Abstract
TALL-104 is a human leukemic T cell line that expresses markers characteristic of both cytotoxic T lymphocytes and natural killer cells. TALL-104 cells are potent tumor killers, and the use of lethally irradiated TALL-104 as cellular therapy for a variety of tumors has been explored. We investigated the interactions of TALL-104 cells with human brain tumor cells. TALL-104 cells mediated increased lysis of a panel of brain tumor cells at low effector-to-target ratios over time. We obtained evidence that TALL-104 cells injured glioma cells by both apoptotic and necrotic pathways. A 7-amino actinomycin D flow cytometry assay revealed that the percentages of both apoptotic and necrotic glioma cells increased after TALL-104 cell/glioma cell coincubations. Fluorescent microscopy studies and a quantitative morphologic assay confirmed that TALL-104 cell/glioma cell interactions resulted in tumor cell apoptosis. Cytokines are secreted when TALL-104 cells are coincubated with brain tumor cells; however, morphologic analysis assays revealed that the soluble factors contained within clarified supernates obtained from 4 h coincubates added back to brain tumor cell cultures did not trigger the glioma apoptosis. TALL-104 cells do not express Fas ligand, even upon coincubation with glioma targets, which suggests that the Fas/Fas ligand apoptotic pathway is not likely responsible for the cell injury observed. We obtained evidence that cell injury is calcium dependent and that lytic granule exocytosis is triggered by contact of TALL-104 cells with human glioma cells, suggesting that this pathway mediates glioma cell apoptosis and necrosis.
Conventional therapy for primary malignant brain tumors consists of surgical debulking followed by chemotherapy and radiation (Laws, 1998; Prados et al., 1998). Unfortunately, WHO grade III and IV astrocytomas in particular do not respond well to these therapies. Immune therapy approaches have been actively investigated for brain tumor treatment because of their potential for selective destruction of malignant cells while leaving normal cells unharmed (Paul and Kruse, 2001; Virasch and Kruse, 2001).
TALL-104 cells were derived from a 3-year-old patient with acute lymphocytic leukemia (Cesano and Santoli, 1992; O’Connor et al., 1991). They are an interleukin 2 (IL-2)3-dependent leukemic T cell line that has surface markers typical of those found on both cytotoxic T lymphocyte (CTL) and natural killer (NK) cells (cluster of differentiation 3+ [CD3+]/T-cell receptor α and β+ chains, CD8+, CD56+). The TALL-104 cells lyse tumor cells in a non-major histocompatibility complex (MHC)-restricted fashion and do not lyse normal cells. In preclinical studies, lethally irradiated TALL-104 cells have been used to treat subcutaneously implanted U87-MG human glioma in mice with severe combined immunodeficiency (Cesano et al., 1995), and the antitumor activity of TALL-104 cells has been investigated in a brain tumor xenograft model (Geoerger et al., 2000). Work performed in our laboratory with a variety of primary brain tumor types supports the idea that localized administration of lethally irradiated TALL-104 cells may be useful as a cellular therapy for glioma patients (Kruse et al., 2000). TALL-104 cells lysed pediatric and adult brain tumor cells, were innocuous to normal brain cells, and trafficked through brain parenchyma, which indicates that they may have the ability to reach pockets of infiltrating tumor cells. Irradiation did not adversely affect TALL-104 cell mediated brain tumor cell lysis in vitro (Kruse et al., 2000), and lysis was observed even after IL-2 withdrawal. Thus, cellular therapy trials involving intracranial administrations of lethally irradiated TALL-104 cells could be performed without concomitant IL-2 treatment, which is expected to reduce toxicity (Kruse and Merchant, 1997; Kruse et al., 1997; Saris et al., 1989). Irradiated TALL-104 cells have been systemically administered as effector cells in other cellular therapy cancer trials. A phase 1 clinical trial for canines with advanced tumors given TALL-104 cellular therapy was shown to be nontoxic and, in some cases, able to induce the disappearance of tumor (Cesano et al., 1996a; Visonneau et al., 1997, 1999a, Visonneau et al., b). Subsequently, a phase 1 study of patients with refractory metastatic breast cancer demonstrated that TALL-104 cells were well tolerated (Visonneau et al., 2000). Additionally, one pediatric patient with an ependymoma received multiple, low doses of irradiated TALL-104 cells intracranially with no untoward effects (Freeman et al., 1999).
Despite their promise as an antitumor cellular therapy, the mechanism(s) by which TALL-104 cells specifically recognize and eliminate human brain tumor cells remains unknown. In other model systems, TALL-104 cells eradicate NK-resistant, NK-sensitive, and leukemic target cells through both apoptotic and necrotic pathways (Cesano and Santoli, 1992; Cesano et al., 1996b). In this report, we provide data to further elucidate TALL-104 cell interactions with brain tumor cells. We show that (1) necrotic and apoptotic cell death is induced in glioma cells exposed to TALL-104 cells, (2) soluble factors, such as the cytokines that are upregulated upon coincubation of TALL-104 cells with gliomas, do not induce apoptosis in glioma cells, (3) cytokine production is depressed in the presence of dexamethasone, (4) the Fas/Fas ligand (FasL) system is not likely involved in cell injury because TALL-104 cells do not express FasL, and (5) granule exocytosis likely mediates the lytic interaction between TALL-104 cells and gliomas.
Materials and Methods
Effector TALL-104 Cell Culture
TALL-104 cells were maintained in Iscove’s modified Dulbecco’s medium (IMDM; Life Technologies, Grand Island, N.Y.) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gemini Bioproducts, Woodland, Calif.) and recombinant human IL-2 (100 units/ml; Chiron Corp., Emeryville, Calif.). They were incubated at 37°C in 10% CO2 and humidified air. For some experiments the TALL-104 cells were gamma-irradiated (60Co, 4000 rads) before the cytotoxicity experiments were performed (Kruse et al., 2000).
Human Tumor Target Cell Culture
The human glioma cell lines or explants used in these experiments, DBTRG-05-MG, 04-11-MG, 10-08-MG, and 14-07-MG, were obtained according to the Colorado Multiple Institutional Review Board guidelines and practices (COMIRB protocol 01-778), established in culture, and characterized (Kleinschmidt-DeMasters et al., 1999; Kruse et al., 1998, 1992). Cultured ependymoma explants, 01-23-PBT and 13-02-PBT, were also placed into culture as described (Kruse et al., 2000). The cells were maintained in F12/Dulbecco’s modified essential medium (1:1 v/v, Life Technologies) containing 10% FBS in a humidified 5% CO2 incubator.
Cytotoxicity Assays
The 51Cr release assay was used to determine the lytic activity when TALL-104 cells were incubated with glioma tumor cells (Kruse et al., 1989). Briefly, 5 × 106 target cells suspended in 0.1 ml of their growth medium were labeled with 100 μCi of Na251 CrO4 (Amersham, Park Ridge, Ill.) for 90 min at 37°C. Cells were washed twice with Hank’s balanced salt solution (Life Technologies) and suspended in IMDM growth medium. In a final volume of 0.2 ml, 104 target cells were placed into 96-well, round-bottom microtitration plate wells that contained various concentrations of irradiated or nonirradiated TALL-104 effector cells. In some experiments, blockade of the granule-exocytosis pathway was achieved by adding 10 mM ethylene glycol-bis(2-aminoethyl) N,N,N′,N′-tetraacetic acid (EGTA) (Calbiochem, LaJolla, Calif.), a calcium chelator, to the assay medium (Lyubchenko et al., 2001; Zweifach, 2000). The plates were centrifuged at 200 × g for 5 min and incubated for 4 or 18 h at 37° C in a humidified 10% CO2 atmosphere. Following centrifugation at 200 × g for 10 min, 50% of the well volume was harvested and counted. Maximal release was produced by incubation of the targets with 2% Triton X-100 (Sigma-Aldrich, St. Louis, Mo.). Spontaneous release was the cpm of targets in assay medium alone. The percentage of specific release was calculated by the formula [(cpmexperimental − cpmspontaneous)/(cpmmaximal − cpmspontaneous)] × 100%. Values were reported as the mean specific release of triplicate wells, provided that the standard error did not exceed 10%. An analysis of variance was used to statistically compare the experimental groups (Winer, 1971).
