Abstract
Donor-acquired solid organ malignancy is a rare complication of organ transplantation. We report a case of a patient who received bilateral lung transplants for pulmonary fibrosis from a donor with known glioblastoma multiforme (GBM). The lungs, heart, liver, and kidneys were harvested after a lethal intracranial bleed and accepted for transplantation by four centers. An enlarged hilar lymph node sampled at the time of transplant was found to contain GBM. Four months later, the patient developed diffuse interstitial pulmonary infiltrates with mediastinal lymphadenopathy. Lung biopsy confirmed metastatic GBM. The patient died 2 weeks after the diagnosis was established. The patient receiving the donor liver also developed GBM. We present a case study, review of the literature, and suggested interventions to minimize the risk of transmission.
Case Study
A 28-year-old male diagnosed with systemic sclerosis 12 years earlier was evaluated for bilateral lung transplant for progressive pulmonary fibrosis. The patient suffered from diffuse cutaneous systemic sclerosis with recurrent digital ulcers, myositis, and arthritis, along with his lung disease. For 3 years, he was maintained on methotrexate and prednisone for control of the myositis. Because of progressive dyspnea, he was evaluated for lung transplantation. At the time of initial evaluation, his pulmonary function studies demonstrated a severe restrictive ventilatory defect with a diffusion capacity for carbon monoxide 35% of predicted and minimal pulmonary hypertension by right heart measurements. He was placed on the transplant waiting list and during the ensuing months developed worsening dyspnea requiring high-flow oxygen.
Sixteen months later, a 29-year-old patient was identified as a donor. The donor had presented 3 years earlier with a headache and was found to have a 9- by 7-cm parietal mass. Surgery was deferred, and the patient was treated with steroids for 2 years until he developed seizures. Stereotactic brain biopsy revealed glioblastoma multiforme (GBM).2 One month after completing whole-brain radiation, he presented with a severe headache and respiratory arrest due to a large intracranial hemorrhage. The donor was evaluated by four medical centers that determined all organs were appropriate for use in transplantation according to the United Network for Organ Sharing (UNOS) criteria (Kauffman et al., 2002). The lungs, heart, kidneys and liver were harvested and transplanted into five different recipients.
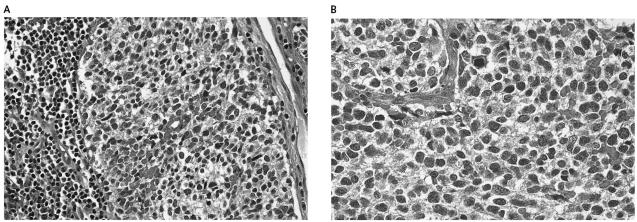
The transplant recipient underwent bilateral lung transplantation with modest difficulty because of extensive pleuroparenchymal fibrosis. He suffered a brief cardiac arrest after undergoing cardiopulmonary bypass, which resulted in bilateral parietal watershed strokes that remained subclinical. One enlarged hilar lymph node was noted while the lungs were being placed into the recipient. This was removed, and formal pathologic examination revealed poorly differentiated cells consistent with metastatic GBM of the small cell type (Fig. 1A). To rule out an oligodendroglioma, we performed fluorescent in situ hybridization for EGFR, the epidermal growth factor receptor gene, as well as for 1p, 10q and 19q, as previously described (Fuller, 2003). Fluorescent in situ hybridization studies revealed deletion of 10q and showed no evidence for either EGFR gene amplification or loss of either 1p or 19q. Baseline studies obtained postoperatively, including an MRI of the brain and CAT scans of the chest and abdomen, were negative. The patient and his family were informed of the risk of disseminated GBM, and retransplant was offered. However, given the risks of reoperative surgery and recent watershed strokes, the patient and family declined. Chemotherapy was not recommended, given its marginal efficacy in this disease and the risk of complications with concurrent immune-suppressive therapy. Radiation therapy was also not recommended, given the need for a large radiation field as well as the risks of radiation to newly transplanted lungs and the esophagus in a patient with scleroderma.
Fig. 1.
Pathology analysis. A. H&E stain of donor bronchial lymph node sampled at the time of transplantation showing GBM. B. Transbronchial biopsy of the recipient 14 weeks after transplant with identical histology. Both sections were positive for glial fibrillary acid protein.
