Abstract
Cell adhesion molecules of the immunoglobulin superfamily are aberrantly expressed in malignant glioma. Amongst these, the human poliovirus receptor CD155 provides a molecular target for therapeutic intervention with oncolytic poliovirus recombinants. Poliovirus has been genetically modified through insertion of regulatory sequences derived from human rhinovirus type 2 to selectively replicate within and destroy cancerous cells. Efficacious oncolysis mediated by poliovirus derivatives depends on the presence of CD155 in targeted tumors. To prepare oncolytic polioviruses for clinical application, we have developed a series of assays in high-grade malignant glioma (HGL) to characterize CD155 expression levels and susceptibility to oncolytic poliovirus recombinants. Analysis of 6 HGL cases indicates that CD155 is expressed in these tumors and in primary cell lines derived from these tumors. Upregulation of the molecular target CD155 rendered explant cultures of all studied tumors highly susceptible to a prototype oncolytic poliovirus recombinant. Our observations support the clinical application of such agents against HGL.
Human pathogenic viruses are increasingly common vehicles for the development of novel therapeutic agents against malignant glioma (Gromeier, 2001; Gromeier and Wimmer, 2001; Gromeier et al., 2000a). The first reported incidences of viral oncolysis were due to nonintended exposure to naturally occurring viruses or after administration of live attenuated vaccine strains (DePace, 1912; reviewed in Sincovics and Horvath [1993]). New prospects for genetically manipulating viruses have opened possibilities for increasing the tumor specificity and lowering the toxicity of oncolytic viral agents (reviewed in Gromeier [2002]). These efforts have given rise to oncolytic adenoviruses (Bischoff et al., 1996), herpesviruses (Martuza et al., 1991), reoviruses (Coffey et al., 1998), vesicular stomatitis virus (Stojdl et al., 2000), and most recently, polioviruses (Gromeier et al., 2000b).
The antineoplastic effects of oncolytic viruses are subject to multifaceted interactions with the host cell. The primary prerequisite for viral oncolysis is expression of cellular receptors mediating viral entry in malignant cell types. In preparation for clinical applications using oncolytic viruses, analysis of viral receptor expression in the target tissue is highly desirable in order to select tumor types and patients most likely to respond favorably to therapeutic intervention.
The critical influence of virus tropism determinants on therapeutic efficacy has recently become evident with oncolytic adenoviruses and adenovirus-derived gene delivery vehicles directed to malignant tissues. Targeting of adenoviral vectors to malignant tissues is greatly limited by low or absent expression of a critical adenovirus attachment and entry molecule, the Coxsackie adenovirus receptor (CAR3; Asaoka et al., 2000; Bergelson et al., 1997; Douglas et al., 2001; Li, D., et al., 1999; Li, Y., et al., 1999; Tomko et al., 1997). Moreover, CAR expression levels in established cell lines frequently used for pre-clinical testing of these agents did not correspond to expression in tumor tissues. The failure of adenovirus-based therapeutic agents to reach and infect the intended target due to low CAR expression has inspired efforts to alter receptor specificity of adenoviruses in order to broaden its tropism (reviewed in Curiel [1999]).
Cellular entry of poliovirus appears to be mediated by a single cell-surface molecule, the immunoglobulin superfamily (IGSF) member CD155 (Mendelsohn et al., 1989). Although a physiological function for CD155 is unknown, the protein binds specifically to the extracellular matrix component vitronectin (Lange et al., 2001). Transcriptional regulation of the CD155 gene is tightly controlled (Solecki et al., 1999, 2000). In accordance with the restricted tropism of poliovirus for anterior horn spinal cord motor neurons, activity of the CD155 promoter was restricted to the floor plate, notochord, and the anterior spinal cord (Gromeier et al., 2000a). Confirming the activity pattern of the CD155 promoter in the developing spinal cord, morphogenic factors active in the floor plate and notochord — the transcription factors sonic hedgehog (shh) and its downstream gli effectors—strongly activate the CD155 promoter and induce CD155 expression (Solecki et al., 2002).
Both shh and gli transcription factors have been implicated in the oncogenesis of neuroectodermal tumors (Goodrich et al., 1997; Kinzler et al., 1987). Thus, the role of shh and gli transcription factors in CD155 gene regulation suggested that CD155 expression may occur ectopically in neuroectodermal malignancies. TagE4, a rodent member of the nectin family recently proposed to be the true rodent CD155 homolog (Baury et al., 2001), is ectopically upregulated in colon and mammary tumors in mice (Denis, 1998). CD155 has been reported to be overexpressed in human colorectal tumors (Masson et al., 2001).
Evidence for CD155 expression in neuroectodermal tumors stems mainly from studies of neuroectodermal tumor cell lines that are susceptible to oncolytic poliovirus-based agents (Gromeier et al., 2000b). However, no systematic CD155 expression analysis has been performed on CNS tumor tissues. Determination of CD155 expression levels is notoriously difficult because of the exceedingly low expression levels, even in cell lines routinely used for the propagation of poliovirus and in confirmed sites of expression, such as human spinal cord motor neurons (Bernhardt et al., 1994). To address this problem, we have generated new polyclonal α-CD155 antibodies directed against a recombinant soluble segment of the antigen. Combining solid-phase antigen capture procedures with Western blot detection of CD155 using our new antibodies proved to be an efficient means of conducting comprehensive and reliable expression analyses in tissue samples and cell lines.
Analysis of 6 cases indicated that CD155 overexpression is commonly associated with high-grade malignant glioma (HGL). CD155 expression levels in tumor tissues corresponded to those in primary tissue cultures derived from the tumors. Furthermore, CD155 expression in primary glioma explant cultures was equivalent to that found in established glioma cell lines used in preclinical evaluations of oncolytic poliovirus recombinants. In accordance with consistent expression of CD155, primary explant cultures of glioma tissue from all 6 cases displayed exquisite sensitivity to the oncolytic poliovirus recombinant PVS-RIPO. We have thus provided evidence for aberrant expression of a molecular target for oncolytic poliovirus recombinants in HGL. Our findings demonstrate that individual tumor characterization for viral cellular receptor expression can aid in the identification of targets most likely to respond to oncolytic virus therapy.