Flow Cytometric Assay with 7-Amino Actinomycin D to Determine Cell Viability/Injury
Glioma cells (04-11-MG, 10-08-MG, and 14-07-MG) were plated into sterile 6-well plates for 24 h. The adherent glioma cells were labeled with 0.25 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) in phosphate-buffered saline (PBS) following the manufacturer instructions (Molecular Probes, Eugene, Oreg.). TALL-104 cells were then added to the wells at a 10:1 effector-to-target (E:T) ratio. Coincubation for 4 h at 10% CO2 in a 37°C humidified chamber was followed by collection of the cells in suspension and harvesting the adherent cells with 0.025% trypsin treatment. The adherent cells were combined with the nonadherent cells. After centrifuging, a 100-μl volume of a 20-μg/ml 7-amino actinomycin D (7AAD) in PBS solution was added to the cells in suspension. After staining of the cells for 20 min at 4°C, they were pelleted by centrifugation, resuspended in 500 μl of PBS, and analyzed by flow cytometry within 30 min at the University of Colorado Flow Cytometry Core facility. Scattergrams were generated by combining forward light scatter with 7AAD fluo-rescence of the CFSE-labeled glioma population. Regions were drawn around clear-cut populations having negative (live cells), bright (dead cells), and dim (apoptotic cells) fluorescence (Philpott et al., 1996; Schmid et al., 1994). After confirming that the segregation was appropriate with one of the gliomas, by confirming that ⩾75% of the cells were positive by fluoresceinated annexin-V staining for the early apoptotic cell marker phosphatidylserine (Lecoeur et al., 2002), the percentages of glioma cells within the segregated live, apoptotic, and necrotic populations were determined.
Fluorescent Microscopy Assay to Discern Apoptosis
Adherent glioma cells were labeled in a 37°C incubator for 30 min with CellTracker Orange (5 μM CMTMR, Molecular Probes, Inc.) in serum-free Dulbecco’s modified essential culture medium according to the manufacturer instructions. Pelleted, nonadherent TALL-104 cells were resuspended and similarly labeled with CellTracker Green (5 μM CMFDA, Molecular Probes) in serum-free IMDM culture medium. After labeling, the probe-containing medium was replaced by non-probe-containing medium for another 30 min, and the cells were then washed twice with PBS. The cells were then incubated overnight in serum-containing medium. The glioma cells were harvested with 0.125% trypsin, washed, and coincubated with TALL-104 cells at a 10:1 E:T ratio. Incubations proceeded for 0, 2, and 4 h. Hoechst 33342 dye (Sigma-Aldrich) was added to the cell mixture at a final concentration of 1 μg/ml for the last 10 min of the incubation period, except for the 0-h incubation, where cells were labeled prior to mixing. The mixed cells were pelleted, washed twice with PBS, fixed with 2% paraformaldehyde in PBS, and stored at 4°C overnight. They were examined on coverslipped slides by fluorescence microscopy (Olympus BH microscope equipped with epi-fluorescence accessories [Olympus America, Inc., Melville, N.Y.]). Appropriate filters (exciter filter UG-1, dichroic mirror U, and barrier filter L-420) were used to discern the cell type by its orange or green labeling, and the glioma cell numbers with condensed or fragmented nuclei were determined by Hoechst dye at each of the incubation times. The mean percentage of apoptotic cells observed ± standard error was determined from 4 separate experiments for each of 3 different malignant glioma cell lines exposed to TALL-104 cells.
Quantitative Cell Morphology Assay to Determine Apoptosis
Cell morphology studies were performed to discern apoptotic figures in hematoxylin and eosin (H&E)-stained cells after 1-, 2-, or 4-h coincubation of TALL-104 cells with glioma cells. The glioma cells (4 × 104) were plated onto sterile Lab-Tek 4-well glass chamber slides (Nalge Nunc International, Naperville, Ill.) for 48 h. Then medium was gently aspirated from the wells and replaced with medium containing TALL-104 cells (4 × 105) in suspension. The mixtures were incubated at 10% CO2 in a 37°C humidified chamber for 4 h. The medium was removed, the chambers were gently rinsed with Hank’s balanced salt solution (Life Technologies), and the cells were fixed in 10% phosphate-buffered formalin for 30 min before H&E staining. Glioma cell nuclear morphology was examined by light microscopy (Olympus BX40, Olympus America, Inc.). Cell counts (800 total) were segregated into groups identified as normal live, apoptotic, or mitotic. From 3 separate experiments, the percentages of each cell type present were calculated at different incubation periods for 3 different human gliomas.
Testing to Determine if Soluble Factors Mediate Apoptosis
To ascertain if a soluble factor(s) mediated the induction of apoptosis, conditioned medium from coincubated TALL-104 with tumor cells was clarified by centrifugation at 400 × g for 10 min and added back to glioma cell cultures. Induction of apoptosis was examined by obtaining cell type counts described for the morphologic analysis above at the end of a 4-h incubation. As in the above experiment, the clarified medium, or dilutions of it with fresh medium, were placed onto glioma cells that had been plated for 48 h.
Cytokine Secretion by Enzyme-Linked Immunosorbent Assay
Levels of interferon (IFN)-γ, tumor necrosis factor (TNF)-α, TNF-β, and granulocyte macrophage-colony stimulating factor (GM-CSF) were measured in clarified supernates obtained 18 h after coincubation of TALL-104 cells with brain tumor cells in a dexamethasone-containing (10−6 M) culture medium at an E:T ratio of 10:1. Cytokine-specific enzyme-linked immunosorbent assay kits (Endogen, Boston, Mass.) were used according to the manufacturer protocol. The sensitivities of the assays were 20 pg/ml for IFN-γ and TNF-α, 8 pg/ml for TNF-β, and 7.8 pg/ml for GM-CSF.
Flow Cytometric Phenotypic Analysis of FasL on TALL-104 Cells and of Fas on Glioma Cells
To determine if upregulation of FasL occurred on TALL-104 cells after a 16-h coincubation with glioma cells (E:T 1:1), glioma cells plated onto 12-well culture plates for 24 h were labeled with 0.25 μM CFSE so that they could be distinguished from TALL-104 cells by flow cytometry. Adherent glioma cells were harvested with a solution of 2 mM ethylene diamine tetraacetic acid in PBS. Glioma cells or nonadherent TALL-104 cells were resuspended after washing and centrifuging in flow cytometry wash buffer (PBS, 1% FBS, 0.2% sodium azide). The cells were incubated with purified primary antibodies at 4°C in the dark for 15 min with CD95 (Fas on gliomas) or CD95L (FasL on TALL-104 cells) (BD Biosciences Pharmingen, San Diego, Calif.). The cells were rinsed and washed in flow cytometry wash buffer. Goat anti-mouse IgG phycoerythrin-conjugated antibodies (Beckman Coulter, Miami, Fla.) were added to the cell suspensions next, and they incubated for 15 min prior to fixation. Samples were then delivered to the University of Colorado Cancer Center Flow Cytometry Core for analysis. After gating the cells stained with isotype control antibody, the percentage of cells positive for the primary antibodies and their relative antigen densities (expressed as mean fluorescence intensities [MFIs]) were determined.
Assay to Monitor for Glioma Cell Injury by Granule Exocytosis Induced by TALL-104 Effector Cells
04-11-MG glioma cells were loaded with calcein by incubating them with 1 μM calcein-AM (Molecular Probes, Inc.) in cell culture medium for 15 min at room temperature. They were allowed to settle acutely onto poly-L-lysine-coated coverslips. The extracellular solution for imaging experiments (Ringer’s solution) contained the following, in millimoles per liter: 145 NaCl, 4.5 KCl, 1 MgCl2, 2 CaCl2, 5 HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), and 10 glucose (pH 7.4 with NaOH), and imaging experiments were performed at room temperature with an imaging system described previously (Zweifach, 2000). A Nikon 40 X oil-immersion objective (numerical aperture 1.4 [Nikon, Melville, N.Y.]) was used. Fluorescence data were analyzed off-line by using macros written in Igor Pro (Wavemetrics, Lake Oswego, Oreg.).
Results
Irradiated TALL-104 Cells Lyse Human Brain Tumor Cells at Low E:T Ratios
Table 1 presents the results of 4- and 18-h 51Cr-release assays started simultaneously to study the tumoricidal effects of TALL-104 cells against several glioma (10-08-MG, 14-07-MG) and ependymoma (01-23-PBT, 13-02-PBT) brain tumor cell explants. Low, clinically achievable E:T ratios were used. At the E:T ratios examined, statistically significant increases of brain tumor cell lysis at 18 h were observed by analysis of variance (P < 0.05). Although this particular assay does not distinguish between the lysis of brain tumor cells by necrotic or apoptotic pathways, these data do suggest that a continuum of glioma cell killing by TALL-104 cells occurs over this time frame.
Table 1.
TALL-104 cell cytolysis of human brain tumor cells increases over time at low E:T ratiosa
| Percent lysis | ||||||
|---|---|---|---|---|---|---|
| Tumor | Length of assay | Tumor type | 10:1 | 5:1 | 2.5:1 | 1.25:1 |
| 10-08-MG | 4 h | Glioblastoma | 26.3 | 26.0 | 18.0 | 14.4 |
| 10-08-MG | 18 h | 65.2 | 61.0 | 52.3 | 41.0 | |
| 14-07-MG | 4 h | Glioblastoma | 69.0 | 62.0 | 53.0 | 38.0 |
| 14-07-MG | 18 h | 97.3 | 99.0 | 94.0 | 74.0 | |
| 01-23-PBT | 4 h | Ependymoma | 63.0 | 55.0 | 49.0 | 40.0 |
| 01-23-PBT | 18 h | 81.0 | 81.0 | 78.0 | 78.0 | |
| 13-02-PBT | 4 h | Ependymoma | 53.0 | 44.0 | 33.0 | 37.0 |
| 13-02-PBT | 18 h | 77.0 | 68.0 | 75.0 | 79.0 | |
Abbreviations: MG, malignant gliomas; PBT, pediatric brain tumors.
