Abstract
Treatment strategies for CNS germinoma are currently evolving. Current approaches include reducing the volume and dose of radiation by adding pre-irradiation chemotherapy. Very accurate staging is necessary with such an approach to prevent failures. Eight consecutive patients with pineal germinoma at one institution underwent endoscopic surgery for tumor biopsy, direct visualization of the third ventricular region, and third ventriculostomy for those with hydrocephalus. All patients were treated with 4 cycles of chemotherapy. Conformal field radiation therapy followed, with the dose to the tumor bed dependent on the response to chemotherapy. Patients who had MRI, endoscopic, or cerebrospinal fluid evidence of multicentric or disseminated disease also received craniospinal radiation. Six patients had diabetes insipidus (DI) at presentation. All 6 had tumor studding the floor of the third ventricle on endoscopic visualization, while only 4 of those patients had MRI evidence of disease in that region. All patients have completed therapy and are alive, with no evidence of disease at median follow-up of 31.5 months from diagnosis. Direct endoscopic visualization of the third ventricular region may be more sensitive than MRI for evaluating the presence of suprasellar disease and appears to add important information. This parameter should be added to the staging evaluation when feasible. In this series, the presence of DI was 100% predictive of suprasellar disease, even when the MRI was negative for involvement of that region. Patients should be evaluated for DI as part of the initial staging, and if it is present, the patients should be treated for suprasellar disease regardless of MRI findings.
Central nervous system germinoma is a relatively rare neoplasm, accounting for no greater than 5% of pediatric brain tumors (Jellinger, 1973; Jennings et al., 1985). The most common subtype of CNS germ cell tumor, germinoma usually arises in midline diencephalic structures, particularly the pineal and suprasellar regions (Jennings et al., 1985; Glenn and Barkovich, 1996; Wara et al., 1979). Synchronous involvement of these sites, sometimes referred to as multiple midline or multicentric germinoma, is present in 2% to 13% of reported cases (Glenn and Barkovich, 1996; Sugiyama et al., 1992). Diabetes insipidus (DI)3 is a common presenting sign of patients with suprasellar or third ventricular tumors (Mootha et al., 1997). DI has occasionally been observed in patients with pineal region germinoma without radiographic evidence of suprasellar/third ventricular disease (Hardenbergh et al., 1997; Dodek and Sadeghi-Nejad, 1998).
The diagnosis of pure germinoma is made histologically after tumor biopsy. Since the biopsy material is often quite small and germ cell tumors may be heterogeneous, nongerminomatous elements are often presumptively excluded by assaying serum and lumbar cerebrospinal fluid (CSF) for tumor markers, such as alpha-fetoprotein (AFP) and β-human chorionic gonadotropin (β-HCG). High levels of these markers are typically associated with more malignant germ cell tumors (Balmaceda et al., 1996; Horowitz and Hall, 1991; Kretschmar, 1997). Elevation in AFP is associated with nongerminomatous germ cell tumors, particularly endodermal sinus tumor and embryonal carcinoma. Although mild elevation of β-HCG can be found in pure germinoma, marked elevation is associated with choriocarcinoma and embryonal carcinoma. There is some debate over the β-HCG level that should be considered a marked elevation, with most authors suggesting that serum or CSF level greater than 50 mIU/ml places the patient in a high-risk or mixed germ cell category (Calaminus et al., 1997; Diez et al., 1999). This paper focuses on the staging and treatment of pure CNS germinoma, which is referred to as germinoma for the remainder of the paper.
For radiotherapy treatment purposes, patients with germinoma are typically divided into 2 groups: (1) those with localized disease and (2) those with disseminated disease based on the presence of tumor in more than one location on pretreatment neuroimaging or positive CSF cytology. With more advanced multimodality protocols utilizing pre-radiotherapy chemotherapy and response-dependent radiation therapy, the dose and volume of radiation are critically dependent on the initial staging. For example, patients with localized disease at diagnosis who have a complete radiographic response to chemotherapy may receive involved-field radiation only at a reduced dose. Patients with disseminated disease at diagnosis who have a complete response will receive both involved-field and whole-brain or craniospinal radiation at a reduced dose. The more accurate the initial staging, the less likely that radiographically occult disease will be missed.
