 18 initial patients responded to treatment, 14 patients would be added. The treatment regimen would be considered worthy of further evaluation if 4 or more of the total 32 patients responded.
The following characteristics were true of this study design: (1) the probability of erroneously concluding a treatment was active (P ⩾ 0.2) when it was actually ineffective (P ⩽0.05) was less than 0.1, that is, α = 0.1; (2) the probability of erroneously concluding that the treatment was ineffective (P ⩽0.05) when the treatment was actually active (P ⩾0.2) was 0.1, that is, β = 0.1. Under the null hypothesis, the probability of early termination was 0.40.
Time to progression (TTP) and overall survival (OS) were measured from the date of enrollment and analyzed by the Kaplan-Meier method including 95% CIs (Brookmeyer and Crowley, 1982; Klein, 1991); the influence of baseline patient characteristics on these end points was explored by using multivariate Cox regression.
Results
Patient Characteristics
A total of 76 patients were enrolled onto this study between December 2000 and June 2001. Patient characteristics (Table 1) were comparable among newly diagnosed patients, recurrent patients, those receiving EIACs, and those not receiving EIACs. The median age was 48 (range, 22–76), and 48 (63%) were male. All patients had a Karnofsky performance status ⩾60%. Thirty-seven patients with newly diagnosed tumors were enrolled, including 28 (76%) with GBM and 9 (24%) with AA. Thirty-nine patients had recurrent disease, including 28 (72%) with GBM, 9 with AA (24%), and 2 with AO (3%). Nearly all patients had a substantial tumor burden. Among newly diagnosed patients, 19 (51%) had a biopsy and 18 (49%) had a subtotal resection prior to chemotherapy. Among recurrent patients, 36 (92%) had either no surgery or a biopsy, while 3 (8%) underwent a subtotal resection prior to treatment. Recurrent patients were also heavily pretreated. Thirty-two (85%) failed 1 or more prior chemotherapy regimens. In addition, 20 patients (51%) had 2 or more recurrences before study enrollment.
Table 1.
Patient characteristics at study enrollment
|
Newly Diagnosed (n = 37)
|
Recurrent (n = 39)
|
||||
|---|---|---|---|---|---|
| Characteristic | +EIAC (n = 22) | No EIAC (n = 15) | +EIAC (n = 24) | No EIAC (n = 15) | Study Total (N = 76) |
| Age, years | |||||
| Median | 51 | 48 | 46 | 44 | 48 |
| Range | 26–61 | 35–69 | 27–63 | 22–76 | 22–76 |
| Sex | |||||
| Male | 14 (64%) | 11 (73%) | 16 (67%) | 7 (47%) | 48 (63%) |
| Female | 8 (36%) | 4 (27%) | 8 (33%) | 8 (53%) | 28 (37%) |
| Histology | |||||
| GBM | 16 (73%) | 12 (80%) | 17 (71%) | 11 (73%) | 56 (74%) |
| AA | 6 (27%) | 3 (20%) | 6 (25%) | 3 (20%) | 18 (24%) |
| AO | 0 | 0 | 1 (4%) | 1 (7%) | 2 (3%) |
| KPS | |||||
| 100 | 5 (23%) | 4 (27%) | 2 (8%) | 2 (13%) | 13 (17%) |
| 90 | 9 (41%) | 5 (33%) | 15 (63%) | 11 (73%) | 40 (53%) |
| 80 | 2 (9%) | 5 (33%) | 2 (8%) | 0 | 9 (12%) |
| 70 | 5 (23%) | 1 (7%) | 3 (13%) | 2 (13%) | 11 (14%) |
| 60 | 1 (5%) | 0 | 2 (8%) | 0 | 3 (4%) |
| Surgery | |||||
| STR | 12 (55%) | 6 (40%) | 2 (8%) | 1 (7%) | 19 (25%) |
| Biopsy | 10 (45%) | 9 (60%) | 3 (13%) | 3 (20%) | 25 (33%) |
| None | 0 | 0 | 19 (79%) | 11 (73%) | 30 (39%) |
| Prior XRT | — | — | 24 (100%) | 14 (93%) | — |
| Prior chemo agents | |||||
| Median No. | — | — | 1.5 | 1 | |
| Range | 0–5 | 0–3 | |||
| No. of prior chemo regimen failures | |||||
| 0 | — | — | 5 (21%) | 2 (13%) | — |
| 1 | — | — | 8 (33%) | 12 (80%) | — |
| 2 | — | — | 9 (38%) | 1 (7%) | — |
| 3 | — | — | 2 (8%) | 0 | — |
| Median | — | — | 1 | 1 | — |
| Range | 0–3 | 0–2 | |||
Abbreviations: AA, anaplastic astrocytoma; AO, anaplastic oligodendroglioma; chemo, chemotherapeutic; EIAC, enzyme-inducing anticonvulsants (phenytoin, carbamazepine, and phenobarbital); GBM, glioblastoma multiforme; KPS, Karnofsky performance status; NTR, near total resection; STR, subtotal resection; XRT, external beam radiotherapy.