Mean percent lysis obtained at 4 or 18 h in 51Cr-release assays by TALL-104 cells against various primary cultures of brain tumor cells are shown at various E:T ratios. The standard error of the means ranged between 0.4% and 3.6% for the 4-h assays and ranged between 1.1% and 10% for the 18-h assays. Statistically significant differences (P ≤ 0.05) were obtained by analysis of variance between the 4- and 18-h lytic values at the E:T ratios examined.
Evidence That Glioma Cell Injury by TALL-104 Cells Shows Features of Necrosis and Apoptosis
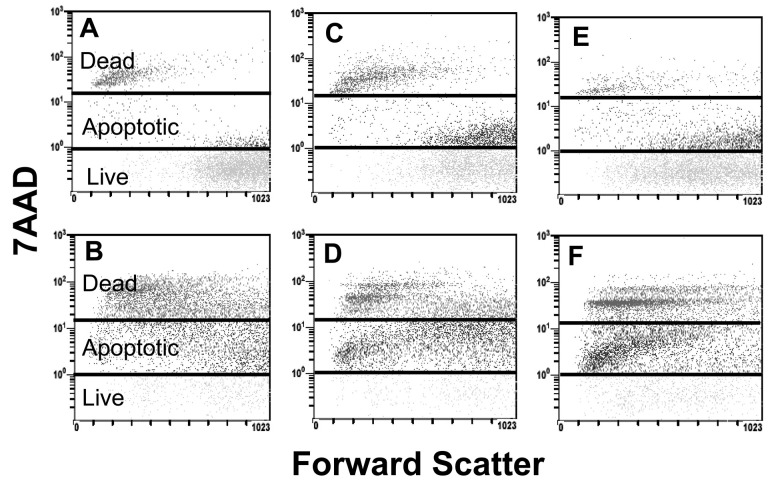
During cell injury the plasma membrane becomes increasingly permeable, and a fluorescent DNA dye, 7AAD, which selectively binds to guanosine/cytosine regions of the DNA, is taken up by the cells in proportion to the degree of injury (Schmid et al., 1994). The scattergrams in Fig. 1 that were obtained from CFSE-labeled 10-08-MG, 14-07-MG, and 04-11-MG glioma cells are shown without (Figs. 1A, 1C, 1E, respectively) or with (Figs. 1B, 1D, 1F, respectively) a 4-h coincubation with TALL-104 cells. It is apparent that cell injury of all 3 gliomas occurred after their coincubation with TALL-104 cells by upward shifts of cells into the segregated apoptotic and necrotic areas. The percentages of apoptotic and dead glioma cells that were detected by the 7AAD assay in CFSE-labeled glioma cells that were or were not coincubated with TALL-104 cells at a 10:1 E:T for 4 h are shown in Table 2. The percentages of dead cells obtained for each of the glioma cell populations correlate with those obtained by trypan blue dye exclusion. The percentages of apoptotic cells for the glioma cell populations are high; however, the assay detects cell injury at early stages, some injury of which may be reversible. In addition, this particular assay analyzes both adherent cells and cells in suspension. Therefore, the numbers of apoptotic cells would be higher than in assays that analyze only adherent cells. The percentages of both apoptotic and dead cell populations increase dramatically after glioma cell exposure to TALL-104 cells.
Fig. 1.
Detection of apoptotic and necrotic cells by the 7AAD assay. Images show carboxyfluorescein diacetate succinimidyl ester-labeled glioma cells that were and those that were not coincubated with TALL-104 cells at a 10:1 E:T for 4 h. A. 10-08-MG cells. B. 10-08-MG + TALL-104 cells. C. 14-07-MG cells. D. 14-07-MG + TALL-104 cells. E. 04-11-MG cells. F. 04-11-MG + TALL-104 cells.
Table 2.
Increases in apoptotic and necrotic glioma cells after coincubation with TALL-104 cells as determined by the 7AAD assay
| Cells in 7AAD assaya | % Live | % Apoptotic | % Dead |
|---|---|---|---|
| 04-11-MG | 56.7 | 37.0 | 6.2 |
| 04-11-MG + TALL-104 | 9.5 | 49.4 | 41.3 |
| 10-08-MG | 68.1 | 21.3 | 9.9 |
| 10-08-MG + TALL-104 | 7.5 | 49.3 | 42.7 |
| 14-07-MG | 45.4 | 40.5 | 13.5 |
| 14-07-MG + TALL-104 | 6.8 | 61.5 | 32.7 |
Abbreviation: 7AAD, 7-amino actinomycin D.
The malignant glioma cells were or were not coincubated with TALL-104 cells for 4 h at a 10:1 E:T ratio. All cells were labeled with 7AAD, and the glioma cells were also labeled with carboxyfluorescein diacetate succinimidyl ester so that the glioma cells could be gated for analysis by flow cytometry. The values are for the glioma cells after coincubation.
Fluorescent Microscopic Evidence for Apoptotic Glioma Cell Death
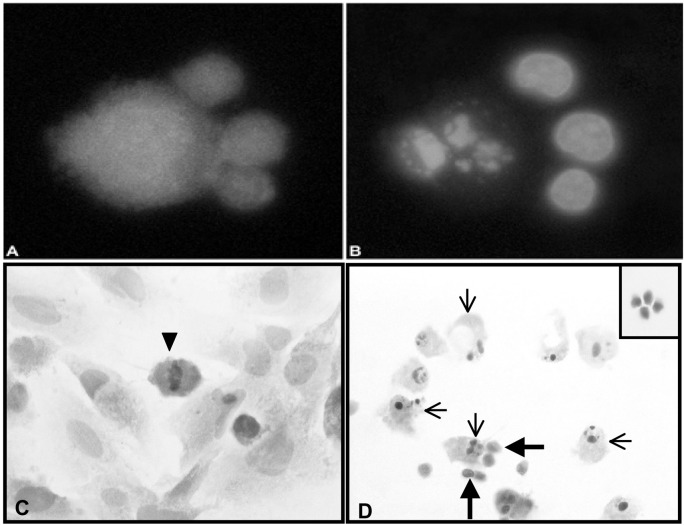
To further examine the possibility that TALL-104 cells injure glioma cells by apoptosis, a fluorescent microscopy technique was used to quantify glioma cells with condensed or fragmented nuclei after their coculture with TALL-104 cells. TALL-104 cells were labeled with Cell-Tracker Green probes, and glioma cells (04-11-MG, 10-08-MG, 14-07-MG) were labeled with CellTracker Orange probes. The nuclei of the cells were labeled with Hoechst 33342 dye. After incubation for 0, 2, and 4 h at an E:T ratio of 10:1, the mean percentages of apoptotic glioma cells were obtained from 4 separate experiments (Fig. 2). Apoptotic cell percentages observed at 0 h were considered baseline for each of the gliomas and ranged from 6% to 10%. Even at a low E:T ratio of 10:1, one of the gliomas, 04-11-MG, exhibited high percentages of apoptotic cells at 2 and 4 h (41%–48%) relative to the other two gliomas (10-08-MG, 14-07-MG), which exhibited more moderate amounts of apoptosis at those time points (17%–29%). Figure 3A demonstrates 3 green-labeled TALL-104 cells in contact with a larger orange-labeled glioma cell. Figure 3B is the respective Hoechst dye-labeled view of the same cells demonstrating the glioma cell with a fragmented nucleus.
Fig. 2.
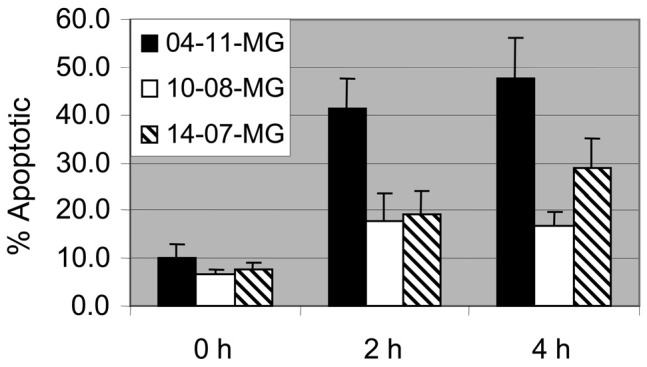
Detection of glioma cell apoptosis caused by TALL-104 cells. TALL-104 cells were coincubated with 04-11-MG, 10-08-MG, and 14-07-MG glioma cells for 0, 2, and 4 h. The percentages of apoptotic glioma cells were determined from Hoechst dye–stained fragmented or condensed nuclei visualized by fluorescence microscopy. The mean percentages ± standard error are given from counts taken from 4 separate experiments.