In addition, symptomatic DI almost invariably reflects a disturbance in the hypothalamic-pituitary regulation of antidiuretic hormone. In the CT era, it has been noted that secondary or acquired DI may be related to radiographically occult tumor infiltration of the infundibulum. Although more sensitive, the MRI may miss measurable disease in some patients early in the course of disease. Thus, the detection of DI in patients with biopsy-confirmed pineal region germinoma, or the observation of microscopic metastases on the walls of the third ventricle during neuroendoscopy, should constitute additional staging criteria for assigning a patient to multicentric or disseminated disease category.
Recent advances in neurosurgical techniques have allowed for endoscopic tumor biopsy and, in patients who require relief of hydrocephalus from a pineal region tumor, endoscopic third ventriculostomy (ETV) (Ellenbogen and Moores, 1997; Gangemi et al., 2001; Robinson and Cohen, 1997). This procedure is being used with increasing frequency to diagnose and treat patients with suspected germinoma. It also allows the surgeon to directly visualize the third ventricular region and inspect for disease. This method may prove a more accurate method to assess extent of disease than MRI.
CNS germinoma is highly responsive to both radiation and chemotherapy. Historically, this tumor was treated with whole-brain radiation alone or with craniospinal radiation, with long-term survival rates approaching 90% or better (Hardenbergh et al., 1997; Huh et al., 1996). Other studies have reported very good long-term survival rates with limited field radiation (Matsutani et al., 1997; Shibamoto et al., 2001). The field of radiation varied from primary tumor only to a generous local field that included the primary tumor site, the third and lateral ventricles, and the sellar and pineal regions. Rare recurrence (approximately 10%) outside the radiation port, including distant spinal metastases, have been reported in these and other studies (Alapetite et al., 2003; Jenkin et al., 1990).
Patients with germinoma who have received radiation have been reported to have a decline in neuropsychological performance and endocrine function, as well as physical and psychosocial health (Sands et al., 2001; Sawamura et al., 1998a). The severity of these effects seems to correlate with the age of the patient at the time of treatment, as well as with the dose and field of radiation treatment. Patients who receive involved-field treatment may have fewer long-term side effects. Matustani et al. (1997) report a good quality of life involving school or employment in 19 (83%) of 23 patients from the study mentioned above still living after more than 10 years.
The addition of pre-irradiation chemotherapy to the treatment of CNS germinoma has allowed the volume and dose of radiation to be reduced while maintaining excellent outcome (Allen et al., 1994; Fouladi et al., 1998; Sawamura et al., 1998b). Several small series have successfully incorporated this strategy, basing dose of radiation on response to chemotherapy, and field on the extent of disease at diagnosis. Treatment of CNS germinoma with chemotherapy alone has been investigated in at least one study (Balmaceda et al., 1996). In this study, 37 of 45 assessable patients (82%) had a complete response to chemotherapy, but 20 of these patients relapsed at a median time of 18 months from diagnosis. Almost all of these patients were salvaged with further therapy that included radiation. Although some patients were cured without radiation, these data suggest that some form of radiation is likely necessary to maintain good long-term control for most patients.
In an attempt to reduce radiation-related sequelae, efforts have continued to combine chemotherapy and radiation. A recent Pediatric Oncology Group (POG) protocol treated patients with CNS germ cell tumors, including germinoma, with chemotherapy that included cisplatin, etoposide, vincristine, and cyclophosphamide, followed by response-adjusted irradiation.4 If patients had disseminated or multicentric disease at diagnosis, craniospinal radiation was also given as a means to prevent distant recurrence. Since 1998, we have managed patients with germinoma at the Children’s Hospital of Alabama (TCHA) with endoscopic surgery followed by the same chemotherapeutic regimen that was used in the low-risk arm of the POG protocol. TCHA patients have also been treated with radiation doses similar to those used in the POG study. Patients with any evidence of multicentric disease, including endoscopic evidence only, receive craniospinal radiation.
Materials and Methods
During the period from 1998 to the present, all patients who presented to TCHA with suspected pineal region germinoma underwent staging evaluation at the time of diagnosis that included brain and spine MRI with and without gadolinium, serum and CSF analysis of AFP and β-HCG, and CSF cytology. Patients had preoperative evaluation for DI that included serum sodium and osmolarity, urine specific gravity, and questioning regarding history for polydipsia and polyuria.