Toxicity
All patients were evaluable for toxicity. A total of 166 courses of chemotherapy were administered, with an average of 2.2 courses per patient (range, 1–9 courses). Of note, most toxicities occurred with comparable frequency among patient subgroups, including those with either newly diagnosed or recurrent tumors, as well as those receiving or not receiving EIAC (Table 2). Grade 3 or greater thromboses and infections occurred in 9 (12%) and 10 (13%) patients, respectively. These rates are typical for this clinical population receiving chemotherapy in our experience. Four infections were fatal, including 2 episodes of sepsis, and single episodes of Pneumocystis carinii pneumonia (PCP) and Clostridium difficile enteritis. The latter 2 infections occurred during the first 1 to 2 weeks of treatment and were likely not related to the study regimen. Also of note, all fatal infections occurred in recurrent patients who were heavily pretreated and receiving prolonged corticosteroid therapy. The only other study-related death occurred in a patient with pneumonitis as described below.
Table 2.
Summary of grade 3 or greater toxicity
| A. Newly diagnosed patients | |||||||
|---|---|---|---|---|---|---|---|
| No EIAC (25 courses) | + EIAC (46 courses) | ||||||
| Toxicity | Grade 3 | Grade 4 | Grade 5 | Grade 3 | Grade 4 | Grade 5 | Total (71 courses) |
| Thrombocytopenia | — | 0 | 0 | — | 0 | 0 | 0 |
| Neutropenia | — | 5 (20%) | 0 | — | 1 (2%) | 0 | 6 (8%) |
| Fever/neutropenia | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 1 (1%) |
| Diarrhea/dehydration | 2 (8%) | 2 (8%) | 0 | 4 (9%) | 1 (2%) | 0 | 9 (13%) |
| Infection | 3 (12%) | 0 | 0 | 1 (2%) | 0 | 0 | 4 (6%) |
| Pulmonary | 0 | 2 (8%) | 1 (4%) | 0 | 0 | 0 | 3 (4%) |
| Thrombosis | 0 | 1 (4%) | 0 | 3 (7%) | 2 (4%) | 0 | 6 (8%) |
| Transaminase | 0 | 0 | 0 | 1 (2%) | 0 | 0 | 1 (1%) |
| Ileus | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 1 (1%) |
| B. Recurrent patients | |||||||
|---|---|---|---|---|---|---|---|
| No EIAC (35 courses) | + EIAC (60 courses) | ||||||
| Toxicity | Grade 3 | Grade 4 | Grade 5 | Grade 3 | Grade 4 | Grade 5 | Total (95 courses) |
| Thrombocytopenia | — | 1 (3%) | 0 | 0 | 0 | 0 | 1 (1%) |
| Neutropenia | — | 4 (11%) | 0 | 0 | 3 (5%) | 0 | 7 (7%) |
| Fever/neutropenia | 0 | 0 | 0 | 0 | 1 (2%) | 0 | 1 (1%) |
| Diarrhea/dehydration | 3 (9%) | 0 | 0 | 4 (7%) | 0 | 0 | 7 (7%) |
| Infection | 0 | 0 | 3 (9%) | 2 (3%) | 1 (2%) | 6 (6%) | |
| Pulmonary | 0 | 0 | 0 | 0 | 1 (2%) | 0 | 1 (1%) |
| Thrombosis | 0 | 1 (3%) | 0 | 1 (2%) | 1 (2%) | 0 | 3 (3%) |
Abbreviation: EIAC = enzyme-inducing anticonvulsants (phenytoin, carbamazepine, phenobarbital).
Hematologic Toxicity
Grade 4 neutropenia occurred in 7% of administered courses. Two episodes had accompanying fever; 1 of these patients had pneumonia, while no documented infection was identified in the other. Both episodes resolved with broad-spectrum antibiotics. Of note, only 1 patient developed grade 4 thrombocytopenia.
Gastrointestinal Toxicity
As expected, diarrhea was the most common grade 3 or greater nonhematologic toxicity and occurred with 10% of courses (16 of 166 total courses). Thirteen of the 16 episodes (81%) were grade 3 and responded to aggressive loperamide therapy or CPT-11 dose modification in most cases. However, 3 patients developed grade 4 diarrhea and were taken off study according to protocol guidelines.
Pulmonary Toxicity
Three patients developed grade 4 interstitial pneumonitis characterized by a significant decline in baseline DLCO and negative workup for thrombotic or infectious etiologies. In each case, pneumonitis developed after only 2 cycles of chemotherapy and prompted study discontinuation. Two patients were nonsmokers, while the third had a 15 pack-year cigarette history and borderline DLCO at study entry. One patient recovered spontaneously, and the other 2 responded to corticosteroids. A fourth patient, however, developed fever, shortness of breath, and hypoxia after the second cycle of chemotherapy, requiring intubation and mechanical ventilation. No pulmonary arterial emboli were detected, and a bronchioalveolar lavage, performed after initiating empiric, broad-spectrum antibiotics with PCP coverage, was negative for viral, fungal, bacterial, or acid-fast organisms and PCP. Although the patient initially improved, subsequent pulmonary failure developed, and findings were consistent with adult respiratory distress syndrome, leading to death approximately 2.5 weeks from symptom onset. All patients with grade ⩾ 4 pulmonary toxicity on this study had received no prior chemotherapy.