Materials and Methods
Dissociation of Tissues Using the Cold Trypsinization Method
Dissociation of tumor tissues was carried out essentially as described by Banker and Goslin (1998). Briefly, tissue fragments (10–30-mm3 volume) obtained at craniotomy for removal of tumor were immersed in sterile phosphate-buffered saline (PBS) containing 0.25% crude trypsin (type IV [Sigma, St. Louis, Mo.]) for 6 h on ice. The tissue fragments were incubated for 5 min at 37°C, centrifuged for 30 s at 1200 rpm to collect the partly dispersed tissue material, dissociated through vigorous pipetting into growth medium containing 10% fetal bovine serum (Invitrogen, Carlsbad, Calif.), and plated out on culture dishes. Cultures were grown and propagated according to standard procedures.
Virus Infection of Primary Cultures
Growth medium was collected from cultures prior to infection and passed through a 0.45-μm syringe filter. Cultures grown in 60-mm dishes were treated either with 1 ml of filtered growth medium alone or with filtered growth medium containing the appropriate amount of virus. Tumor cultures were infected with PVS-RIPO at a multiplicity of infection of 10. After incubating while gently rocking for 30 min at room temperature to allow for virus attachment, the cultures were thoroughly rinsed 3 times with growth medium to remove unbound virus particles. Thereafter, 1 ml of filtered growth medium was added to the cultures, and they were transferred to a 37°C incubator. At indicated time intervals, cultures were removed from the incubator and cell morphology was documented. Viral titers in individual cultures were determined by freeze-thawing and subsequent plaque analysis from the resulting lysate according to standard procedures (Gromeier et al., 2000b). Where indicated, infected cultures were processed for Western blot analysis of viral gene products (see below).
Immunocytochemistry
Unpassaged primary explant tumor cells (DU0722) were grown on plastic chamber well slides (Labtek, Campbell, Calif.) to 80% confluence and fixed with freshly prepared 4% paraformaldehyde for 5 min at room temperature. Cells were washed in PBS and incubated with 1% bovine serum albumin in PBS for 20 min. Cells were washed in PBS and incubated in 2% goat serum for 30 min to prevent nonspecific binding of antibodies. Cells were incubated with the α-EGFRvIII antibody (L8A4; 20 μg/ml) or an isotype-matched control antibody (20 μg/ml) for 1 h. Cells were then washed in PBS and incubated with a biotin-conjugated goat α-mouse immunoglobulin G (40 μg/ml [Vector Labs, Burlingame, Calif.]) for 1 h. Cells were washed in PBS and then treated with a streptavidin/horseradish peroxidase complex (Roche, Indianapolis, Ind.) for 30 min. The cells were developed with a precipitating diaminobenzidine (DAB) substrate according to the manufacturer’s protocol (Pierce, Rockford, Ill.). The cells were counterstained with hematoxylin. The slides were air dried and mounted in Permount (Fisher Scientific, Fairlawn, N.J.).
Production of Polyclonal α-CD155 Antibodies
Polyclonal α-CD155 antibodies were raised against recombinant soluble his-tagged CD155 produced with a baculovirus expression system (BaculoGold; PharMingen, San Diego, Calif.). Briefly, co-transfection of BaculoGold DNA (containing lethal mutations preventing virus replication) with a pVL vector encoding the CD155 extracellular domains N-terminally fused to the his-tag led to recombination events producing replicating virus that expresses soluble his-tagged CD155. This product was purified according to standard affinity chromatography procedures. Purified recombinant CD155 was conjugated to keyhole limpet hemocyanin (KLH) by the glutaraldehyde method. KLH-crosslinked CD155 (0.4 mg) and Freund’s adjuvant were used to immunize 3 New Zealand rabbits following standard procedures. Four boosts with 0.15 mg of soluble recombinant CD155 were performed at 3, 6, 12, and 26 weeks following initial immunization. Serum samples obtained at appropriate intervals following immunization were evaluated by Western blot assays for reactivity against soluble recombinant CD155 and HeLa cell extracts subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Polyclonal sera used in this study were obtained 12 days following the last boost with the recombinant protein. Polyclonal sera were affinity-purified on a protein A column following established procedures (Harlow and Lane, 1988).
Antigen Capture/Western Blot Analyses of CD155
CD155 expression levels were determined by using a combined solid-phase antigen capture/Western blot procedure. Monolayer cultures of tumor cells were solubilized in PBS containing 0.5% NP40 (Sigma) and protease inhibitor cocktail (Roche). Similarly, tumor tissue was homogenized in the same buffer by Dounce homogenization. The detergent in the solubilization buffer (0.5% NP40) did not affect CD155 detection in the antigen capture/Western blot assay. The total protein concentration of cell or tissue homogenates was determined by using the Bradford assay and confirmed by subjecting aliquots of the homogenate to SDS-PAGE and silver staining (data not shown). Equivalent amounts of total solubilized protein were used for antigen capture. To this end, individual wells in ELISA plates (Falcon) were prepared by treatment with 100 μl of carbonate buffer (0.2 M sodium carbonate, 0.2 M sodium bicarbonate pH 9.4) containing 7.5 μg of affinity-purified monoclonal α-CD155 antibody D171 (Nobis et al., 1985). Cell or tissue homogenate was added to pretreated wells at the determined concentration and was incubated while gently rocking for 4 h at room temperature. The unbound homogenate was removed, and treated wells were washed three times with PBS. Bound antigen was removed by adding 50 μl of boiling sample buffer (10% glycerol, 150 mM Tris base, 150 mM Tris HCl, 2% SDS, 2 mM EDTA, pH 8.5) to each well. The sample buffer was collected from the wells, and β-mercaptoethanol (Merck, West Trenton, N.J.) was added at a final concentration of 5% before the samples were subjected to SDS-PAGE and Western blot analysis.