Fig. 3.
Confirmation of apoptosis induction in glioma cells by a fluorescent microscopy technique and by an in vitro morphologic assay. A. Three CellTracker Green–labeled TALL-104 cells (smaller cells) in contact with a CellTracker Orange–labeled glioma cell (larger cell). B. Hoechst 33342 dye–labeled nuclei of the same cells demonstrating that the glioma cell has a fragmented nucleus. C. H&E-stained brain tumor cell monolayer cultured in the absence of TALL-104 cells demonstrating that the normal, nonapoptotic brain tumor cells are large, contain abundant cytoplasm, and have large oval nuclei. A mitotic figure in a glioma cell at metaphase is seen (arrowhead). D. A view of an H&E-stained brain tumor cell monolayer after coincubation with TALL-104 cells for 4 h. The coincubation of TALL-104 cells with the human brain tumor cells resulted in an increase in apoptotic tumor cells with a concurrent decrease in the number of brain tumor cells with mitotic figures. Cells identified as apoptotic (thin arrows) demonstrated classic morphological changes: either condensed nuclei, fragmented nuclei, or shedding of apoptotic bodies and membrane blebbing. TALL-104 cells were visualized in direct contact with apoptotic brain tumor cells (thick arrows). The inset shows H&E staining of TALL 104 cells.
TALL-104 Cell Coincubations with Brain Tumors Cause a Decrease in Gliomas with Mitotic Figures and an Increase in Apoptosis
To further confirm that TALL-104 cells injure adherent glioma cells by apoptosis, a quantitative morphologic analysis of glioma cell monolayers cocultured with TALL-104 cells was performed. Glioma cells cultured in the absence of TALL-104 cells had normal nuclear morphology, and mitotic figures were evident (Fig. 3C). In contrast, upon 4-h coincubation of TALL-104 cells with glioma cells, numerous apoptotic glioma cells were present (Fig. 3D). Glioma cells exposed to TALL-104 cells displayed morphologic changes indicative of apoptosis: nuclear DNA condensation, fragmented nuclear DNA, and shedding of apoptotic bodies. TALL-104 cells were also seen in direct contact with apoptotic glioma cells. Glioma cell morphology was examined by light microscopy after TALL-104 cells were coincubated with them for 4 h at a 10:1 E:T ratio. For each glioma cell line, 800 H&E-stained adherent cells were scored as either live normal, mitotic, or apoptotic. Morphologic analysis revealed changes relative to control, glioma-alone cultures. For the 3 glioma cultures, there was an approximate 3-fold decrease in the percentage of glioma cells with mitotic figures, and an 11- to 35-fold increase in the percentage of apoptotic cells at 4 h (Table 3). In correlation with the findings by fluorescent microscopy, one glioma cell line, 04-11-MG, was exquisitely more sensitive to TALL-104 cell injury and therefore analyzed at earlier times because, at 4 h, fewer adherent glioma cells were available for morphologic analysis.
Table 3.
Decrease in mitosis of glioma cells and increase in apoptosis upon coincubation with TALL-104 cells as determined by an in vitro morphologic assaya
| % Mitotic | % Apoptotic | |||||
|---|---|---|---|---|---|---|
| Tumor ± TALL-104 | 1h | 2h | 4h | 1h | 2h | 4h |
| 04-11-MG | 1 | 1 | 10.7 | 6.6 | 5.5 | 2.7 |
| 04-11-MG + TALL | 0 | 0 | 3.4 | 51.9 | 45.9 | 29.1 |
| 10-08-MG | 10.4 | 1.6 | ||||
| 10-08-MG + TALL | 2.7 | 55.7 | ||||
| 14-07-MG | 4.1 | 3.2 | ||||
| 14-07-MG + TALL | 1.3 | 36.9 | ||||
Values obtained from microscopic counts of H&E-stained glioma cells attached to 4-well chamber tissue culture slides. Apoptotic and mitotic figures were determined by light microscopy at 1, 2, or 4 h after coincubation of TALL-104 cells with glioma cells. Eight hundred cells were examined and scored as normal, mitotic, or apoptotic. The E:T ratio was 10:1.
Cytokines Secreted upon Coincubation of TALL-104 Cells with Glioma Cells Are Decreased in the Presence of Dexamethasone
Three human glioma cells lines were cocultured with irradiated TALL-104 cells for 18 h with and without dexamethasone at 10−6 M. The clarified supernates, analyzed for IFN-γ, GM-CSF, TNF-α, and TNF-β cytokines, indicated that the production of the cytokines was decreased or suppressed in the presence of high-concentration dexamethasone (Table 4).
Table 4.
Cytokines are secreted when irradiated TALL-104 cells are coincubated with brain tumor cells, but secretion is decreased in the presence of dexamethasone (units in pg/ml)
| Coincubated agentsa | IFN-γ | GM-CSF | TNF-α | TNF-β |
|---|---|---|---|---|
| DBTRG-05MG + TALL | 4.8 | 68.4 | 45 | 21.8 |
| DBTRG-05MG + TALL + Dex | 0 | 14.2 | 0 | 0 |
| 04-11-MG + TALL | 0 | 19.4 | 45 | 0 |
| 04-11-MG + TALL + Dex | 0 | 2.8 | 6.6 | 5 |
| 10-08-MG + TALL | 16 | 93.4 | 0 | 33.6 |
| 10-08-MG + TALL + Dex | 0 | 10.2 | 0 | 0 |
DBTRG-05MG, 04-11-MG, and 10-08-MG are all derived from glioblastoma cell explants at passages 12, 18, and 8, respectively. Human brain tumor cells were cocultured with irradiated TALL-104 cells for 18 h. Supernates were harvested, clarified, and tested for the presence of cytokines by enzyme-linked immunosorbent assay. Cytokines were not detected in the supernates from brain tumor cells or TALL-104 cells cultured alone.
Soluble Factors Produced upon Coincubation of TALL-104 Cells with Glioma Cells Do Not Induce Apoptosis, but May Decrease Mitosis
To determine if a soluble factor(s) mediated the induction of apoptosis and/or inhibition of cell proliferation, clarified supernates were obtained from 4-h coincubates of TALL-104 cells with brain tumor cells. The clarified supernates, or dilutions of it, were added to glioma cell monolayers, and 4 h later morphologic analysis was performed. Morphologic analysis revealed that for all 3 glioma cell lines the soluble factors present in the medium do not cause an induction of apoptosis (Table 5). The positive control, addition of TALL-104 cells, was placed into parallel cultures, and in each instance the percentage of apoptotic cells increased. The number of cells with mitotic figures was decreased approximately 5- to 10-fold upon exposure to varying dilutions of the clarified supernates. These data imply that either cell contact alone, or cell contact with soluble factors, may be required to induce apoptosis of glioma cells.
Table 5.
Soluble factors produced upon coincubation of TALL-104 cells with glioma cells do not induce apoptosis but may influence mitosisa
| Glioma | + | Supernate ± Medium (v:v) | % Mitotic | % Apoptotic |
|---|---|---|---|---|
| 04-11-MG | + | None / new medium | 4 | 5.0 |
| 04-11-MG | + | Coincubation supernate / new medium (1:1) | 0 | 3.0 |
| 04-11-MG | + | Coincubation supernate / new medium (2:1) | 3 | 3.0 |
| 04-11-MG | + | Coincubation supernate / none | 1 | 3.0 |
| 04-11-MG | + | TALL-104 cell controlb | 1 | 23.0 |
| 10-08-MG | + | None / new medium | 10 | 1.6 |
| 10-08-MG | + | Coincubation supernate / new medium (1:1) | 1 | 4.8 |
| 10-08-MG | + | Coincubation supernate / new medium (2:1) | 2 | 4.2 |
| 10-08-MG | + | Coincubation supernate / none | 0 | 4.0 |
| 10-08-MG | + | TALL-104 cell controlb | 0 | 64.8 |
| 14-07-MG | + | None / new medium | 6 | 2.0 |
| 14-07-MG | + | Coincubation supernate / new medium (1:1) | 1 | 2.0 |
| 14-07-MG | + | Coincubation supernate / new medium (2:1) | 3 | 3.0 |
| 14-07-MG | + | Coincubation supernate / none | 2 | 5.0 |
| 14-07-MG | + | TALL-104 cell controlb | 0 | 40.0 |
Four-hour morphologic assessment of H&E-stained glioma cells attached to 4-well chamber slides. After coincubation of human glioma cells with TALL-104 cells, supernate was collected and clarified. Clarified supernate, either neat or diluted (v/v) with fresh medium, was then added to brain tumor cell monolayers. After a 4-h incubation period, 400 cells were examined and scored as normal, mitotic, or apoptotic.
The 3 glioma cells explants were coincubated with TALL-104 cells as a positive control.