Efficacy
Newly Diagnosed Patients
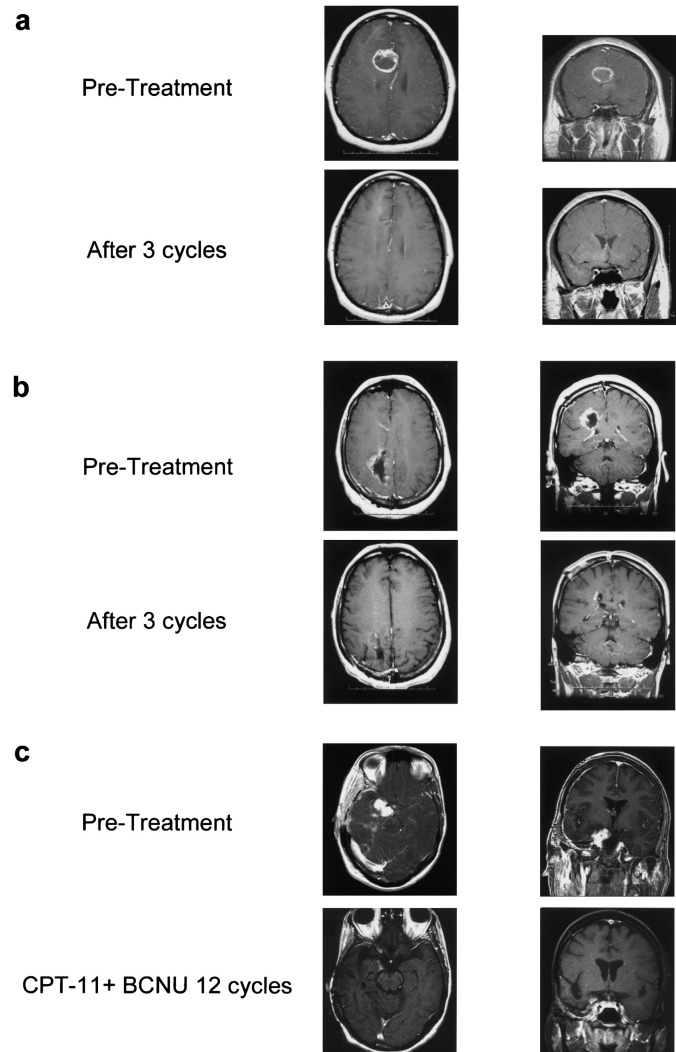
Radiographic response while on study was used to assess efficacy in newly diagnosed patients since the study design mandated that these patients come off study to initiate external beam radiotherapy after no more than 3 cycles of therapy. A confirmed objective response (CR or PR) was observed in 5 patients with newly diagnosed tumors (Table 3A), resulting in an intent-to-treat (ITT) response rate of 14% (95% CI, 5%–29%), including 1 patient with a CR and 4 who achieved a PR (Fig. 1). Sixteen (43%) patients achieved SD, including 2 with MR. Sixteen (43%) had PD, including 1 patient who came off study prior to completion of cycle 1 because of toxicity. Table 4 lists response rate by histology. With a median follow-up of 68.3 weeks, the median OS was 51.3 weeks (95% CI, 32.1–62.6 weeks) for all newly diagnosed patients, including a median 39.8 weeks (95% CI, 29.6–58.6 weeks) for those with GBM. The median OS for newly diagnosed AA/AO patients had not been reached at the time of manuscript preparation. The 1-year survival probability for patients with newly diagnosed GBM was 37% (95% CI, 22%–44%).
Table 3.
Summary of overall response data
| A. Newly diagnosed patients | |||
|---|---|---|---|
| Response | No EIAC n = 15 | + EIAC n = 22 | Total n = 37 |
| Complete (CR) | 0 | 1 (5%) | 1 (3%) |
| Partial (PR) | 2 (13%) | 2 (9%) | 4 (11%) |
| Stable disease | 6 (40%) | 10 (45%) | 16* (43%) |
| CR + PR + MR | 3 (20%) | 4 (18%) | 7 (19%) |
| Progression** | 7 (47%) | 9 (41%) | 16 (43%) |
| B. Recurrent patients | |||||||
|---|---|---|---|---|---|---|---|
| Response | No EIAC n=15 | + EIAC n=24 | Total n=39 | ||||
| Complete (CR) | 1 (7%) | 0 | 1 (3%) | ||||
| Partial (PR) | 1 (7%) | 3 (13%) | 4 (10%) | ||||
| Stable disease | 5 (33%) | 12 (50%) | 17# (44%) | ||||
| CR + PR + MR | 3 (20%) | 3 (13%) | 6 (15%) | ||||
| Progression§ | 8 (53%) | 9 (38%) | 17 (44%) | ||||
Abbreviations: EIAC, enzyme-inducing anticonvulsants (phenytoin, carbamazepine; phenobarbital); MR, minor response.