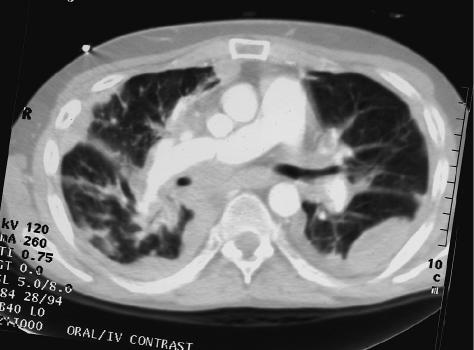
The patient’s posttransplant course was complicated by multiple hospitalizations for dyspnea. A CAT scan 3 months posttransplant revealed diffuse bilateral pulmonary infiltrates and pleural effusions. Bronchoscopic examination at that time revealed atypical cells suspicious for malignancy. There was no evidence of rejection. Two weeks later the patient had increasing dyspnea at rest and atrial arrhythmias. Repeat bronchoscopy and transbronchial biopsy revealed metastatic GBM with histologic features identical to those seen in the lymph node removed at the time of transplantation (Fig. 1B). A CAT scan revealed no extrathoracic metastases but rapid tumor progression with lymphangitic spread, as well as mediastinal, pericardial, and great vessel involvement (Fig. 2). The patient was bed bound, requiring 80% oxygen by face mask. He died one week later, four months after transpant.
Fig. 2:
Chest CAT scan 4 months after bilateral lung transplant: Pleural masses, as well as small pulmonary nodules with tumor encasement of the great vessels and central airways
Information on the other patients who received organs from the same donor was provided by their treating physicians. The patient who received the donor liver developed metastatic GBM. One of the kidney recipients had the donor kidney removed and remains on dialysis. The recipients of the heart and other kidney remain without evidence of metastases at this time.
Discussion
This case highlights the potential problems of using donors with GBM in an effort to increase the availability of solid tumor organs for transplantation. In 2002, there were more than 24,000 solid organ transplants performed, 1000 of which were lung transplants (UNOS, 2003). In recent years, the problem of lack of supply and increasing demand for these transplants and the significant shortage of donor organs has been underlined by the fact that one third of all patients awaiting solid organs, including 4000 patients for lungs, die on the waiting list because of lack of donor availability (UNOS, 2003). Furthermore, among those considered, only 7% to 22% of potential multiorgan donors are deemed suitable for lung transplantation because of trauma or other measures of fragility of the lungs in brain-dead patients (Weill, 2002). This has led to an attempt to systematically reconsider the criteria for donor suitability using large databases (Weill, 2002). Traditionally, these have included age, sputum gram stain, and history of chest trauma. In past years, to bridge this discrepancy, patients with low-grade skin cancer (basal or squamous cell), in situ malignancies, and primary brain tumors have been included. While infections and graft rejection constitute the majority of life-threatening problems for post-lung-transplant patients, malignancies account for only a small percentage, namely Epstein-Barr virus–associated posttransplant lympho-proliferative disease (PTLD) and nonmelanoma skin cancers. These malignancies are recipient derived and are related to immune-suppressive therapy.
Retrospective studies have attempted to define the risk of donor-derived malignancies. The first review of the UNOS Transplant Tumor Registry studied 14,705 cadaveric donors, of whom 257 had a past history of cancer, including 188 with a history of CNS tumors. Of a total 650 organs (397 kidneys, 178 livers, and 75 hearts) transplanted, there were no documented donor-derived malignancies recorded after a 45-month mean follow-up (Kauffman et al., 2000). A more recent update of the UNOS registry evaluated the risk of transmission from 397 donors with a history of a CNS tumor who donated 1220 organs (Kauffman et al., 2002). Again, no donor-derived malignancies were identified after a mean follow-up of 36 months. Despite these studies, since 1987 there have been at least nine known published case reports (our study being the tenth) of transmission of donor-derived CNS malignancies to at least 15 recipients (including the lung and liver noted in our case) (Bosmans et al., 1997; Colquhoun et al., 1994; Frank et al., 1998; Jonas et al., 1996; Konigsrainer et al., 1993; Lefrancois et al., 1987; Morse et al., 1990; Ruiz et al. 1993; Val-Bernal et al., 1993). Seven of the 10 cases have involved a donor with glioblastoma; the remaining cases involved 1 malignant meningioma, 1 central nervous system lymphoma, and 1 medulloblastoma. Our case is the first report of transmission through lung transplantation.