The Fas/FasL Pathway Is Not Likely Involved as an Antiglioma Mechanism
Secretion of TNF often results when an apoptotic pathway of cell injury is enacted. One of many possibilities is a Fas/FasL interaction. To determine whether this interaction may be involved between TALL-104 cells and glioma cells, the expression of FasL on TALL-104 cells and the expression of Fas on gliomas were determined by flow cytometric analysis (Table 6). Although nearly all cells of the 3 gliomas tested did express Fas at moderate levels (99.8%–100% positive, MFIs 5.5–7.8), the TALL-104 cells did not express FasL (1.2% positive, MFI 0.27).
Table 6.
Phenotypic expression of FasL by TALL-104 cells and Fas by glioma cells
| Cells assayeda | Coincubated cells | Antibody | % cells positive | MFIb |
|---|---|---|---|---|
| TALL-104 | None | Anti FasL | 1.23 | 0.26 |
| TALL-104 | 10-08-MG | Anti FasL | 0.1 | 1.0 |
| TALL-104 | 04-11-MG | Anti FasL | 2.2 | 3.6 |
| TALL-104 | 14-07-MG | Anti FasL | 0.1 | 1.0 |
| 10-08-MG | none | Anti Fas | 100 | 7.83 |
| 04-11-MG | none | Anti Fas | 99.8 | 6.21 |
| 14-07-MG | none | Anti Fas | 99.8 | 5.52 |
Abbreviations: FasL, Fas ligand; MG, malignant glioma.
The cell type assayed was either incubated alone or coincubated with carboxyfluorescein diacetate succinimidyl ester–labeled MG cells for 16 h prior to assessing for upregulation of FasL on the TALL-104 cells by flow cytometry.
MFI is mean fluorescence intensity reflective of the relative antigen density.
Conceivably, the coincubation of an effector with a target might upregulate the expression of such molecules. However, even after a 16-h coincubation of TALL-104 cells with glioma cells, the expression of FasL was not altered on the TALL-104 cells (0.1%–2.2% positive, MFIs 1.0–3.6). Thus, it is unlikely that this particular cell-cell recognition system is used by TALL-104 cells to engender injury of glioma cells.
Evidence That Glioma Cell Injury by TALL-104 Cells Is Calcium Dependent
Three human gliomas were placed into 4- and 18-h cytotoxicity assays performed with or without EGTA, a calcium chelator. EGTA was placed into the assay medium to prevent injury due to the granule-exocytosis pathway since perforin-mediated lysis is calcium dependent. The data show that 2 of the 3 gliomas (10-08-MG and 14-07-MG) were killed exclusively by a Ca2+-dependent mechanism, while the other (04-11-MG) was killed largely by a Ca2+-dependent mechanism (Fig. 4). The data shown are for the 4 h assay time, but similar findings were obtained at 18 h.
Fig. 4.
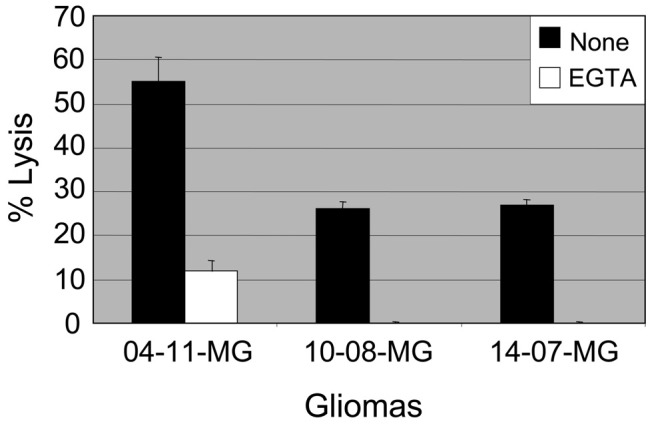
Calcium-dependent lysis of glioma cells by TALL-104 cell effectors is demonstrated. Three human gliomas (04-11-MG, 10-08-MG, and 14-07-MG) were placed into 4-h cytotoxicity assays with (□) or without (▪) 10 mM ethylene glycol-bis (2-amino ethyl) N′N′N′,N′-tetraacetic acid (EGTA) in the assay medium. At a 10:1 E:T ratio, a complete or partial inhibition of glioma cell lysis occurred when EGTA, a calcium chelator, was present. The mean percent lysis ± standard error is given.
Direct Evidence for Lytic Granule Exocytosis During the Interaction of TALL-104 Cells with Malignant Brain Tumors
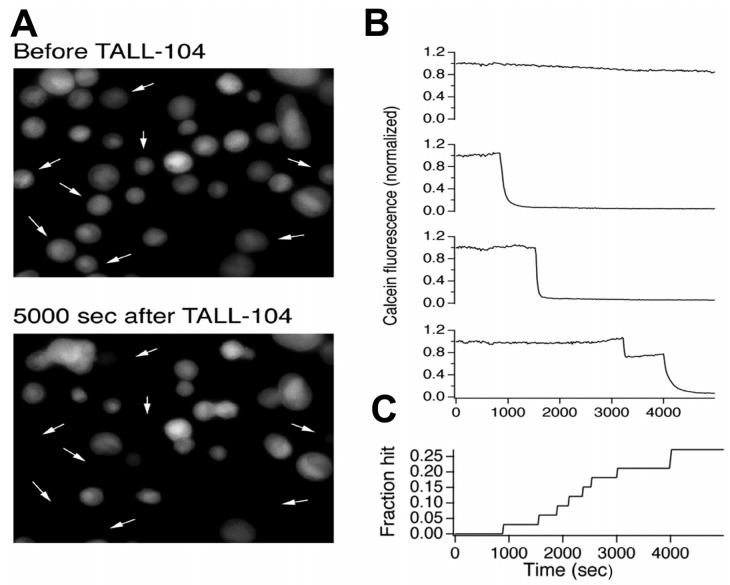
The evidence presented thus far suggests that TALL-104 cells do not kill malignant brain tumors by either the Fas/FasL pathway or by secretion of soluble proapoptotic cytokines. We surmised that since granule-dependent killing of malignant brain tumors is primarily dependent on extracellular calcium, lysis could be due to granule exocytosis. As a further test of this idea, we used a method we developed that directly monitors target cell membrane integrity due to perforin pore formation (Fig. 5). We monitored interactions of TALL-104 cells with 04-11-MG cells in these experiments. We loaded 04-11-MG cells with calcein, adhered them to poly-L-lysine coated coverslips, and allowed an excess of unlabeled TALL-104 cells to settle into contact with them. Figure 5A shows a representative field of 04-11-MG cells before (top) and 5000 s after (bottom) the addition of TALL-104 cells. Some of the cells lost calcein during the course of the experiment (arrows). Figure 5B shows fluorescence traces for individual 04-11-MG cells from the experiment shown in A. Two types of behavior were observed. In some cells (like the one shown in the top trace), fluorescence at the end of the experiment was approximately 80% of its initial value. Examination of the time course of the fluorescence signal revealed that the decrease in fluorescence was gradual and monotonic, likely because of photobleaching. In contrast, traces from cells like those highlighted by arrows in Fig. 5A reveal abrupt drops in calcein fluorescence. (The bottom 3 traces in Fig. 5B are representative.) This behavior is characteristic of perforin-mediated membrane damage following lytic granule exocytosis. Figure 5C shows that over the 5000-s (83-min) duration of the experiment performed at room temperature, approximately 25% of 04-11-MG cells were hit by perforin. If hitting continued at this rate, we would expect >70% of 04-11-MG cells to be hit in 4 h at 37°C. This rate of hitting is more than sufficient to account for killing of 04-11-MG (Table 2).
Fig. 5.
Evidence that granule exocytosis occurs during the interaction between TALL-104 and glioma cells. A. Calcein-loaded 04-11-MG glioma cells before (upper panel) and then at 5000 s after the addition of TALL-104 cells (lower panel). The arrows in each of the panels point to the locations of adherent fluorescent cells initially present that lost fluorescence during the experiment. B. Fluorescence traces from individual cells shown in A. The upper trace is an example of a cell that did not release calcein; the second, third, and bottom three panels monitor individual cells that displayed calcein release at 900 s, 1500 s, and 3100 s, respectively, after the addition of the TALL-104 cells. C. Time course of hitting for the experiment shown in A and B. Approximately 25% of cells were hit over the 5000-s (83-min) duration of the experiment.
Discussion
Several mechanisms for effector-mediated cytotoxicity have been described for specific and nonspecific effector cell populations: (1) antibody-dependent cell-mediated cytotoxicity, (2) necrosis that is thought to reflect Ca2+-dependent granule exocytosis-mediated cytotoxicity, (3) apoptosis that may be either Ca2+ dependent, or Ca2+ independent if the damage is mediated through CD95/CD95L (Fas/FasL) interactions, and (4) cytokine-mediated cytotoxicity (Berke, 1994; Cappello et al., 2002; Chong et al., 1989; Geske and Gerschenson, 2001; Hehner et al., 1998; Henkart, 1994; Li et al., 1998). The latter 3 were investigated as possible mechanisms for glioma cell injury by TALL-104 cells.