Includes 2 patients with minor responses.
One patient did not complete cycle 1 because of toxicity and was considered progressive for the intent-to-treat response analysis.
Includes 1 patient with minor responses.
Three patients did not complete cycle 1 because of toxicity and were considered progressive for the intent-to-treat response analysis.
Fig. 1.
T-1 weighted MRIs of representative responses to BCNU plus CPT-11. a. A patient with newly diagnosed glioblastoma multiforme (GBM) who achieved a complete response. b. A patient with newly diagnosed anaplastic astrocytoma who achieved a partial response. c. A patient with recurrent GBM who achieved a partial response.
Table 4.
Tumor response data by histologic diagnosis
| Tumor Histology | Disease Status (n) | Response (CR + PR) | Stable Disease* | Progressive Disease |
|---|---|---|---|---|
| GBM | Newly diagnosed (28) | 3 (11%) | 11 (39%) | 14 (50%) |
| Recurrent (28) | 3 (11%) | 13 (47%) | 12 (43%) | |
| AA/AO | Newly diagnosed (9) | 2 (22%) | 5 (56%) | 2 (22%) |
| Recurrent (11) | 2 (18%) | 5 (45%) | 4 (36%) |
Abbreviations: AA, anaplastic astrocytoma; AO, anaplastic oligodendroglioma; CR, complete response; GBM, glioblastoma multiforme; PR, partial response.
Includes minor responses.
Recurrent Patients
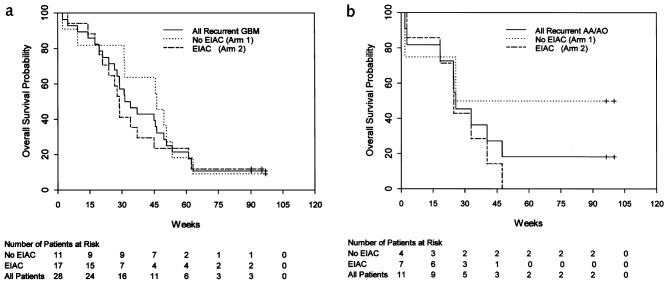
Confirmed objective responses were observed in 5 patients with recurrent tumors (Table 3B), resulting in an ITT response rate of 13% (95% CI, 4%–27%), including 1 patient with a CR and 4 patients who achieved a PR (Fig. 1). All patients who responded did so within 3 cycles of therapy. Among responding patients, 1 patient who achieved a PR developed PD and died 2 months later because of fulminant recurrent disease. The remaining responses were durable. Two responders subsequently developed PD 14 and 29 months after study enrollment, but remain alive with active disease 28 and 30 months from study enrollment. Two responders remain alive without active disease 31 and 32 months from study enrollment. Seventeen (44%) patients achieved SD, including 1 patient with an MR. Seventeen (44%) had PD, including 3 patients who came off study prior to completing 1 cycle of therapy because of toxicity. There was no difference in response rate between the ITT population and those who completed at least the first cycle of therapy. Response duration ranged from 12 to 108 weeks. Table 4 lists response rate by histology. There was no correlation between radiographic response and either the number of prior recurrences or prior chemotherapy agents (data not shown). The median TTP for all recurrent patients was 11.4 weeks (95% CI, 6.0–14.3 weeks). Patients with recurrent AA/AO tumors had a median TTP of 16.9 weeks (95% CI, 4.9–26.4 weeks) compared to 11.3 weeks (95% CI, 6.0 –12.1 weeks) for patients with recurrent GBM (Fig. 2). Median OS for all recurrent patients was 31.3 weeks (95% CI, 25.7– 45.6 weeks), including 32.6 weeks (95% CI, 27.7–46.3 weeks) for those with GBM and 25.7 weeks (95% CI, 18.3–47.4 weeks) for those with recurrent AA/AO tumors (Fig. 3). The unexpectedly low median OS among patients with recurrent AA/AO reflects the impact of 2 deaths early during cycle 1 in this relatively small patient subset. The 1-year survival probability for patients with recurrent GBM was 21% (95% CI, 11%–44%). Median TTP and OS did not differ significantly on the basis of EIAC use (data not shown).
Fig. 2.
Kaplan-Meier estimate of time to progression stratified by use of enzyme-inducing anticonvulsant (EIAC) for patients with (a) recurrent glioblastoma multiforme (GBM) and (b) recurrent anaplastic astrocytoma or anaplastic oligodendroglioma (AA/AO)
Fig. 3.