Samples were run on 4% to 12% bis-tris polyacrylamide gels (Invitrogen) and subjected to electrophoretic transfer onto nitrocellulose membranes following standard procedures. Membranes were blocked with 3% fat-free dry milk in Tris-buffered saline Tween-20 (100 mM Tris, 150 mM NaCl, 0.05% Tween-20) and treated with affinity-purified primary polyclonal α-CD155 antibody D480 diluted 1:2000 in Tris-buffered saline Tween-20 containing 3% dry milk for 1 h at room temperature. After 3 consecutive washes, the blots were treated with secondary biotinylated α-species antibody (Vector Labs) for 1 h. After thorough rinsing of the blots, they were treated with a streptavidin/horseradish peroxidase complex (Roche) and subsequently developed with a chemiluminescent peroxidase substrate (ECL; Pharmacia, Piscataway, N.J.).
Western Blot Analysis of Viral Gene Expression
Cell cultures in 60-mm dishes infected with PVS-RIPO and incubated for varying intervals were freeze-thawed. The cell lysate was centrifuged for 1 min at 2000 rpm to collect cell debris. The resulting pellet was dissolved in homogenization buffer (PBS, 0.5% NP40), and the total protein concentration was determined by Bradford assay. Samples containing equivalent amounts of total protein were subjected to SDS-PAGE and Western blot to determine rates of synthesis of viral polypeptides according to procedures outlined by Dufresne et al. (2002).
Results
Viral Oncolysis of Primary Glioma Cultures
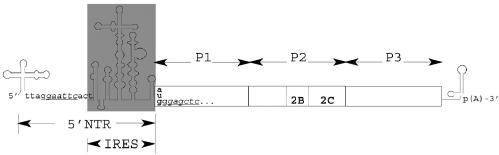
PVS-RIPO is a novel viral oncolytic agent, consisting of the genome of the live attenuated vaccine strain polio-virus type 1 (Sabin strain), containing the human rhinovirus type 2 (HRV2) internal ribosomal entry site (IRES) (Fig. 1). Tumor specificity of PVS-RIPO is based on affinity for CD155, ectopically upregulated in neuroectodermal malignancies (Gromeier et al. 2000b; Solecki et al., 2002), and on functional growth deficits of the HRV2 IRES element in normal cells of neuronal derivation (Gromeier et al., 1996, 1999).
Fig. 1.
Genetic structure of PVS-RIPO. The genome of the life-attenuated vaccine strain poliovirus type 1 (Sabin) was modified by exchange of the internal ribosomal entry site (IRES; gray box) with that of HRV2 (Gromeier et al., 1996). EcoRI (5′ terminal IRES) and SacI (5′ open reading frame, introduced through silent mutagenesis) restriction endonuclease sites were used for insertion of the foreign sequences into the PV1(S) genome. The main proteolytic cleavage products of the single polyprotein are indicated; viral gene products 2B, 2C, and 2BC are released through proteolytic cleavage of P2.
Typically, preclinical studies of viral oncolysis are limited to treatment of established glioma cell lines in tissue culture or xenotransplantation animal models. This limitation is problematic in view of the fact that expression profiles of molecular targets in established glioma cell lines may not correspond to those in actual tumors. Therefore, to study the association of the molecular target CD155 with malignant glioma, we extended our analyses conducted in established glioma cell lines (Gromeier et al., 2000b) to tumor material explanted from glioma patients. To this end, a panel of HGLs and primary, low-passage cultures derived from these tumors were subjected to analyses of CD155 expression and PVS-RIPO susceptibility.
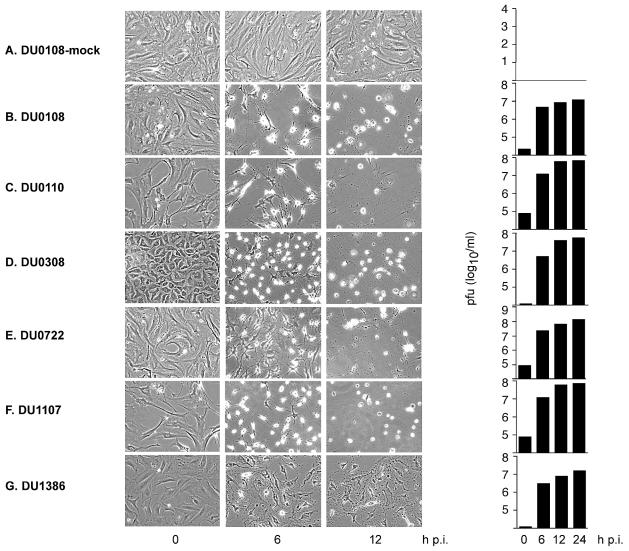
To test the oncolytic activity of PVS-RIPO in primary, low-passage glioma cultures, we obtained tissue specimens from 6 patients undergoing craniotomy for removal of a malignant glioma. Biopsies of the tumors were histologically classified as either grade III anaplastic astrocytoma or grade IV glioblastoma multiforme (Zülch et al., 1979; Table 1). All viral oncolysis experiments, assays of viral growth kinetics, immunocytochemistry analyses, and Western blot analyses of relative CD155 expression levels were performed with primary tumor cultures at their third passage (Fig. 2).
Table 1.