Each patient with hydrocephalus underwent ETV with a rigid 0-degree ventriculoscope that was guided through the lateral and third ventricles, where pertinent landmarks, including the foramen of Monro, mammillary bodies, infundibular recess, and floor of the third ventricle, were observed. These areas were inspected for tumor studding the floor of the third ventricle just prior to making a midline perforation between the mammillary bodies and infundibular recess. Presence or absence of tumor in this area was documented in the operative report. Tumor was not biopsied from this area because of the significant risk to the hypothalamic region and the relative safety of biopsy of the pineal region tumor for diagnosis. Only after the ETV was performed and the hydrocephalus presumably treated was the pineal region tumor endoscopically biopsied.
The definitive diagnosis of CNS germinoma was made based on histologic confirmation by pathology and the absence or low levels of tumor markers in both the patient’s serum and CSF. Low levels of tumor markers were defined as AFP less than 1.5 ng/ml and β-HCG ⩽50 mIU/ml in both lumbar CSF and serum. Patients were considered to have disseminated disease if they had any evidence of multicentric disease either radiographically, by CSF cytology, or by direct visual inspection.
After recovering from surgery, each patient underwent induction chemotherapy, off study, but according to POG protocol 9530. This regimen included 2 cycles of cisplatin and etoposide alternating with 2 cycles of cyclophosphamide and vincristine. After completing the 4 cycles of chemotherapy, the patients were restaged with MRI, and if initially abnormal, cytology and tumor marker studies were repeated. Radiation was administered on the basis of response to chemotherapy. The POG protocol was again followed, with lower doses of radiation being given to patients with a complete response to chemotherapy. A complete response was defined as disappearance of all radiographically discernible lesions. Those patients with a partial response, defined as ⩾50% reduction in tumor size as measured by the sum of the products of the maximum perpendicular diameters of all measurable lesions, received higher doses of radiation at the discretion of the treating physician.
Following completion of therapy, patients have been followed closely in the neuro-oncology clinic at TCHA with repeat imaging at the following intervals: one month after completion of radiation, every 3 months for the first year, then every 6 months for 2 years, and then annually. In addition to neurologic follow-up, patients also receive routine endocrinologic and psychological follow-up through the neuro-oncology clinic.
Results
Between 1998 and 2002, 8 patients were diagnosed with pineal region germinoma at TCHA. Six of the 8 patients with pineal region germinoma had DI as a presenting symptom (Table 1). Of these 6, 4 patients had enhancing lesions on MRI in both the suprasellar and pineal regions (Fig. 1). The other 2 patients had no radiographic evidence of disease in the suprasellar region at presentation (Fig. 2). Intraoperatively, however, all 6 patients with DI had evidence of disease on the floor of the third ventricle on endoscopic inspection. Figure 3 demonstrates the intra-operative findings of third ventricular