Kaplan-Meier estimate of overall survival stratified by use of enzyme-inducing anticonvulsant (EIAC) for patients with (a) recurrent glioblastoma multiforme (GBM) and (b) recurrent anaplastic astrocytoma or anaplastic oligodendroglioma (AA/AO)
Discussion
The rationale for combining BCNU with CPT-11 in the current study is based on several key observations. First, both agents have independent activity against MG. Several clinical trials demonstrate a modest increase in progression-free survival for patients with MG treated with nitrosourea-based chemotherapy (Fine et al., 1993; Levin et al., 1985, 1990; Walker et al., 1978), and nitrosourea-containing regimens have been shown to achieve radiographic responses in the neoadjuvant setting (Grossman et al., 1997, 2003; Rajkumar et al., 1999). The activity of CPT-11 was established in a recent phase 2 study. In that study, monotherapy with CPT-11 resulted in an overall ITT response rate of 15%, and an additional 55% of patients achieved SD for more than 2 courses (Friedman et al., 1999). Second, BCNU and CPT-11 target different but interdependent components of the cell cycle. Nitrosoureas alkylate DNA, forming adducts that induce intrastrand cross-links, whereas CPT-11 inhibits topoisomerase I. Preclinical in vivo studies with MG xenografts confirmed that BCNU plus CPT-11 exhibits schedule-dependent, synergistic antitumor activity, suggesting that BCNU-induced adducts or cross-links prior to CPT-11 administration are critical for the enhanced activity of this regimen (Castellino et al., 2000; Coggins et al., 1998). Additional preclinical studies have begun to elucidate the mechanisms by which alkylators potentiate the activity of topoisomerase I inhibitors (Pourquier et al., 2001). Third, the primary dose-limiting toxicity (DLT) attributes of both agents are well characterized and nonoverlapping. Cumulative myelosuppression is dose limiting for nitrosoureas, whereas CPT-11 is associated with dose-limiting gastrointestinal toxicity.
In a previous phase 1 study, we established the MTD of CPT-11 administered weekly for the first 4 weeks of each 6-week cycle to be 225 mg/m2 for patients on EIAC and 125 mg/m2 for those not on EIAC when combined with 100 mg/m2 of BCNU (Quinn et al., 2004). Dose-limiting toxicity was primarily hematologic and gastrointestinal, although one episode of pulmonary DLT also occurred. Despite the dose escalation design, 5 patients (7%) achieved a PR, while 38 patients (52%) achieved SD, including 8 patients (11%) who completed 6 or more cycles.
In the current study, the overall frequency and severity of hematologic, gastrointestinal, infectious, and thrombotic toxicity were comparable to those observed with single-agent BCNU or CPT-11 therapy (Friedman et al., 1999; Levin et al., 1985, 1990; Stewart et al., 2002; Walker et al., 1978). Eleven patients (14%) came off study because of toxicity, including grade 4 or greater infectious (n = 4), pulmonary (n = 4), and gastrointestinal (n = 3) events. Two of the infectious events occurred in patients following only 1 or 2 CPT-11 doses of cycle 1 and therefore were likely not related to the study regimen. The other events that led to study discontinuation were likely attributable to the study regimen. Although nitrosoureas are associated with interstitial pneumonitis (Aronin et al., 1980), we were concerned about the frequency and severity of pulmonary toxicity noted on the current study, as well as its unexpected development early in the course of therapy. Three patients (4%) developed reversible grade 4 interstitial pneumonitis after only 2 cycles of therapy. A fourth patient developed fatal pneumonitis after only 2 cycles of therapy, although the precise etiology was unclear. One patient treated in our prior phase 1 study developed a grade 3 decline in DLCO after only 2 cycles of therapy, although the patient remained asymptomatic (Quinn et al., 2004). The frequency, acuteness, and early onset of pneumonitis observed among patients on our phase 1 and 2 studies with BCNU plus CPT-11 suggest that this combination may be associated with a heightened risk of pulmonary toxicity.
In the current phase 2 study, BCNU plus CPT-11 was associated with clear antitumor activity as measured by radiographic response. As expected, the rate of response was better among patients with AA/AO (WHO grade III) tumors than among those with recurrent GBM; however, the true activity of BCNU plus CPT-11 in this subset of patients remains to be determined because of the small number enrolled in this study. Among patients with GBM, 2 achieved a CR, while 8 achieved a PR, which resulted in an ITT response rate of 13%. An additional 33 patients (43%) achieved SD, including 3 with MR. The rate of response observed on the current study was comparable to that observed on our prior phase 2 study in which patients with recurrent MG who had failed no more than 1 prior chemotherapy regimen were treated with CPT-11 alone (Friedman et al., 1999). In fairness, patients enrolled on the current study were more heavily pretreated, with more than 40% having received more than 2 prior chemotherapy agents.