Histological classification and location of tumors investigated in this study*
| Tumor/Cell line no. | Location | Histological classification |
|---|---|---|
| DU0108 | L temporal | Glioblastoma multiforme, grade IV |
| DU0110 | L parietal | Anaplastic astrocytoma, grade III |
| DU0308 | R temporal | Glioblastoma multiforme, grade IV |
| DU0722 | R temporal | Glioblastoma multiforme, grade IV |
| DU1107 | R parietal | Glioblastoma multiforme, grade IV |
| DU1386 | R temporal | Anaplastic astrocytoma, grade III |
Fig. 2.
Progression of cytopathic changes in PVS-RIPO infected primary tumor cell cultures. The incubation intervals following infection are indicated below. A. Mock-infected DU0108 cells did not show signs of cytopathic change stemming from the infection procedure. Infection with PVS-RIPO uniformly produced prominent cytopathic effects and lysis of DU0108 (B), DU0110 (C), DU0308 (D), DU0722 (E), DU1107 (F), and DU1386 (G) cell cultures. In infected cultures, no intact cells could be detected 12 h p.i. with PVS-RIPO. Cultures were freeze-thawed after documentation of their morphology to determine the virus yield (plaque-forming units [pfu]) by plaque assay (right panel).
To analyze the oncolytic potential of PVS-RIPO in primary low-passage glioma cultures, we infected the panel of primary cell lines derived from tumors listed in Table 1. The infection procedure employed (see Materials and Methods) did not affect viability or morphological appearance of mock-treated cultures (Fig. 2A). All primary glioma cell lines rapidly succumbed to the cytopathic effects of PVS-RIPO infection as indicated by drastic changes in cell morphology that are characteristic for the cytopathic effect in poliovirus-infected cells and, eventually, by cell lysis (Figs. 2B–G). Extensive damage to primary glioma cultures was evident by 6 h postinfection (p.i.) and had progressed to complete lysis of the culture by 12 h p.i. (Figs. 2B–G). The rate and extent of virus-induced cytolysis in primary glioma cultures did not differ from the effects of infection on established glioma cell lines (Gromeier et al., 2000b). Also, we did not observe significant differences in susceptibility amongst the panel of tumor cell lines tested, indicating susceptibility to PVS-RIPO.
In accordance with our observations of uniform cytolysis upon infection with PVS-RIPO, we observed efficient viral propagation in all primary glioma cell lines (Fig. 2; right panel). The maximal progeny yield was reached at approximately 6 h p.i. with only minor increases thereafter. Efficient replication of PVS-RIPO in primary glioma cultures indicated that the cytopathic effects observed relate directly to virus propagation.
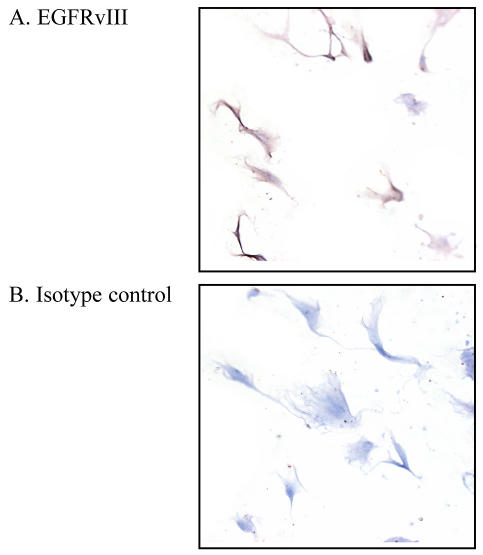
Importantly, we have documented that these primary cell lines are predominantly glioblastoma in origin. Epidermal growth factor receptor (EGFR) vIII is an EGFR mutant commonly associated with HGL (Bigner et al., 1990) and absent in normal tissue. Immunocytochemical analysis of DU0722 revealed EGFRvIII expression in this primary culture, confirming a cancerous phenotype (Fig. 3A).
Fig. 3.
Immunocytochemical analysis of EGFRvIII expression in DU0722 primary glioblastoma cells. A. DU0722 cells stained positively (brown, DAB) for EGFRvIII, a glioma-associated antigen, demonstrating that the cells are derived from the HGL, not from contaminating normal cells. B. DU0722 cells failed to stain with DAB after treatment with a nonspecific isotype-matched control primary antibody.
Viral Gene Expression in Glioma Versus Neuronal Cell Types
PVS-RIPO, following exchange of the cognate poliovirus IRES element with its counterpart from HRV2, acquired a neuronal propagation deficit documented in Sk-N-Mc neuroblastoma cells, HEK-293 cells,4 mice transgenic for the human poliovirus receptor CD155, and nonhuman primates (Gromeier et al., 1996, 1999). Cell type–specific dysfunction of the HRV2 IRES within PVS-RIPO drastically decreased the inherent neuropathogenic properties of poliovirus and prevented the appearance of clinical symptoms in infected CD155 transgenic mice (Gromeier et al., 1996) and nonhuman primates (Gromeier et al., 1999).
We tested whether the morphological changes of PVS-RIPO-infected primary glioma cell lines corresponded to rates of viral gene expression. To that end, we generated one-step growth curves of PVS-RIPO replication in primary glioma cultures and nonpermissive HEK-293 cells. At appropriate intervals, infected cultures were analyzed with regard to the viral yield and viral gene expression levels. We utilized 293 cells transformed with sheared adenovirus 5 DNA as our noncancerous neuronal model because this transformation strategy selects for neuro-blast cells in the embryonic kidney (Shaw et al., 2002).4
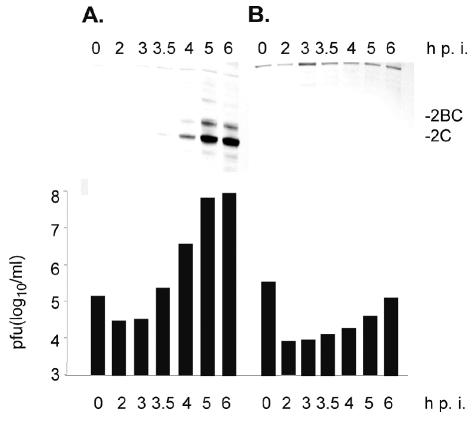
The kinetics of PVS-RIPO propagation in primary glioma cultures correspond to those observed in established glioma cell lines commonly used in preclinical investigations (Fig. 4A; Gromeier et al., 2000b). Accordingly, Western blot analysis of the expression of the viral gene product P2 and the P2 proteolytic cleavage products 2BC and 2C (see Fig. 1) revealed efficient viral protein synthesis in primary explant glioma cell lines in step with rates of viral particle production (Fig. 4A). Figure 4 shows the results of studies of primary explant glioma cell line DU0722 only, but kinetics of viral propagation and gene expression were identical in all 6 primary cell lines evaluated.