Multiple lines of evidence suggest that glioma cells killed by TALL-104 cells show features characteristic of both apoptotic and necrotic cell death. Despite the fact that TNF is among the cytokines secreted upon coincubation of gliomas with TALL-104 cells (Table 4; Kruse et al., 2000), soluble factors that include those cytokines with half-lives that are present within the 4- to 8-h time frame of our experiment (Table 5) are not responsible for the glioma apoptosis observed. Immune T cells can trigger apoptotic signals through the Fas receptor or by the exocytosis of granzyme B and perforin (Barry et al., 2000; Bossi and Griffiths, 1999; Cappello et al., 2002). Since TALL-104 cells did not express FasL (Table 6), Fas/FasL interactions probably play little to no role in the cell injury observed. Since recent evidence suggests that the appearance of FasL on the surface of CTL can be mediated by exocytosis of lytic granules (Bossi and Griffiths, 1999) and that a Ca2+-dependent induction requires a transcriptional component that requires 4 to 5 h after activation (Vignaux et al., 1995), we coincubated TALL-104 cells with glioma targets for 16 h before looking at FasL expression on the TALL-104 cells. We found no upregulation of FasL expression (Table 6). Although calcium is required for FasL induction, the binding and activation of FasL are calcium independent (Bossi and Griffiths, 1999; Vignaux et al., 1995). Indeed, granule exocytosis-dependent killing is absolutely dependent on extracellular Ca2+, as the release of granules by CTL, the insertion of perforin monomers into the target cell membrane, and the polymerization of perforin monomers to form functional pores all require extracellular Ca2+ (Henkart et al., 1984; Takayama and Sitkovsky, 1987; Uellner et al., 1997). The cytotoxicity experiments with EGTA (Fig. 4) indicate that cell injury is mediated largely by a calcium-dependent mechanism(s). In the case where lysis of one glioma was partially inhibited, the data suggest that a pathway of cell injury other than Fas/FasL is enacted.
Previous work that imaged the dynamics of single TALL-104 cells interacting with bispecific antibody-treated Raji B cells directly demonstrated the release of lytic granules (labeled with a lysosomotrophic dye, Lyso-Tracker Red ) followed approximately 15 s later by efflux of calcein from the target cells (Lyubchenko et al., 2001). We also observed evidence of lytic granule exocytosis during TALL-104/glioma cell interactions (Fig. 5). Could lytic granule exocytosis mediate both necrosis and apoptosis of glioma cells? Several lines of evidence suggest that this is the case. When granzymes are secreted in the presence of perforin, they are internalized and trigger apoptotic cell death (Waterhouse and Trapani, 2002). Furthermore, we know that one TALL-104 cell is capable of successive and rapid lysis of multiple Raji tumor targets (i.e., 3 targets killed within <8 min).4 Therefore, necrotic cell injury is also likely to be one mechanism used for target cell destruction.
Dexamethasone is an immunosuppressive glucocorticoid often administered to glioma patients to control brain edema. We have tested for the effects of dexamethasone on lysis and cytokine secretion, using a range of dexamethasone concentrations from 10−6 to 10−8 M (5.16 – 516 × 10−6 g/liter dexamethasone sodium phosphate), when combining effector cells of various types (lymphokine-activated killer cells, lectin-stimulated lymphocytes, alloreactive CTL, TALL-104 cells) with glioma target cells (Kruse and Merchant, 1997; Kruse et al., 2000; Read et al., 2003). The in vitro concentrations of dexamethasone tested were at or exceeded the serum level doses that might be achieved in patients given 1 or 8 mg/day the previous day. For 1- or 8-mg/day doses, clinical laboratory test ranges are 140 to 295 ng or 1600 to 2850 ng dexamethasone/dl of serum, respectively (Esoterix, 2002). The lower and upper values translate to 1.4 to 28.5 × 10−6 g/liter. We assume that the serum levels of dexamethasone would be higher in patients given bolus steroid (up to 20 mg/day), such as might be given at surgery. Assuming that brain parenchyma levels do not exceed those in serum or CSF, the 10−6 M concentration tested with TALL-104 cells is at, or more likely above, the serum levels achieved at bolus therapeutic application. With alloactivated lymphocytes, dexamethasone concentrations of 10−6, 10−7, and 10−8 M did not have an effect on 4- or 18-h lytic activity (Read et al., 2003). However, the secretion of T helper 1 (TH1)-type cytokines at 18 h was depressed in a concentration-dependent manner, whereas TH2 cytokine secretion was unaffected or even enhanced as the concentration of dexamethasone increased (Read et al., 2003). With TALL-104 cells, high-concentration dexamethasone (10−6 M) was able to impair TALL-104-mediated brain tumor cell lysis only in 18-h cytotoxicity assays but did not do so in 4-h assays (Kruse et al., 2000). Since adoptively transferred, lethally irradiated TALL-104 cells are likely to exert their direct, lytic effector function quickly, the impairment at 18 h was not considered to be a detriment. We also showed that TH1 cytokine secretions, variably upregulated upon TALL-104 cell coincubations with cultured brain tumor cells (Kruse et al., 2000), were decreased when high-concentration dexamethasone was present (Table 4). The implication of this finding is that patients on extremely high-dose steroid therapy may exhibit an altered response to TALL-104 cellular therapy. It is unknown what the overall effects of a depressed TH1 cytokine microenvironment would be. In vivo, the cytokines produced may be required to activate a beneficial endogenous immune response directed toward glioma cells. Alternatively, the inflammation that may be induced in the brain by the therapy may be beneficially reduced by dexamethasone to make the therapy tolerable.
The interactions between glioma cells and TALL-104 cells remain enigmatic, despite work performed in 3 separate laboratories specifically with brain tumors and TALL-104 cells (Cesano et al., 1995; Geoerger et al., 2000; Kruse et al., 2000). It is not clear what cell adhesion and recognition molecules give TALL-104 cells the ability to differentiate between normal and tumor cells. Nearly 70% of TALL-104 cells express the T-cell receptor α and β chains (O’Connor et al., 1991). Although the brain tumor cells used in these experiments express HLA class I antigens, blocking antibodies to class I had little to no effect on the lysis of gliomas at 4 or 18 h.5 Therefore, lysis of gliomas is likely largely non-MHC-restricted, as has been observed for TALL-104 cell interactions with other tumor cell types. In some instances, lysis was actually enhanced with addition of the anti–class I antibody,6 which suggests that the blocking of the class I sites may in fact allow better recognition of other sites involved in the cytotoxicity. Furthermore, leukocyte function antigen-1/intercellular adhesion molecule-1 (LFA-1/ICAM-1) interactions do not appear to be involved in TALL-104 cell interactions with gliomas. Although TALL-104 cells are 100% positive (MFI = 33.6) for LFA-1 expression and 2 of the 3 gliomas examined had greater than 90% expression of ICAM-1 (the other had 18% expression), blocking antibodies to ICAM-1 failed to inhibit lysis at either 4 or 18 h.7 Therefore, ICAM-1/LFA-1 interactions are probably not important for TALL-104 cell-induced glioma cell injury. Furthermore, homotypic binding between neural cell adhesion molecules is also not likely to be involved in the lytic interaction because while 81% to 94% of TALL-104 cells express CD56 or neural cell adhesion molecules (O’Connor et al., 1991), cultured glioma cells lose their expression of the adhesion molecule (Kleinschmidt-DeMasters et al., 1999). More work is needed to determine how TALL-104 cells recognize glioma cells.
Our laboratory’s research interests have developed around the use of allogeneic CTL, reactive to the tumor-bearing host’s MHC antigens, as potent brain tumor cell effectors (Fleshner et al., 1992; Kruse et al., 1990, 1994; Redd et al., 1992). Follow-up on 6 patients enrolled into a phase 1 trial where alloreactive CTL cells were repeatedly infused intracranially into the resected tumor beds of recurrent brain tumors (Kruse and Rubinstein, 2001; Kruse et al., 1997) demonstrated that 3 of 6 patients responded to treatment. Alloreactive CTL cells require individualization of the biologic (Kruse and Beck, 1997). If other well-defined allogeneic effector cells, such as lethally irradiated TALL-104 cells, showed the same promise but the effector cell production could be batched, it could offer advantages for intratumorally administered cellular antiglioma therapy. Studies on systemically administered TALL-104 cells in various animal model systems (including severe combined immunodeficient animals, immunocompetent mice, and canines) have shown that TALL-104 cells are potent tumor cell killers. Importantly, detailed toxicological evaluations performed with rodents, dogs, monkeys, and human patients have shown that TALL-104 cells are nontoxic (Cesano et al., 1994, 1996a, b; 1997). Despite their promise, however, trials of locally administered TALL-104 cells as cellular therapy for brain tumors have not yet been conducted.