Because of concern that radiographic response may not correlate with a benefit in overall outcome (Grossman et al., 2003), we also evaluated TTP as a secondary end point among patients with recurrent tumors treated on our study. TTP observed on the current study was comparable to that reported previously with other salvage regimens (Wong et al., 1999). There are several possible explanations for the disappointing activity of this regimen noted in our study. First, it is possible that we underestimated TTP because patients without measurable tumor were not eligible for participation in this trial. Second, it is possible that the MTD established in our prior phase 1 study did not yield sufficient tumor exposures to drug for synergistic activity (Quinn et al., 2004). Third, as pharmacokinetic studies were not performed in our study, it is also possible that detrimental drug metabolism or metabolite interactions may have occurred. Fourth, the results achieved in our preclinical in vivo xenograft studies with BCNU and CPT-11 using established MG cell lines may not reflect the true activity of this combination in the primary tumor setting. Although preclinical in vivo studies are the best available means of assessing activity prior to actual clinical trials and therefore remain a critical step in cancer drug development, inherent differences between primary tumors and cell lines, as well as between patients and animal models, often limit the applicability of preclinical results to the clinical arena. Finally, because all recurrent and/or progressive patient categories were combined regardless of histologic grade, it is possible that the study was underpowered to detect a statistically significant improvement in outcome for either recurrent grade IV or III subpopulations.
In conclusion, the results of this phase 2 trial indicate that BCNU plus CPT-11, via a 6-week dosing schedule as described, is as active as CPT-11 alone for patients with MG and may be associated with increased pulmonary toxicity. As an alternative to BCNU plus CPT-11, we have initiated a phase 1 clinical trial of CPT-11 combined with temozolomide, an imidazotetrazine-derived alkylating agent with significant antiglioma activity (Friedman et al., 2000), based on highly encouraging preclinical data demonstrating synergistic antitumor activity of this combination against MG (Patel et al., 2000).
Footnotes
This work was supported by NIH Grants NS20023 and CA11898; NIH Grant MO1 RR 30, GCRC Program, NCRR; and NCI SPORE 1 P20 CA096890.
Abbreviations used are as follows: AA, anaplastic astrocytoma; AO, anaplastic oligodendroglioma; BCNU, 1,3-bis(2-chloroethyl)-1-nitrosourea (carmustine); CR, complete response; DLCO, diffusing capacity of the lung for carbon monoxide; DLT, dose-limiting toxicity; EIAC, enzyme-inducing anticonvulsant; GBM, glioblastoma multiforme; ITT, intent to treat; MG, malignant glioma; MTD, maximum tolerated dose; MR, minor response; OS, overall survival; PCV, procarbazine, CCNU [lomustine], and vincristine; PCP, Pneumocystis carinii pneumonia; PD, progressive disease; PR, partial response; SD, stable disease; TTP, time to progression.
References
- Aronin PA, Mahaley MS, Jr, Rudnick SA, Dudka L, Donohue JF, Selker RG, Moore P. Prediction of BCNU pulmonary toxicity in patients with malignant gliomas: An assessment of risk factors. N Engl J Med. 1980;303:183–188. doi: 10.1056/NEJM198007243030403. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- Cairncross JG, Ueki K, Zlatescu MC, Lisle DK, Finelstein DM, Hammond RR, Silver JS, Stark PC, Macdonald DR, Ino Y, Ramsay DA, Louis DN. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90:1473–1479. doi: 10.1093/jnci/90.19.1473. [DOI] [PubMed] [Google Scholar]
- Castellino RC, Elion GB, Keir ST, Houghton PJ, Johnson SP, Bigner DD, Friedman HS. Schedule-dependent activity of irinotecan plus BCNU against malignant glioma xenografts [erratum in Cancer Chemother. Pharmacol. 46, 84, 2000] Cancer Chemother Pharmacol. 2000;45:345–349. doi: 10.1007/s002800050050. [DOI] [PubMed] [Google Scholar]
- Chang CH, Horton J, Schoenfeld D, Salazer O, Perez-Tamayo R, Kramer S, Weinstein A, Nelson JS, Tsukada Y. Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. A joint Radiation Therapy Oncology Group and Eastern Cooperative Oncology Group study Cancer. 1983;52:997–1007. doi: 10.1002/1097-0142(19830915)52:6<997::aid-cncr2820520612>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Coggins CA, Elion GB, Houghton PJ, Hare CB, Keir S, Colvin OM, Bigner DD, Friedman HS. Enhancement of irinotecan (CPT-11) activity against central nervous system tumor xenografts by alkylating agents. Cancer Chemother Pharmacol. 1998;41:485–490. doi: 10.1007/s002800050771. [DOI] [PubMed] [Google Scholar]