Fig. 4.
Western blot detection of poliovirus gene products 2C in DU0722 primary glioblastoma (A) and noncancerous 293 cells (B). The lower panel depicts the viral yield (plaque-forming units [pfu]) recovered from infected cultures at indicated time points.
In contrast to robust viral propagation in DU0722 cells, the noncancerous 293 cells infected with PVS-RIPO failed to exhibit viral progeny production exceeding the input or viral gene expression by 6 h p.i. (Fig. 4B). This neuronal replication deficit is due to the presence of the HRV2 IRES in PVS-RIPO (Gromeier et al., 1996, 1999), since wild-type poliovirus propagation is uninhibited in 293 cells.4
Expression Analysis of CD155 in Malignant Glioma and the Normal CNS
We analyzed CD155 expression in the panel of 6 HGLs and derived cell lines to determine whether (a) CD155 expression occurs in HGL tissues, (b) CD155 expression is affected by subcultivation of tumor cells, (c) CD155 expression levels in primary glioma cell lines are equivalent to those in established cell lines, and (d) CD155 expression levels in primary glioma cell lines correlate with PVS-RIPO susceptibility.
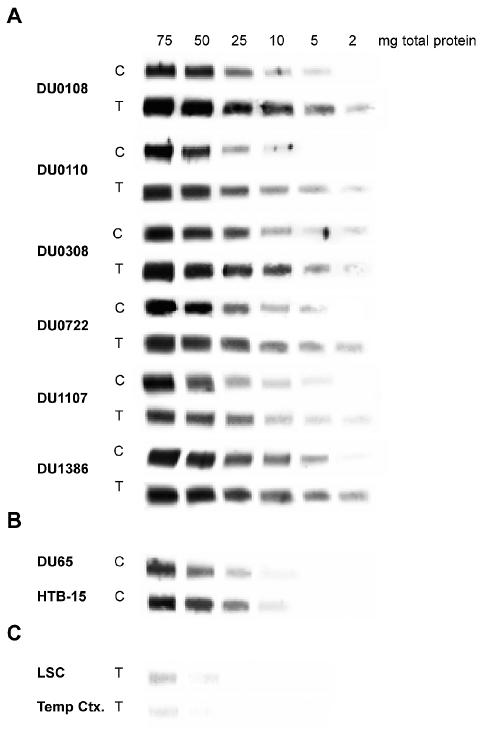
Because of the exceedingly low CD155 expression levels in tissue, conventional Western blot analysis is inconclusive (Bernhardt et al., 1994). We resolved this problem by combining solid-phase antigen capture with Western blot, permitting detection of proteins of low abundance in tissue homogenates (Fig. 5). We analyzed paired homogenates, prepared either from glioma tissue or from the derived primary cell lines for CD155 expression (Fig. 5A). Furthermore, we analyzed CD155 expression in established glioma cell lines known to be susceptible to PVS-RIPO (Fig. 5B) and in normal human CNS tissues with confirmed CD155 expression (Fig. 5C).
Fig. 5.
Combined antigen capture/Western blot analysis of CD155 expression in cell and tissue homogenates of normal and cancerous CNS tissues. A. The amount of total protein analyzed is indicated at the top of the figure. Antigen capture/Western blot assays of cell and tissue homogenates are labeled “C” and “T,” respectively. The case origin of the homogenate is indicated on the left. B. DU65 and HTB-15 are widely used established glioma cell lines. C. Tissue homogenates of lumbar spinal cord (LSC) and temporal cortex (Temp Ctx.) were analyzed in parallel with tumor tissue lysates.
All 6 primary glioma cell lines included in this study expressed CD155 at levels equivalent to established glioma cell lines (compare the cell homogenate panels [C panels] in Fig. 5A with Fig. 5B). Parallel CD155 expression profiles explain the universal susceptibility of primary glioma cell lines (see Fig. 2) and established glioma lines (Gromeier et al., 2000b) to PVS-RIPO. Most importantly, we found that CD155 expression levels in primary cell lines corresponded to those in the tumors from which they were derived (Fig. 5A, compare panels C and T). This result indicates that the universal susceptibility to PVS-RIPO of primary explant glioma cell lines may extend to the tumors from which they originate.
CD155 expression is exceedingly low in normal human CNS. Analysis of normal human lumbar spinal cord and temporal cortex homogenates revealed significantly reduced CD155 expression levels when compared with cancerous tissues (compare Figs. 5A and 5C). Resistance of human cortical cell populations to poliovirus in infected patients and low expression rates of CD155 in normal human CNS tissues suggest that lack of receptor expression on most CNS cells may contribute to their resistance to PVS-RIPO.
Discussion
The association of cell adhesion molecules (CAMs) of the IGSF with neuroectodermal malignancies is well documented. Expression of IGSF-CAMs, physiologically associated with the developing embryonic CNS, appears to be commonly deregulated in neuroectodermal tumors. Examples for this phenomenon include L1/NgCAM (Izumoto et al., 1996), NCAM (Sasaki et al., 1998), TAX-1 (Rickman et al., 2001), NrCAM/Bravo (Sehgal et al., 1998), and CD155. IGSF-CAMs, through their interactions with extracellular matrix (ECM) proteins, are believed to convey invasive properties to malignant glioma cells (reviewed in Goldbrunner et al. [1999]). Since local invasion is a standard feature of malignant glioma posing severe limits on surgical and radiological treatment regimens, the functional significance of IGSF-CAM expression in malignant glioma has been under intense investigation.