disease in a patient with DI and pineal region germinoma but no suprasellar or third ventricular mass on MRI. Two patients had no DI and no third ventricular disease by both MRI and direct visual inspection. Tumor histology, serum, and CSF markers were consistent with the diagnosis of pure germinoma in all 8 patients. Six patients were considered to have disseminated disease on the basis of the finding of multicentric disease on MRI and/or endoscopic examination. Spinal MRI and CSF cytology were negative for disease in all patients.
Table 1.
TCHA patients with pineal region germinoma Pt age
| Pt | Age at dx | Sex | Preoperative symptoms | Preoperative MRI | Surgery | Intraoperative findings | Post-chemo MRI | Post-XRT MRI | Post-treatment symptoms/most recent MRI | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 21 | M | HA, N/V, Parinaud’s | 1) Pineal lesion | Clean 3rd vent floor | CR | CCR | Partial Parinaud’s | 27 | |
| 2) 3rd vent floor clear | Biopsy* | No | ||||||||
| 3) HCP | ETV | HCP | CCR | |||||||
| 2 | 11 | M | DI | 1) Pineal lesion | Tumor bulging through Monro | CR | CCR | DI | 21 | |
| 2) Suprasellar lesion | Biopsy | No | CCR | |||||||
| 3) No HCP | HCP | |||||||||
| 3 | 12 | F | HA, N/V, DI | 1) Pineal lesion | Tumor studding on floor of 3rd vent | CR | CCR | DI | 36 | |
| 2) Enhancing 3rd vent floor | Biopsy | No | CCR | |||||||
| 3) HCP | ETV | HCP | ||||||||
| 4 | 12 | F | N/V, Hemiparesis, DI | 1) Pineal lesion | Tumor studding on floor of 3rd vent | PR | CR | Improved hemiparesis | 44 | |
| 2) BG lesion | Biopsy | No | ||||||||
| 3) HCP | ETV | HCP | DI CCR | |||||||
| 5 | 21 | M | HA, ataxia, LOC, DI | 1) Pineal lesion | Tumor studding on floor of 3rd vent | PR | CR | DI | 59 | |
| 2) Enhancing 3rd | Biopsy | No | CCR | |||||||
| vent floor, suprasellar | ETV | HCP | ||||||||
| Region | ||||||||||
| 3) HCP | ||||||||||
| 6 | 14 | M | Diplopia, HA, DI | 1) Pineal lesion | Tumor studding on floor of 3rd vent | CR | CCR | DI | 47 | |
| 2) 3rd vent floor clear | Biopsy | No | ||||||||
| 3) HCP | ETV | |||||||||
| 7 | 14 | M | HA, N/V, DI | 1) Pineal lesion | Tumor studding on floor of 3rd vent | CR | CCR | DI | 19 | |
| 2) Mass on 3rd vent floor | Biopsy | No | CCR | |||||||
| 3) HCP | ETV | HCP | ||||||||
| 8 | 16 | M | HA, N/V, Diplopia | 1) Pineal lesion | Clean 3rd vent floor | CR | CCR | No symptoms | 15 | |
| 2) 3rd vent floor clear | Biopsy | CCR | ||||||||
| 3) HCP | ETV |
Biopsy indicates endoscopic tumor biopsy.
Abbreviations used: BG, basal ganglia; CCR, continued complete response; CR, complete response; DI, diabetes insipidus; dx, diagnosis; ETV, endoscopic third ventriculostomy; F, female; HA, headache; HCP, hydrocephalus; LOC, loss of consciousness; M, male; N/V, nausea and vomiting; PR, partial response; Pt, patient; 3rd vent, third ventricle.
Fig. 1.
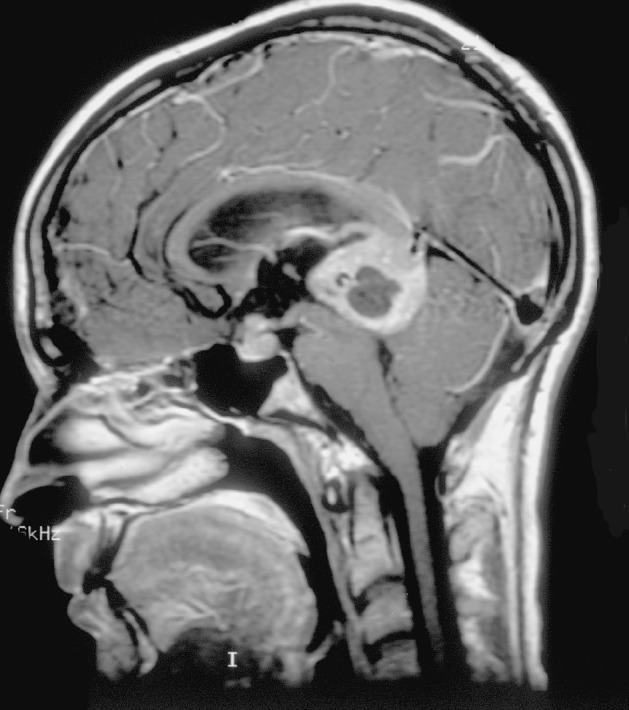
Sagittal post-gadolinium MRI demonstrates both suprasellar and pineal region masses in a patient who presented with headaches and diabetes insipidus.
Fig. 2.
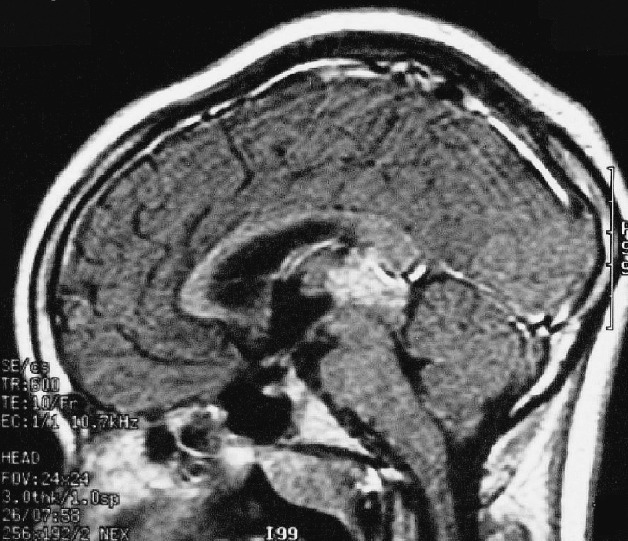
Sagittal post-gadolinium MRI demonstrates pineal region mass but no evidence of suprasellar or third ventricular abnormality in a patient with germinoma and diabetes insipidus. This patient had evidence of disease on endoscopic examination.