- Deutsch M, Green SB, Strike TA, Burger PC, Robertson JT, Selker RG, Shapiro WR, Mealey J, Jr, Ransohoff J, 2nd and, Paoletti P. Results of a randomized trial comparing BCNU plus radiotherapy, streptozotocin plus radiotherapy, BCNU plus hyperfractionated radiotherapy, and BCNU following misonidazole plus radiotherapy in the postoperative treatment of malignant glioma. Int J Radiat Oncol Biol Phys. 1989;16:1389–1396. doi: 10.1016/0360-3016(89)90939-5. [DOI] [PubMed] [Google Scholar]
- Fine HA, Dear KB, Loeffler JS, Black PM, Canellos GP. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71:2585–2597. doi: 10.1002/1097-0142(19930415)71:8<2585::aid-cncr2820710825>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Friedman HS, Petros WP, Friedman A.H, Schaaf LJ, Kerby T, Lawyer J, Parry M, Houghton PJ, Lovell S, Rasheed K, Cloughesy T, Stewart ES, Colvin OM, Provenzale JM, McLendon RE, Bigner DD, Cokgor I, Haglund M, Rich J, Ashley D, Malczyn J, Elfring GL, Miller LL. Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol. 1999;17:1516–1525. doi: 10.1200/JCO.1999.17.5.1516. [DOI] [PubMed] [Google Scholar]
- Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000;6:2585–2597. [PubMed] [Google Scholar]
- Green SB, Byar DP, Walker MD, Pistenmaa DA, Alexander E, Jr, Batzdorf U, Brooks WH, Hunt WE, Mealey J, Jr, Odom GL, Paoletti P, Ransohoff J, 2nd, Robertson JT, Selker RG, Shapiro WR, Smith KR, Jr, Wilson CB, Strike TA. Comparisons of carmustine, procarbazine, and high-dose methylprednisolone as additions to surgery and radiotherapy for the treatment of malignant glioma. Cancer Treat Rep. 1983;67:121–132. [PubMed] [Google Scholar]
- Grossman SA, O’Neill A, Grunnet M, Pearlman JL, Wagner H, Gilbert M, Newton HB, Hellman R, Eastern Cooperative Oncology Group. Phase III study comparing three cycles of infusional carmustine and cisplatin followed by radiation therapy with radiation therapy and concurrent carmustine in patients with newly diagnosed supratentorial glioblastoma multiforme: Eastern Cooperative Oncology Group Trial 2394. J. Clin. Oncol. 2003;21:1485–1491. doi: 10.1200/JCO.2003.10.035. [DOI] [PubMed] [Google Scholar]
- Grossman SA, Wharam M, Sheidler V, Kleinberg L, Zeltzman M, Yue N, Piantadosi S. Phase II study of continuous infusion carmustine and cisplatin followed by cranial irradiation in adults with newly diagnosed high-grade astrocytoma. J Clin Oncol. 1997;15:2596–2603. doi: 10.1200/JCO.1997.15.7.2596. [DOI] [PubMed] [Google Scholar]
- Hare CB, Elion GB, Houghton PJ, Houghton JA, Keir S, Marcelli SL, Bigner DD, Friedman HS. Therapeutic efficacy of the topoisomerase I inhibitor 7-ethyl-10-(4-[1-piperidino]-1-piperidino)-carbonyloxy-camptothecin against pediatric and adult central nervous system tumor xenografts. Cancer Chemother Pharmacol. 1997;39:187–191. doi: 10.1007/s002800050558. [DOI] [PubMed] [Google Scholar]
- Houghton PJ, Cheshire PJ, Hallman JD, 2nd, Lutz L, Friedman HS, Danks MK, Houghton JA. Efficacy of topoisomerase I inhibitors, topotecan and irinotecan, administered at low dose levels in protracted schedules to mice bearing xenografts of human tumors. Cancer Chemother Pharmacol. 1995;36:393–403. doi: 10.1007/BF00686188. [DOI] [PubMed] [Google Scholar]
- Ino Y, Betensky RA, Zlatescu MC, Sasaki H, Macdonald DR, Stemmer-Rachmanimov AO, Ramsay DA, Cairncross JG, Louis DN. Molecular subtypes of anaplastic oligodendroglioma: Implications for patient management at diagnosis. Clin Cancer Res. 2001;7:839–845. [PubMed] [Google Scholar]
- Klein JP. Small sample moments of some estimators of the variance of the Kaplan-Meier and Nelson-Aalen estimators. Scand J Stat. 1991;18:333–340. [Google Scholar]
- Kristof RA, Neuloh G, Hans V, Deckert M, Urbach H, Schlegel U, Simon M, Schramm J. Combined surgery, radiation, and PCV chemotherapy for astrocytomas compared to oligodendrogliomas and oligoastrocytomas WHO grade III. J Neurooncol. 2002;59:231–237. doi: 10.1023/a:1019987116596. [DOI] [PubMed] [Google Scholar]
- Levin VA, Wara WM, Davis RL, Vestnys P, Resser KJ, Yatsko K, Nutik S, Gutin PH, Wilson CB. Phase III comparison of BCNU and the combination of procarbazine, CCNU, and vincristine administered after radiotherapy with hydroxyurea for malignant gliomas. J Neurosurg. 1985;63:218–223. doi: 10.3171/jns.1985.63.2.0218. [DOI] [PubMed] [Google Scholar]
- Levin VA, Silver P, Hannigan J, Wara WM, Gutin PH, Davis RL, Wilson CB. Superiority of post-radiotherapy adjuvant chemotherapy with CCNU, procarbazine, and vincristine (PCV) over BCNU for anaplastic gliomas: NCOG 6G61 final report. Int J Radiat Oncol Biol Phys. 1990;18:321–324. doi: 10.1016/0360-3016(90)90096-3. [DOI] [PubMed] [Google Scholar]
- Medical Research Council Brain Tumor Working Party. Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: A Medical Research Council trial. J Clin Oncol. 2001;19:509–518. doi: 10.1200/JCO.2001.19.2.509. [DOI] [PubMed] [Google Scholar]
- NCI. National Cancer Institute, Cancer Therapy Evaluation Program. Common Toxicity Criteria—Version 2.0, published April 30, 1999. Cited January 5, 2004. Available at https://webapps.ctep.nci.nih.gov/ctcv2/plsql/ctc000w$.startup.