The human poliovirus receptor CD155, through a proposed interaction with the ECM protein vitronectin (Lange et al., 2001), establishes a further link between CAMs of the IGSF and the ECM in glioma. Vitronectin, physiologically expressed in the developing retina and brain, is abundant in HGL and has been reported to promote glioma cell survival and invasion (Gladson et al., 1995; Uhm et al., 1999).
The relation of CD155 with neuroectodermal malignancies renders the CD155-ligand poliovirus an ideal candidate for the generation of oncolytic viral agents targeting malignant glioma cells. CD155 is necessary and sufficient to mediate poliovirus susceptibility. Transgenic mice expressing CD155 develop classic paralytic polio-myelitis after virus infection, unlike their wild-type peers (Koike et al., 1991; Ren et al., 1990). On the basis of its pivotal role in determining poliovirus susceptibility, we can reasonably assume that CD155 expression renders malignant glioma cells vulnerable to PVS-RIPO.
Consideration for therapeutic purposes is possible because poliovirus’s inherent neuropathogenic properties can be selectively ablated without affecting cytolytic properties in malignant cell types through genetic recombination with HRV2 (Gromeier et al., 1996, 1999). Guided by this principle, we have generated a novel oncolytic virus prototype based on poliovirus that has shown strong oncolytic potential against malignant glioma cells in culture and in xenotransplantation experiments (Gromeier et al., 2000b).
Expression of cell surface markers in established tumor cell lines often does not correlate with their distribution profiles in actual tumors. This applies equally to viral receptors of the IGSF, as has become evident with the adenovirus receptor CAR (see Introduction). Correlation of expression of critical susceptibility determinants (e.g., viral receptors) in cell culture systems used in pre-clinical studies and actual tumor tissues is a priority for preparing oncolytic viruses for clinical applications.
Considering that upcoming phase 1 clinical trials of PVS-RIPO will be conducted in patients with grade III-IV malignant gliomas, we focused our study on HGLs. Since expression levels of many IGSF molecules and their ECM affinities frequently vary according to histological grade of malignant gliomas (e.g., vitronectin is most abundant in HGLs but virtually absent from low-grade tumors [Gladson et al., 1995]), comparative CD155 expression analyses in low-grade tumors would shed more light on the relationship of CD155 with neuroectodermal malignancy. Because of the difficulty of accruing a sufficient number of cases where biopsy material can be obtained for the necessary studies, the analysis of the relationship of CD155 expression with low-grade glioma will take more time.
Our expression analyses rely on tests of tissue or cell homogenates that do not permit an evaluation of CD155 distribution within a tumor. We therefore cannot exclude the possibility that tumor cells lacking CD155 expression could give rise to cell populations refractory to PVS-RIPO treatment. To evaluate the distribution and local abundance of CD155 in tumor tissues, immunohisto-chemical studies would be highly desirable. Unfortunately, we have been consistently unable to achieve unambiguous immunohistochemical staining for CD155, including in the spinal cord anterior horn, even with the use of immunological probes specially generated for this purpose. A cumbersome combined antigen capture/Western blot procedure was required to detect CD155, even by Western blot (see Materials and Methods), because of its exceedingly low expression rate in any human tissues and in cell lines (Bernhardt et al., 1994; Gromeier et al., 2000a).
Despite our inability to demonstrate CD155 expression at the single-cell level, we feel that patient screening with our assay prior to PVS-RIPO treatment constitutes a considerable advantage over therapies with oncolytic viruses administered without any information on receptor expression. We were surprised to find that, amongst established glioma cell lines, CD155 expression is ubiquitous (our tests included a vast array of commonly used cell lines [Gromeier et al., 2000b]). This situation is mirrored in glioma tissues and primary cell lines derived therefrom. While this finding greatly encourages therapeutic use of PVS-RIPO, the lack of CD155-negative cell lines deprives us of a valuable research tool for distinguishing cell-external factors (receptors) from cell-internal (cellular replication factors) determinants of virus susceptibility. We have recently reported expression analyses of CD155 in breast cancer cell lines and tissues (Ochiai et al., 2004). In contrast to malignant glioma, breast cancers as well as cell lines derived therefrom display a range of CD155 expression levels with correspondingly varying susceptibility to PVS-RIPO. Regulation of the CD155 gene by transcription factors associated with malignancy may indicate their involvement in determining expression in individual tumors (Solecki et al., 2002).
Cancer treatments directed against molecular targets specifically associated with malignancy, for example, oncolytic viral agents selectively targeting tumor cells, offer significant advantages over conventional cytostatic or radiation therapy associated with serious toxic side effects to nonmalignant tissues. To optimize treatment decisions with agents recognizing such molecular targets, tumors need to be characterized individually to verify the presence of the targeted molecule. We have developed a comprehensive series of tests, including analysis of CD155 expression levels and virus oncolysis assays on primary explant tumor cultures, that may permit prediction of malignant glioma susceptibility to prototype oncolytic poliovirus recombinants. Our study provides the first precedent for a viral attachment protein serving as a molecular target in cancer, and this work strongly supports the use of antineoplastic agents directed against the human poliovirus receptor CD155.
Footnotes
This project is supported by Public Health Service Grant CA87537 (to M.G.); the American Cancer Society; The Cleveland Clinic Foundation “Finding Cures for Glioblastoma” program; the ABC2 Foundation; NIH Grants NS20023 and CA11898; NIH Grant MO1 RR 30, General Clinical Research Centers Program, National Center for Research Resources; and NCI SPORE 1 P20 CA096890. M.G. is a recipient of a Burroughs Wellcome Fund Career Award in the Biomedical Sciences.