Acknowledgments
We thank C.J. Gup for her technical assistance, Tom Nicholas for the statistical analyses, and the University of Colorado Cancer Center Flow Cytometry Core personnel for their help.
Footnotes
Partially supported by NIH grants RO1 AI42964 (A.Z.), RO1 NS28905 and R21 NS46463 (C.A.K.), F31 NS46463 (G.G.G.), and RO1 CA20833 (D.S.), and by funds donated in memory of Lindsey Melvey, Adam Wolken, Ceil Baker, Jay Kruse, and Brad Yackle. Research activity was also supported by the R. Herbert and Alma S. Manweiler Memorial Research Fund.
Abbreviations used are as follows: 7AAD, 7-amino actinomycin D; CD, cluster of differentiation; CFSE, carboxyfluorescein diacetate succinimidyl ester; CTL, cytotoxic T lymphocyte; EGTA, ethylene glycol-bis(2-aminoethyl)N,N,N′,N′-tetraacetic acid; ELISA, enzyme-linked immunosorbent assay; E:T, effector-to-target ratio; FasL, Fas ligand; FBS, fetal bovine serum; GM-CSF, granulocyte macrophage-colony stimulating factor; H&E, hematoxylin and eosin; IFN, interferon; IL-2, interleukin 2; ICAM, intercellular adhesion molecule; IMDM, Iscove’s modified Dulbecco’s medium; LFA, leucocyte function antigen; MFI, mean fluorescence intensity; MHC, major histocompatibility complex; NK, natural killer; PBS, phosphate-buffered saline; TH, T helper cell; TNF, tumor necrosis factor.
Zweifach, A., unpublished data, 2003.
Kruse, C.A., unpublished data, 2003.
Ibid.
Ibid.
References
- Barry M, Heibein JA, Pinkoski MJ, Lee S.-F, Moyer RW, Green DR, Bleackley RC. Granzyme B short-circuits the need for caspase 8 activity during granule-mediated cytotoxic T-lymphocyte killing by directly cleaving Bid. Mol Cell Biol. 2000;20:3781–3794. doi: 10.1128/mcb.20.11.3781-3794.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke G. The binding and lysis of target cells by cytotoxic lymphocytes: Molecular and cellular aspects. Annu Rev Immunol. 1994;12:735–773. doi: 10.1146/annurev.iy.12.040194.003511. [DOI] [PubMed] [Google Scholar]
- Bossi G, Griffiths GM. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat Med. 1999;5:90–96. doi: 10.1038/4779. [DOI] [PubMed] [Google Scholar]
- Cappello P, Novelli F, Forni G, Giovarelli M. Death receptor ligands in tumors. J Immunother. 2002;25:1–15. doi: 10.1097/00002371-200201000-00001. [DOI] [PubMed] [Google Scholar]
- Cesano A, Santoli D. Two unique human leukemic T-cell lines endowed with a stable cytotoxic function and a different spectrum of target reactivity analysis and modulation of their lytic mechanisms. In Vitro Cell Dev Biol. 1992;28A:648–656. doi: 10.1007/BF02631041. [DOI] [PubMed] [Google Scholar]
- Cesano A, Visonneau S, Cioe L, Clark SC, Rovera G, Santoli D. Reversal of acute myelogenous leukemia in humanized SCID mice using a novel adoptive transfer approach. J Clin Invest. 1994;94:1076–1084. doi: 10.1172/JCI117422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesano A, Visonneau S, Santoli D. Treatment of experimental glioblastoma with a human major histocompatibility complex nonrestricted cytotoxic T cell line. Cancer Res. 1995;55:96–101. [PubMed] [Google Scholar]
- Cesano A, Visonneau S, Jeglum KA, Owen J, Wilkinson K, Carner K, Reese L, Santoli D. Phase I clinical trial with a human major histocompatibility complex nonrestricted cytotoxic T-cell line (TALL-104) in dogs with advanced tumors. Cancer Res. 1996a;56:3021–3029. [PubMed] [Google Scholar]
- Cesano A, Visonneau S, Pasquini S, Rovera G, Santoli D. Antitumor efficacy of a human major histocompatibility complex nonrestricted cytotoxic T-cell line (TALL-104) in immunocompetent mice bearing syngeneic leukemia. Cancer Res. 1996b;56:4444–4452. [PubMed] [Google Scholar]
- Cesano A, Visonneau S, Wolfe JH, Jeglum KA, Fernandez J, Gillio A, O’Reilly RJ, Santoli D. Toxicological and immunological evaluation of the MHC-non-restricted cytotoxic T cell line TALL-104. Cancer Immunol Immunother. 1997;44:125–136. doi: 10.1007/s002620050365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong AS, Aleksijevic A, Scuderi P, Hersh EM, Grimes WJ. Phenotypic and functional analysis of lymphokine-activated killer (LAK) cell clones. Ability of CD3+, LAK cell clones to produce interferon-gamma and tumor necrosis factor upon stimulation with tumor targets. Cancer Immunol Immunother. 1989;29:270–278. doi: 10.1007/BF00199215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esoterix (2002) Final Report, Serum Dexamethasone. Calabasas Hills, Calif.: Esoterix, Inc., 2002.
- Fleshner M, Watkins LR, Redd JM, Kruse CA, Bellgrau D. A 9L gliosarcoma transplantation model for studying adoptive immunotherapy into the brains of conscious rats. Cell Transplant. 1992;1:307–312. doi: 10.1177/096368979200100408. [DOI] [PubMed] [Google Scholar]
- Freeman, J.E., Winston, K.R., Foreman, N., Paul, D.B., Gup, C.J., Gomez, G., Visonneau, S., Santoli, D., and Kruse, C.A. (1999) Cellular immunotherapy of brain tumors involving intratumoral placements of the irradiated TALL-104 cytotoxic T cell line. Proceedings of the American Association of Neurological Surgeons/Congress of Neurological Surgeons Section on Pediatric Neurological Surgery 28th Annual Meeting, Atlanta, Ga., pp. 100–101 (abstract).
- Geoerger B, Tang C.-B, Cesano A, Visonneau S, Marwaha S, Judy KD, Sutton LN, Santoli D, Phillips PC. Antitumor activity of a human cytotoxic T-cell line (TALL-104) in brain tumor xenografts. Neuro-oncol. 2000;2:103–113. doi: 10.1093/neuonc/2.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geske FJ, Gerschenson LE. The biology of apoptosis. Human Pathol. 2001;32:1029–1038. doi: 10.1053/hupa.2001.28250. [DOI] [PubMed] [Google Scholar]
- Hehner SP, Hofmann TG, Ratter F, Dumont A, Dröge W, Schmitz ML. Tumor necrosis factor-α-induced cell killing and activation of transcription factor NF-κB are uncoupled in L929 cells. J Biol Chem. 1998;273:18117–18121. doi: 10.1074/jbc.273.29.18117. [DOI] [PubMed] [Google Scholar]
- Henkart PA, Millard PJ, Reynolds CW, Henkart MP. Cytolytic activity of purified cytoplasmic granules from cytotoxic rat large granular lymphocyte tumors. J Exp Med. 1984;160:75–93. doi: 10.1084/jem.160.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkart PA. Lymphocyte-mediated cytotoxicity: Two pathways and multiple effector molecules. Immunity. 1994;1:343–346. doi: 10.1016/1074-7613(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters BK, Orr EA, Savelieva E, Owens GC, Kruse CA. Paucity of retinoic acid receptor alpha (RARα) nuclear immunostaining in gliomas and inability of retinoic acid to influence neural cell adhesion molecule (NCAM) expression. J Neurooncol. 1999;41:31–42. doi: 10.1023/a:1006162211296. [DOI] [PubMed] [Google Scholar]
- Kruse CA, Beck LT. Artificial-capillary-system development of human alloreactive cytotoxic T-lymphocytes that lyse brain tumours. Biotechnol Appl Biochem. 1997;25:197–205. [PubMed] [Google Scholar]
- Kruse, C.A., and Merchant, R.E. (1997) Cellular therapy of brain tumors: Clinical trials. In Kornblith, P.L., and Walker, M.D. (Eds.), Advances in Neuro-Oncology, II Armonk, N.Y.: Futura Publishing Company, pp. 487–504.
- Kruse, C.A., and Rubinstein, D. (2001) Cytotoxic T-lymphocytes reactive to patient major histocompatibility complex proteins for therapy of brain tumors. In Liau, L.M., Becker, D.P., Cloughesy, T.F., and Bigner, D.D. (Eds.), Brain Tumor Immunotherapy. Totowa, N.J.: Humana Press, pp. 149–170.