Fig. 3.
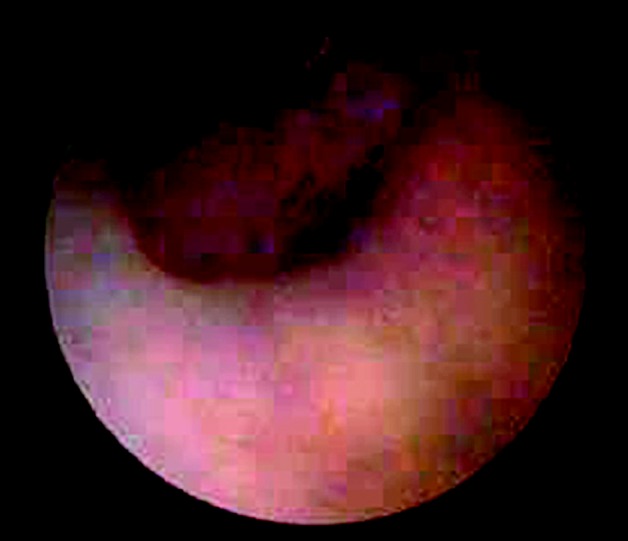
This photo was taken during endoscopic third ventriculostomy in a patient with pineal germinoma and diabetes insipidus but no suprasellar or third ventricular abnormality on MRI. It demonstrates disease studding the floor of the third ventricle.
All patients completed the prescribed course of chemotherapy and irradiation. Six patients had a complete response to chemotherapy, and 2 patients had a partial response. The 2 patients with no DI and pineal region tumors only had a complete response to chemotherapy and received 36 Gy to the prechemotherapy tumor volume. Three of 4 patients with MRI evidence of multicentric disease at diagnosis, involving both suprasellar and pineal regions, had a complete response to chemotherapy. They received radiation as follows: 23.4 to 24 Gy to the craniospinal axis and 36 Gy to the prechemotherapy tumor volume. The fourth patient, with MRI evidence of multicentric disease, had a partial response to chemotherapy and received 25.2 Gy radiation to the craniospinal axis and 45 Gy to the prechemotherapy tumor volume. The 2 patients with only endoscopic evidence of disease in the third ventricle received the following radiation: 1 patient had a complete response to chemotherapy and received 23.4 Gy to the craniospinal axis and 41.4 Gy to the prechemotherapy MRI tumor volume; the other had a partial response and received 23.4 Gy to the craniospinal axis and 45 Gy to the pretreatment MRI tumor volume. For both of these patients, the boosted area of radiation also included the suprasellar/third ventricular region with a 1.5-cm geometric margin. All 8 patients had a complete radiographic response after radiation treatment, and all remain in complete remission at a median follow up of 31.5 months (range, 15–59 months).
Another multiinstitutional series (Beth Israel Consortium study) included 38 fully staged, histologically confirmed germinoma patients.5 The study design included neoadjuvant chemotherapy followed by response-dependent radiotherapy. After a median follow-up of 24 months, 2 patients have developed recurrent disease. Eight of 10 patients on study with isolated pineal region disease had a complete response to neoadjuvant chemotherapy, permitting involved-field radiotherapy to 30 Gy to the pineal region only. One of these 8 patients developed recurrence after 23 months in the suprasellar region that was outside the involved field. He presented with symptomatic DI but had no MRI abnormalities in the suprasellar region at initial presentation.
Discussion
CNS germinoma is a highly treatable disease. As in other studies, this series demonstrates excellent and durable responses to treatment using chemotherapy and reduced-dose radiation. Patients with CNS germinoma are traditionally staged with MRI imaging and CSF evaluation. The application of endoscopic surgery to the management of germinoma allows the surgeon not only to perform a less invasive diagnostic procedure coupled with treatment of hydrocephalus but also to directly inspect the third ventricular region. This procedure may be a more sensitive means to detect disease in this region and the optimal initial intervention for these patients. Visualization of disease should be documented and incorporated into the staging evaluation of CNS germinoma.