Abbreviations used are as follows: CAM, cell adhesion molecule; CAR, Coxsackie adenovirus receptor; DAB, diaminobenzidine; ECM, extracellular matrix; EGFR, epidermal growth factor receptor; HGL, high-grade malignant glioma; HRV2, human rhinovirus type 2; IGSF, immunoglobulin superfamily; IRES, internal ribosomal entry site; PAGE, polyacrylamide gel electrophoresis; PBS, phosphate-buffered saline; p.i., postinfection; SDS, sodium dodecyl sulfate.
Moore et al., unpublished observations (2003).
References
- Asaoka K, Tada M, Sawamura Y, Ikeda J, Abe H. Dependence of efficient adenoviral gene delivery in malignant glioma cells on the expression levels of the Coxsackievirus and adenovirus receptor. J Neurosurg. 2000;92:1002–1008. doi: 10.3171/jns.2000.92.6.1002. [DOI] [PubMed] [Google Scholar]
- Banker, G., and Goslin, K., Eds. (1998) Culturing Nerve Cells Cambridge, Mass.: MIT press.
- Baury B, Geraghty RJ, Masson D, Lustenberger P, Spear PG, Denis MG. Organization of the rat Tage4 gene and herpesvirus entry activity of the encoded protein. Gene. 2001;265:185–194. doi: 10.1016/s0378-1119(01)00343-2. [DOI] [PubMed] [Google Scholar]
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Bernhardt G, Bibb JA, Bradley J, Wimmer E. Molecular characterization of the cellular receptor for poliovirus. Virology. 1994;199:105–113. doi: 10.1006/viro.1994.1102. [DOI] [PubMed] [Google Scholar]
- Bigner SH, Humphrey PA, Wong AJ, Vogelstein B, Mark J, Friedman HS, Bigner DD. Characterization of the epidermal growth factor receptor in human glioma cell lines and xenografts. Cancer Res. 1990;50:8017–8022. [PubMed] [Google Scholar]
- Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- Curiel DT. Strategies to adapt adenoviral vectors for targeted delivery. Ann NY Acad Sci. 1999;886:158–171. doi: 10.1111/j.1749-6632.1999.tb09409.x. [DOI] [PubMed] [Google Scholar]
- Denis MG. Characterization, cloning and expression of the Tage4 gene, a member of the immunoglobulin superfamily. Int J Oncol. 1998;12:997–1005. doi: 10.3892/ijo.12.5.997. [DOI] [PubMed] [Google Scholar]
- DePace N. Sulla scomparsa di un enorme cancro vegetante del collo dell’utero senza cura chirurgica. Ginecologia. 1912;9:82–89. [Google Scholar]
- Douglas JT, Kim M, Sumerel LA, Carey DE, Curiel DT. Efficient oncolysis by a replicating adenovirus (ad) in vivo is critically dependent on tumor expression of primary ad receptors. Cancer Res. 2001;61:813–817. [PubMed] [Google Scholar]
- Dufresne AT, Dobrikova EY, Schmidt S, Gromeier M. Genetically stable picornavirus expression vectors with recombinant internal ribosomal entry sites. J Virol. 2002;76:8966–8972. doi: 10.1128/JVI.76.17.8966-8972.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladson CL, Wilcox JN, Sanders L, Gillespie GY, Cheresh DA. Cerebral microenvironment influences expression of the vitronectin gene in astrocytic tumors. J Cell Sci. 1995;108:947–956. doi: 10.1242/jcs.108.3.947. [DOI] [PubMed] [Google Scholar]
- Goldbrunner RH, Bernstein JJ, Tonn JC. Cell-extracellular matrix interaction in glioma invasion. Acta Neurochir. 1999;141:295–305. doi: 10.1007/s007010050301. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- Gromeier M. Viruses as therapeutic agents against malignant disease of the central nervous system. J Natl Cancer Inst. 2001;93:889–890. doi: 10.1093/jnci/93.12.889. [DOI] [PubMed] [Google Scholar]
- Gromeier M. Viruses for treating cancer. ASM News. 2002;68:438–445. [Google Scholar]
- Gromeier M, Wimmer E. Viruses for the treatment of malignant glioma. Curr Opin Mol Ther. 2001;3:503–508. [PubMed] [Google Scholar]
- Gromeier M, Alexander L, Wimmer E. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc Natl Acad Sci USA. 1996;93:2370–2375. doi: 10.1073/pnas.93.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromeier M, Bossert B, Arita M, Nomoto A, Wimmer E. Dual stem loops within the poliovirus internal ribosomal entry site control neurovirulence. J Virol. 1999;73:958–964. doi: 10.1128/jvi.73.2.958-964.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromeier M, Solecki D, Patel DD, Wimmer E. Expression of the human poliovirus receptor/CD155 gene during embryonic development of the central nervous system: Implications for the pathogenesis of poliomyelitis. Virology. 2000a;273:248–257. doi: 10.1006/viro.2000.0418. [DOI] [PubMed] [Google Scholar]
- Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, Wimmer E. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc Natl Acad Sci USA. 2000b;97:6803–6808. doi: 10.1073/pnas.97.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow, E., and Lane, D. (1988) Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Izumoto S, Ohnishi T, Arita N, Hiraga S, Taki T, Hayakawa T. Gene expression of neural cell adhesion molecule L1 in malignant gliomas and biological significance of L1 in glioma invasion. Cancer Res. 1996;56:1440–1444. [PubMed] [Google Scholar]