- Patel VJ, Elion GB, Houghton PJ, Keir S, Pegg AE, Johnson SP, Dolan ME, Bigner DD, Friedman HS. Schedule-dependent activity of temozolomide plus CPT-11 against a human central nervous system tumor-derived xenograft. Clin Cancer Res. 2000;6:4154–4157. [PubMed] [Google Scholar]
- Pourquier P, Waltman JL, Urasaki Y, Loktionova NA, Pegg AE, Nitiss JL, Pommier Y. Topoisomerase I-mediated cytotoxicity of N-methyl-N′-nitro-N′-nitrosoguanidine: Trapping of topoisomerase I by the O6-methylguanine. Cancer Res. 2001;61:53–58. [PubMed] [Google Scholar]
- Quinn JA, Reardon DA, Friedman AH, Rich JN, Sampson JH, Vredenburgh J, Gururangan S, Provenzale JM, Walker A, Schweitzer H, Bigner DD, Tourt-Uhlig S, Herndon JE, II, Affronti ML, Jackson S, Allen D, Zeigler K, Bohlin C, Lentz C, Friedman HS. Phase 1 trial of irinotecan plus BCNU in patients with progressive or recurrent malignant glioma. Neurooncol. 2004;6:145–153. doi: 10.1215/S1152851703000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar SV, Buckner JC, Schomberg PJ, Pitot HC, 4th, Ingle JN, Cascino TL. Phase I evaluation of preirradiation chemotherapy with carmustine and cisplatin and accelerated radiation therapy in patients with high-grade gliomas. Neurosurgery. 1999;44:67–73. doi: 10.1097/00006123-199901000-00036. [DOI] [PubMed] [Google Scholar]
- Shapiro WR, Green SB, Burger PC, Mahaley MS, Jr, Selker RG, VanGilder JC, Roberston JT, Ransohoff J, Mealey J, Jr, Strike TA, Pistenmaa DA. Randomized trial of three chemotherapy regimens and two radiotherapy regimens in postoperative treatment of malignant glioma. Brain Tumor Cooperative Trial 8001. J Neurosurg. 1989;71:1–9. doi: 10.3171/jns.1989.71.1.0001. [DOI] [PubMed] [Google Scholar]
- Slichenmyer WJ, Rowinsky EK, Grochow LB, Kaufmann SH, Donehower RC. Camptothecin analogues: Studies from the Johns Hopkins Oncology Center. Cancer Chemother Pharmacol. 1994;34:S53–S57. doi: 10.1007/BF00684864. (suppl.) [DOI] [PubMed] [Google Scholar]
- Smith JS, Perry A, Borell TJ, Lee HK, O’Fallon J, Hosek SM, Kimmel D, Yates A, Burger PC, Scheithauer BW, Jenkins RB. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol. 2000;18:636–645. doi: 10.1200/JCO.2000.18.3.636. [DOI] [PubMed] [Google Scholar]
- Stenning SP, Freedman LS, Bleehen NM. An overview of published results from randomized studies of nitrosoureas in primary high grade malignant glioma. Br J Cancer. 1987;56:89–90. doi: 10.1038/bjc.1987.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart LA, Glioma Meta-analysis Trialists (GMT) Group. Chemotherapy in adult high-grade glioma: A systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- Stupp R, Dietrich P.-Y, Ostermann Kraljevic S, Pica A, Maillard I, Maeder P, Meuli R, Janzer R, Pizzolato G, Miralbell R, Porchet F, Regli L, de Tribolet N, Mirimanoff RO, Leyvraz S. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002;20:1375–1382. doi: 10.1200/JCO.2002.20.5.1375. [DOI] [PubMed] [Google Scholar]
- Walker MD, Alexander E, Jr, Hunt WE, MacCarty CS, Mahaley MS, Jr, Mealey J, Jr, Norrell HA, Owens G, Ransohoff J, Wilson CB, Gehan EA, Strike TA. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49:333–343. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritisis AP, Prados MD, Levin VA, Yung WK. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]