It seems logical that patients with pineal region germinoma and DI must have some involvement of the hypothalamic-pituitary axis (suprasellar region). This is not always demonstrated on MRI. In our series, the presence of DI was 100% predictive of suprasellar disease seen with endoscopic visualization, even in the 2 cases when the MRI was negative in that region. The patient who failed in the Beth Israel Medical Center series also presented with DI but had no disease on MRI in the suprasellar region at presentation. We hypothesize that this patient had occult suprasellar disease at diagnosis. The combined results of these 2 series suggest that patients with pineal region germinoma and DI have suprasellar disease regardless of MRI findings. The presence of DI should also be used in the staging evaluation of CNS germinoma, especially when endoscopic surgery is not feasible in patients with pineal region tumors only.
In our series, 6 of 8 patients were considered to have disseminated disease because they had multicentric disease. No patient had spinal dissemination or positive CSF cytology. Only 1 of these patients had disease outside the midline suprasellar and pineal regions, and that was in the basal ganglia, which embryologically are also part of the diencephalon. Theories on the development of CNS germinoma suggest an embryologic event that leads to proliferation of germ cells in the diencephalons through aberrant migration (Glenn and Barkovich, 1996). It has been suggested that the tumor develops either multicentrically or by subependymal laminar infiltration around the third ventricle when it occurs in multiple midline sites, rather than by subarachnoid or CSF pathway metastasis (Halperin et al., 1999). It seems plausible that midline multicentric disease is more common than was previously thought but often undetectable by neuroimaging. The endoscopic findings in our series support this theory.
In our series, all patients with multicentric disease were considered to have disseminated disease and received craniospinal irradiation. It is unclear if patients with multicentric disease involving both pineal and suprasellar regions, or multiple midline germinoma, require treatment as aggressive as that used for patients with more widely disseminated disease. Two radiation questions that arise are what should be included in the “involved field” and whether craniospinal radiation is necessary. A recent French study reported a 14% relapse rate for germinoma patients treated with chemotherapy and focal radiation (Alapetite et al., 2003). Seven of 9 patients who relapsed did so in the ventricle. One could hypothesize that this was from occult disease not seen on neuro-imaging. Some groups have advocated whole ventricular radiation instead of whole-brain or craniospinal radiation (Wolden et al., 1995). The number of patients in our study is small, but the data suggest that patients with both pineal and suprasellar disease should have both areas included in the involved-field radiation port, even if the only sign of suprasellar involvement is DI. This would include the third ventricular region and at least part of the lateral ventricles. Craniospinal or whole-brain irradiation in the setting of multiple midline germinomas, however, may be excessive.
In summary, the findings of this study suggest that multiple midline germinomas may be more common than once thought and that the endoscopic examination of the ventricular region may be a more sensitive means to detect disease than current MRI capabilities. The presence of DI is almost certainly from suprasellar disease. The staging evaluation of CNS germinoma may be enhanced with endoscopic visual examination by the treating surgeon and evaluation for DI, as well as the traditional MRI and CSF studies. The optimal therapy for CNS germinoma remains a work in progress, but adding these parameters to the staging evaluation will likely decrease the failure rate and augment refinements in treatment.
Footnotes
The data in this paper were presented as a poster in abstract form at the Society for Neuro-Oncology Annual Meeting in San Diego, California, November 2002. Some of the patients presented in this study are included in a larger series of patients that details the surgical ramifications of endoscopic procedures in the treatment of germ cell tumors. This work, by Wellons et al., has been accepted for publication elsewhere.
Abbreviations used are as follows: AFP, alpha-fetoprotein; CSF, cerebrospinal fluid; DI, diabetes insipidus; ETV, endoscopic third ventriculostomy; β-HCG, β-human chorionic gonadotropin; Pediatric Oncology Group (POG); The Children’s Hospital of Alabama (TCHA).
Kretschmar, C.S., personal communication, 2003.
Allen, J.C., et al., unpublished data, 2003.