- Kinzler KW, Bigner SH, Bigner DD, Trent JM, Law ML, O’Brien SJ, Wong AJ, Vogelstein B. Identification of an amplified, highly expressed gene in a human glioma. Science. 1987;236:70–73. doi: 10.1126/science.3563490. [DOI] [PubMed] [Google Scholar]
- Koike S, Taya C, Kurata T, Abe S, Ise I, Yonekawa H, Nomoto A. Transgenic mice susceptible to poliovirus. Proc Natl Acad Sci USA. 1991;88:951–955. doi: 10.1073/pnas.88.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange R, Peng X, Wimmer E, Lipp M, Bernhardt G. The poliovirus receptor CD155 mediates cell-to-matrix contacts by specifically binding to vitronectin. Virology. 2001;285:218–227. doi: 10.1006/viro.2001.0943. [DOI] [PubMed] [Google Scholar]
- Li D, Duan L, Freimuth P, O’Malley BW., Jr Variability of adenovirus receptor density influences gene transfer efficiency and therapeutic response in head and neck cancer. Clin Cancer Res. 1999;5:4175–4181. [PubMed] [Google Scholar]
- Li Y, Pong RC, Bergelson JM, Hall MC, Sagalowsky AI, Tseng CP, Wang Z, Hsieh JT. Loss of adenoviral receptor expression in human bladder cancer cells: A potential impact on the efficacy of gene therapy. Cancer Res. 1999;59:325–330. [PubMed] [Google Scholar]
- Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- Masson D, Jarry A, Baury B, Blanchardie P, Laboisse C, Lustenberger P, Denis MG. Overexpression of the CD155 gene in human colorectal carcinoma. Gut. 2001;49:236–240. doi: 10.1136/gut.49.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn CL, Wimmer E, Racaniello VR. Cellular receptor for poliovirus: Molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Nobis P, Zibirre R, Meyer G, Kuhne J, Warnecke G, Koch G. Production of a monoclonal antibody against an epitope on HeLa cells that is the functional poliovirus binding site. J Gen Virol. 1985;66:2563–2569. doi: 10.1099/0022-1317-66-12-2563. [DOI] [PubMed] [Google Scholar]
- Ochiai H, Moore SA, Archer GE, Okamura T, Chewning TA, Marks JR, Sampson JH, Gromeier M. Treatment of intracerebral neoplasia and neoplastic meningitis with regional delivery of oncolytic recombinant poliovirus. Clin Cancer Res. 2004 doi: 10.1158/1078-0432.CCR-03-0694. In press. [DOI] [PubMed] [Google Scholar]
- Ren RB, Constantini F, Gorgacz EJ, Lee JJ, Racaniello VR. Transgenic mice expressing a human poliovirus receptor: A new model for poliomyelitis. Cell. 1990;63:353–362. doi: 10.1016/0092-8674(90)90168-e. [DOI] [PubMed] [Google Scholar]
- Rickman DS, Tyagi R, Zhu XX, Bobek MP, Song S, Blaivas M, Misek DE, Israel MA, Kurnit DM, Ross DA, Kish PE, Hanash SM. The gene for the axonal cell adhesion molecule TAX-1 is amplified and aberrantly expressed in malignant glioma. Cancer Res. 2001;61:2162–2168. [PubMed] [Google Scholar]
- Sasaki H, Yoshida K, Ikeda E, Asou H, Inaba M, Otani M, Kawase T. Expression of the neural cell adhesion molecule in astrocytic tumors: An inverse correlation with malignancy. Cancer. 1998;82:1921–1931. doi: 10.1002/(sici)1097-0142(19980515)82:10<1921::aid-cncr16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Sehgal A, Boynton AL, Young RF, Vermeulen SS, Yonemura KS, Koehler EP, Aldape HC, Simrell CR, Murphy GP. Cell adhesion molecule Nr-CAM is over-expressed in human brain tumors. Int J Cancer. 1998;76:451–458. doi: 10.1002/(sici)1097-0215(19980518)76:4<451::aid-ijc1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Shaw G, Morse S, Ararat M, Graham FL. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. 2002;16:869–871. doi: 10.1096/fj.01-0995fje. [DOI] [PubMed] [Google Scholar]
- Sincovics JG, Horvath J. New developments in the virus therapy of cancer: A historical review. Intervirology. 1993;36:193–214. doi: 10.1159/000150339. [DOI] [PubMed] [Google Scholar]
- Solecki DJ, Wimmer E, Lipp M, Bernhardt G. Identification and characterization of the cis-acting elements of the human CD155 gene core promoter. J Biol Chem. 1999;274:1791–1800. doi: 10.1074/jbc.274.3.1791. [DOI] [PubMed] [Google Scholar]
- Solecki DJ, Bernhardt G, Lipp M, Wimmer E. Identification of a nuclear respiratory factor-1 binding site within the core promoter of the human polio virus receptor/CD155 gene. J Biol Chem. 2000;275:12453–12462. doi: 10.1074/jbc.275.17.12453. [DOI] [PubMed] [Google Scholar]
- Solecki DJ, Gromeier M, Bernhardt G, Wimmer E. Expression of the human poliovirus receptor/CD155 gene is activated by sonic hedgehog. J Biol Chem. 2002;277:25697–25702. doi: 10.1074/jbc.M201378200. [DOI] [PubMed] [Google Scholar]
- Stojdl DF, Litchy B, Knowles S, Marius R, Atkins H, Sonenberg N, Bell JC. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- Tomko RP, Xu R, Philipson L. HCAR and MCAR: The human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhm JH, Dooley NP, Kyritsis AP, Rao JS, Gladson CL. Vitronectin, a glioma-derived extracellular matrix protein, protects tumor cells from apoptotic death. Clin Cancer Res. 1999;5:1587–1594. [PubMed] [Google Scholar]
- Zülch, K.J. (1979) Histological Typing of Tumours of the Central Nervous System (International Histological Classification of Tumours, no. 21). Geneva: World Health Organization, pp. 19–20.