- Kruse CA, Mitchell DH, Lillehei KO, Johnson SD, McCleary EL, Moore GE, Waldrop S, Mierau GW. Interleukin-2-activated lymphocytes from brain tumor patients. A comparison of two preparations generated in vitro. Cancer. 1989;64:1629–1637. doi: 10.1002/1097-0142(19891015)64:8<1629::aid-cncr2820640813>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Kruse CA, Lillehei KO, Mitchell DH, Kleinschmidt-DeMasters B, Bellgrau D. Analysis of interleukin 2 and various effector cell populations in adoptive immunotherapy of 9L rat gliosarcoma: Allogeneic cytotoxic T lymphocytes prevent tumor take. Proc Natl Acad Sci USA. 1990;87:9577–9581. doi: 10.1073/pnas.87.24.9577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse CA, Mitchell DH, Kleinschmidt-DeMasters BK, Franklin WA, Morse HG, Spector EB, Lillehei KO. Characterization of a continuous human glioma cell line DBTRG-05MG: Growth kinetics, karyotype, receptor expression, and tumor suppressor gene analyses. In Vitro Cell Dev Biol. 1992;28A:609–614. doi: 10.1007/BF02631035. [DOI] [PubMed] [Google Scholar]
- Kruse CA, Schiltz PM, Bellgrau D, Kong Q, Kleinschmidt-DeMasters BK. Intracranial administrations of single or multiple source allogeneic cytotoxic T lymphocytes: Chronic therapy for primary brain tumors. J Neurooncol. 1994;19:161–168. doi: 10.1007/BF01306458. [DOI] [PubMed] [Google Scholar]
- Kruse CA, Cepeda L, Owens B, Johnson SD, Stears J, Lillehei KO. Treatment of recurrent glioma with intracavitary alloreactive cytotoxic T lymphocytes and interleukin-2. Cancer Immunol Immunother. 1997;45:77–87. doi: 10.1007/s002620050405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse CA, Varella-Garcia M, Kleinschmidt-DeMasters BK, Owens GC, Spector EB, Fakhrai H, Savelieva E, Liang BC. Receptor expression, cytogenetic, and molecular analysis of six continuous human glioma cell lines. In Vitro Cell Dev Biol Anim. 1998;34:455–462. doi: 10.1007/s11626-998-0078-x. [DOI] [PubMed] [Google Scholar]
- Kruse CA, Visonneau S, Kleinschmidt-DeMasters BK, Gup CJ, Gomez GG, Paul DB, Santoli D. The human leukemic T-cell line, TALL-104, is cytotoxic to human malignant brain tumors and traffics through brain tissue: Implications for local adoptive immunotherapy. Cancer Res. 2000;60:5731–5739. [PubMed] [Google Scholar]
- Laws ER., Jr Central nervous system tumors: What have we learned and where are we heading? Ca: Cancer J. Clin. 1998;48:322–327. 329. doi: 10.3322/canjclin.48.6.327. (editorial) [DOI] [PubMed] [Google Scholar]
- Lecoeur H, de Oliveira-Pinto LM, Gougeon ML. Multiparametric flow cytometric analysis of biochemical and functional events associated with apoptosis and oncosis using the 7-aminoactinomycin D assay. J Immunol Methods. 2002;265:81–96. doi: 10.1016/s0022-1759(02)00072-8. [DOI] [PubMed] [Google Scholar]
- Li JH, Rosen D, Ronen D, Behrens CK, Krammer PH, Clark WR, Berke G. The regulation of CD95 ligand expression and function in CTL. J Immunol. 1998;161:3943–3949. [PubMed] [Google Scholar]
- Lyubchenko TA, Wurth GA, Zweifach A. Role of calcium influx in cytotoxic T lymphocyte lytic granule exocytosis during target cell killing. Immunity. 2001;15:847–859. doi: 10.1016/s1074-7613(01)00233-3. [DOI] [PubMed] [Google Scholar]
- O’Connor R, Cesano A, Lange B, Finan J, Nowell PC, Clark SC, Raimondi SC, Rovera G, Santoli D. Growth factor requirements of childhood acute T-lymphoblastic leukemia: Correlation between presence of chromosomal abnormalities and ability to grow permanently in vitro. Blood. 1991;77:1534–1545. [PubMed] [Google Scholar]
- Paul DB, Kruse CA. Immunologic approaches to therapy for brain tumors. Curr Neurol Neurosci Rep. 2001;1:238–244. doi: 10.1007/s11910-001-0024-8. [DOI] [PubMed] [Google Scholar]
- Philpott NJ, Turner AJ, Scopes J, Westby M, Marsh JC, Gordon-Smith EC, Dalgleish AG, Gibson FM. The use of 7-amino actinomycin D in identifying apoptosis: Simplicity of use and broad spectrum of application compared with other techniques. Blood. 1996;87:2244–2251. [PubMed] [Google Scholar]
- Prados MD, Berger MS, Wilson CB. Primary central nervous system tumors: Advances in knowledge and treatment. Ca: Cancer J Clin. 1998;48:331–360. doi: 10.3322/canjclin.48.6.331. [DOI] [PubMed] [Google Scholar]
- Read SB, Kulprathipanja NV, Gomez GG, Paul DB, Winston KR, Robbins JM, Kruse CA. Human alloreactive CTL interactions with gliomas and with those having upregulated HLA expression from exogenous IFN-γ or IFN-γ gene modification. J Interferon Cytokine Res. 2003;23:379–393. doi: 10.1089/107999003322226032. [DOI] [PubMed] [Google Scholar]
- Redd JM, Lagarde AC, Kruse CA, Bellgrau D. Allogeneic tumor-specific cytotoxic T lymphocytes. Cancer Immunol Immunother. 1992;34:349–354. doi: 10.1007/BF01741557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saris SC, Patronas NJ, Rosenberg SA, Alexander JT, Frank J, Schwartzentruber DJ, Rubin JT, Barba D, Oldfield EH. The effect of intravenous interleukin-2 on brain water content. J Neurosurg. 1989;71:169–174. doi: 10.3171/jns.1989.71.2.0169. [DOI] [PubMed] [Google Scholar]
- Schmid I, Uittenbogaart CH, Keld B, Giorgi JV. A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry. J Immunol Methods. 1994;170:145–157. doi: 10.1016/0022-1759(94)90390-5. [DOI] [PubMed] [Google Scholar]
- Takayama H, Sitkovsky MV. Antigen receptor-regulated exocytosis in cytotoxic T lymphocytes. J Exp Med. 1987;166:725–743. doi: 10.1084/jem.166.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uellner R, Zvelebil MJ, Hopkins J, Jones J, MacDougall LK, Morgan BP, Podack E, Waterfield MD, Griffiths GM. Perforin is activated by a protolytic cleavage during biosynthesis which reveals a phospholipid-binding C2 domain. EMBO J. 1997;16:7287–7296. doi: 10.1093/emboj/16.24.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignaux F, Vivier E, Malissen B, Depraetere V, Nagata S, Golstein P. TCR/CD3 coupling to Fas-based cytotoxicity. J Exp Med. 1995;181:781–786. doi: 10.1084/jem.181.2.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virasch N, Kruse CA. Strategies using the immune system for therapy of brain tumors. Hematol Oncol Clinics North Am. 2001;15:1053–1071. doi: 10.1016/s0889-8588(05)70267-7. [DOI] [PubMed] [Google Scholar]
- Visonneau S, Cesano A, Tran T, Jeglum KA, Santoli D. Successful treatment of canine malignant histiocytosis with the human major histocompatibility complex nonrestricted cytotoxic T-cell line TALL-104. Clin Cancer Res. 1997;3:1789–1797. [PubMed] [Google Scholar]
- Visonneau S, Cesano A, Jeglum KA, Santoli D. Adjuvant treatment of canine osteosarcoma with the human cytotoxic T-cell line TALL-104. Clin Cancer Res. 1999a;5:1868–1875. [PubMed] [Google Scholar]
- Visonneau S, Cesano A, Jeglum KA, Santoli D. Adoptive therapy of canine metastatic mammary carcinoma with the human MHC non-restricted cytotoxic T-cell line TALL-104. Oncol Rep. 1999b;6:1181–1188. doi: 10.3892/or.6.6.1181. [DOI] [PubMed] [Google Scholar]
- Visonneau S, Cesano A, Porter DL, Luger SL, Schuchter L, Kamoun M, Torosian MH, Duffy K, Sickles C, Stadtmauer EA, Santoli D. Phase I trial of TALL-104 cells in patients with refractory metastatic breast cancer. Clin Cancer Res. 2000;6:1744–1754. [PubMed] [Google Scholar]
- Waterhouse NJ, Trapani JA. CTL: Caspases terminate life, but that’s not the whole story. Tissue Antigens. 2002;59:175–183. doi: 10.1034/j.1399-0039.2002.590301.x. [DOI] [PubMed] [Google Scholar]
- Winer, B.J. (1971) Multifactor experiments having repeated measures on the same elements. In Statistical Principles in Experimental Design, 2nd ed., Chapter 7. New York: McGraw-Hill, pp. 514–603.
- Zweifach A. Target-cell contact activates a highly selective capacitative calcium entry pathway in cytotoxic T lymphocytes. J Cell Biol. 2000;148:603–614. doi: 10.1083/jcb.148.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]