References
- Alapetite C, Carrie C, Brisse H, Thiesse P, Habrand J.-L, Gaboriaud G, Frappaz D, Cuilliere J.-C, Moncho V, Baranzelli M.-C, Patte C on behalf of the SFOP (Société Française d’Oncologie Pédiatrique) Patterns of relapse following carboplatin-based chemotherapy and focal irradiation of intracranial germinoma: The SFOP experience. Neuro-oncol. 2003;5:25. (abstract) [Google Scholar]
- Allen JC, DaRosso RC, Donahue B, Nirenberg A. A phase II trial of preirradiation carboplatin in newly diagnosed germinoma of the central nervous system. Cancer. 1994;74:940–944. doi: 10.1002/1097-0142(19940801)74:3<940::aid-cncr2820740323>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Balmaceda C, Heller G, Rosenblum M, Diez B, Villablanca JG, Kellie S, Maher P, Vlamis V, Walker RW, Leibel S, Finlay JL. Chemotherapy without irradiation—a novel approach for newly diagnosed CNS germ cell tumors: Results of an international cooperative trial. The First International Central Nervous System Germ Tumor Study. J Clin Oncol. 1996;14:2908–2915. doi: 10.1200/JCO.1996.14.11.2908. [DOI] [PubMed] [Google Scholar]
- Calaminus G, Andreussi L, Garre ML, Kortmann RD, Schober R, Gobel U. Secreting germ cell tumors of the central nervous system (CNS). First results of the cooperative German/Italian pilot study (CNS sGCT) Klin Padiatr. 1997;209:222–227. doi: 10.1055/s-2008-1043954. [DOI] [PubMed] [Google Scholar]
- Diez B, Balmaceda C, Matsutani M, Weiner HL. Germ cell tumors of the CNS in children: Recent advances in therapy. Childs Nerv Syst. 1999;15:578–585. doi: 10.1007/s003810050546. [DOI] [PubMed] [Google Scholar]
- Dodek AB, Sadeghi-Nejad A. Pineal germinoma presenting as central diabetes insipidus. Clin Pediatr. 1998;37:693–695. doi: 10.1177/000992289803701109. [DOI] [PubMed] [Google Scholar]
- Ellenbogen RG, Moores LE. Endoscopic management of a pineal and suprasellar germinoma with associated hydrocephalus: Technical case report. Minim Invasive Neurosurg. 1997;40:13–15. doi: 10.1055/s-2008-1053406. discussion 16. [DOI] [PubMed] [Google Scholar]
- Fouladi M, Grant R, Baruchel S, Chan H, Malkin D, Weitzman S, Greenberg ML. Comparison of survival outcomes in patients with intracranial germinomas treated with radiation alone versus reduced-dose radiation and chemotherapy. Childs Nerv Syst. 1998;14:596–601. doi: 10.1007/s003810050279. [DOI] [PubMed] [Google Scholar]
- Gangemi M, Maiuri F, Colella G, Buonamassa S. Endoscopic surgery for pineal region tumors. Minim Invasive Neurosurg. 2001;44:70–73. doi: 10.1055/s-2001-16002. [DOI] [PubMed] [Google Scholar]
- Glenn OA, Barkovich AJ. Intracranial germ cell tumors: A comprehensive review of proposed embryologic derivation. Pediatr Neurosurg. 1996;24:242–251. doi: 10.1159/000121046. [DOI] [PubMed] [Google Scholar]
- Halperin, E.C., Constine, L.S., Tarbell, N.J., and Kun, L.E (1999) Supratentorial brain tumors except ependymomas: Brain tumors in babies and very young children. In Pediatric Radiation Oncology, 3rd Ed., Chapter 3. Lippincott Williams & Wilkins, Philadelphia, pp. 67–68.
- Hardenbergh PH, Golden J, Billet A, Scott RM, Shrieve DC, Silver B, Loeffler JS, Tarbell NJ. Intracranial germinoma: The case for lower dose radiation therapy. Int J Radiat Oncol Biol Phys. 1997;39:419–426. doi: 10.1016/s0360-3016(97)00330-1. [DOI] [PubMed] [Google Scholar]
- Horowitz MB, Hall WA. Central nervous system germinomas. Arch Neurol. 1991;48:652–657. doi: 10.1001/archneur.1991.00530180110026. [DOI] [PubMed] [Google Scholar]
- Huh SJ, Shin KH, Kim IH, Ahn YC, Ha SW, Park CI. Radiotherapy of intracranial germinomas. Radiother Oncol. 1996;38:19–23. doi: 10.1016/0167-8140(95)01649-x. [DOI] [PubMed] [Google Scholar]
- Jellinger K. Primary intracranial germ cell tumours. Acta Neuropathol. 1973;25:291–306. doi: 10.1007/BF00691757. [DOI] [PubMed] [Google Scholar]
- Jenkin D, Berry M, Chan H, Greenberg M, Hendrick B, Hoffman H, Humphreys R, Sonley M, Weitzman S. Pineal region germinomas in childhood treatment considerations. Int J Radiat Oncol Biol Phys. 1990;18:541–545. doi: 10.1016/0360-3016(90)90058-r. [DOI] [PubMed] [Google Scholar]
- Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: Natural history and pathogenesis. J Neurosurg. 1985;63:155–167. doi: 10.3171/jns.1985.63.2.0155. [DOI] [PubMed] [Google Scholar]
- Kretschmar CS. Germ cell tumors of the brain in children: A review of current literature and new advances in therapy. Cancer Invest. 1997;15:187–198. doi: 10.3109/07357909709115773. [DOI] [PubMed] [Google Scholar]
- Matsutani M, Sano K, Takakura K, Fujimaki T, Nakamura O, Funata N, Seto T. Primary intracranial germ cell tumors: A clinical analysis of 153 histologically verified cases. J Neurosurg. 1997;86:446–455. doi: 10.3171/jns.1997.86.3.0446. [DOI] [PubMed] [Google Scholar]
- Mootha SL, Barkovich AJ, Grumbach MM, Edwards MS, Gitelman SE, Kaplan SL, Conte FA. Idiopathic hypothalamic diabetes insipidus, pituitary stalk thickening, and the occult intracranial germinoma in children and adolescents. J Clin Endocrinol Metab. 1997;82:1362–1367. doi: 10.1210/jcem.82.5.3955. [DOI] [PubMed] [Google Scholar]
- Robinson S, Cohen AR. The role of neuroendoscopy in the treatment of pineal region tumors. Surg Neurol. 1997;48:360–365. doi: 10.1016/s0090-3019(97)00018-9. discussion 365–367. [DOI] [PubMed] [Google Scholar]
- Sands SA, Kellie SJ, Davidow AL, Diez B, Villablanca J, Weiner HL, Pietanza MC, Balmaceda C, Finlay JL. Long-term quality of life and neuropsychologic functioning for patients with CNS germ-cell tumors: From the First International CNS Germ-Cell Tumor Study. Neuro-oncol. 2001;3:174–183. doi: 10.1093/neuonc/3.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura Y, Ikeda J, Shirato H, Tada M, Abe H. Germ cell tumours of the central nervous system: Treatment consideration based on 111 cases and their long-term clinical outcomes. Eur J Cancer. 1998a;34:104–110. doi: 10.1016/s0959-8049(97)10045-4. [DOI] [PubMed] [Google Scholar]
- Sawamura Y, Shirato H, Ikeda J, Tada M, Ishii N, Kato T, Abe H, Fujieda K. Induction chemotherapy followed by reduced-volume radiation therapy for newly diagnosed central nervous system germinoma. J Neurosurg. 1998b;88:66–72. doi: 10.3171/jns.1998.88.1.0066. [DOI] [PubMed] [Google Scholar]
- Shibamoto Y, Sasai K, Oya N, Hiraoka M. Intracranial germinoma: Radiation therapy with tumor volume-based dose selection. Radiology. 2001;218:452–456. doi: 10.1148/radiology.218.2.r01ja08452. [DOI] [PubMed] [Google Scholar]
- Sugiyama K, Uozumi T, Kiya K, Mukada K, Arita K, Kurisu K, Hotta T, Ogasawara H, Sumida M. Intracranial germ-cell tumor with synchronous lesions in the pineal and suprasellar regions: Report of six cases and review of the literature. Surg Neurol. 1992;38:114–120. doi: 10.1016/0090-3019(92)90088-5. [DOI] [PubMed] [Google Scholar]
- Wara WM, Jenkin RD, Evans A, Ertel I, Hittle R, Ortega J, Wilson CB, Hammond D. Tumors of the pineal and suprasellar region: Children’s Cancer Study Group treatment results 1960–1975: A report from Children’s Cancer Study Group. Cancer. 1979;43:698–701. doi: 10.1002/1097-0142(197902)43:2<698::aid-cncr2820430243>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Wolden SL, Wara WM, Larson DA, Prados MD, Edwards MS, Sneed PK. Radiation therapy for primary intracranial germ-cell tumors. Int J Radiat Oncol Biol Phys. 1995;32:943–949. doi: 10.1016/0360-3016(95)00067-9. [DOI] [PubMed] [Google Scholar]