Each year in the United States, approximately 17,000 patients are diagnosed with malignant primary brain tumors, and more than 13,000 succumb to these diseases (American Cancer Society, 2002). In adults, the majority of these are gliomas, and of these, two thirds are high grade, such as anaplastic oligodendrogliomas, anaplastic astrocytomas (grade III astrocytomas), or GBM (grade IV astrocytoma). The remainder include low-grade astrocytomas (grades I–II), low-grade oligodendrogliomas, and mixed low-grade astrocytoma/oligodendroglioma. In addition, there is the rare adult medulloblastoma, germinoma, or primary CNS lymphoma. As a rule, gliomas are not cured with available therapies. Patients with low-grade gliomas may live for years but ultimately die of recurrent low-grade disease or a tumor that has transformed into an anaplastic astrocytoma or GBM. Patients with high-grade gliomas have a short survival, as typified by GBM patients, where the median survival is 9 to 12 months. A significant percentage of these patients are young and without underlying disease, which makes them excellent candidates for organ donation.
Traditionally, gliomas are considered to be confined to the CNS (Burger, 2002). Patients generally die from complications related to progression of intracranial disease, and thus clinical manifestations outside the CNS are rare and hardly ever diagnostically sought. Autopsies rarely find disseminated metastases, but no formal series have been reported. General estimates of extracranial spread have been quoted at 1% to 4% (Cerame et al., 1985). Notably, one study seeking to estimate the incidence of distant involvement evaluated 20 patients with GBM at diagnosis. All patients underwent staging bone marrow, and one half (10) also had bone scans. All studies were negative for metastatic disease (Frappaz et al., 2001).
In Tables 1 and 2, we summarize 30 reports since 1975 of malignant brain tumors with spread outside the CNS. In general, these diseases could be classified by site into two categories: locally advanced (Table 1) and distant metastasis (Table 2). While the former probably occur as a result of local extension of intracranial disease related to scalp seeding, lymph node involvement, spread after ventriculoperitoneal shunt, or surgical contamination, diseases in the second category are likely related to hematogenous spread. Nineteen patients in the reported cases had regional metastases, and distant systemic metastases occurred in at least 84 patients, with bone, lungs, and bone marrow being the most frequently mentioned sites. The majority of these were a result of GBM.
Table 1.
Reports since 1975 of regional metastasis from malignant brain tumors
| Diagnosis | Site | No. of Patients | Reference |
|---|---|---|---|
| GBM | Needle track | 1 | Aichholzer, M., et al. Minim. Invasive Neurosurg.44, 175–177, 2001. |
| GBM | Spinal drop, cervical node | 4 | Hubner, F., et al. Acta Neurochir.143, 25–29, 2001. |
| Malignant glioma | Scalp, skull, cervical node | 3 | Houston, S.C., et al. Int. J. Radiat. Oncol. Biol. Phys.48, 831–836. 2000. |
| GBM | Ascites after VP shunt | 1 | Kumar, R., et al. Pediatr. Neurosurg.31, 242–245, 1999. |
| GBM | Cervical node | 1 | Datta, C.K., et al. W. V. Med. J. 94, 276–278, 1998. |
| Anaplastic astrocytoma | Needle track | 1 | Perrin, R.G., et al. J. Neurooncol.36, 243–246, 1998. |
| Malignant glioma | Cervical node | 1 | al-Rikabi, A.C., et al. Cytopathology8, 421–427, 1997. |
| GBM | Cervical node | 2 | Wallace, C.J., et al. Am. J. Neuroradiol.17, 1929–1931, 1996. |
| GBM | Neck mass, cervical node | 2 | Vural, G., et al. Diagn. Cytopathol.15, 60–65, 1996. |
| GBM | Peritoneal seeding after VP shunt | 2 | Newton, H.B., et al. Cancer69, 2149–2153. 1992. |
| GBM | Cervical node | 1 | Gonzalez, C.R., et al. Acta Cytol.37, 938–942, 1993. |
Abbreviations: GBM, glioblastoma multiforme, VP, ventriculoperitoneal.
Table 2.
Reports since 1975 of distant metastasis from malignant brain tumors
| Diagnosis | Site | # | Reference |
|---|---|---|---|
| Oligoastrocytoma | B | 1 | Cervio, A., et al. J. Neurooncol.52, 141–148, 2001. |
| GBM | L, H, LN, skull/scalp | 1 | Hata, N., et al. No Shinkie Geka29, 433–438, 2001 (Japanese). |
| GBM | B, L, neck mass, scalp | 6 | Park, C.C., et al. J. Neuropathol. Exp. Neurol.59, 1044–1050, 2000. |
| GBM | B | 1 | Beauchesne, P., et al. J. Neurosurg. 93, 887–890, 2000. |
| GBM | Parotid gland | 1 | Waite, K.J., et al. Clin. Oncol.11, 205–207, 1999. |
| GBM | BM | 2 | Kleinschmidt-Demasters, B.K. Hum. Pathol.27, 197–201, 1996. |
| GBM | LN (Bronchial/mediastinal), L | 1 | Granjon, O., et al. Rev. Mal. Respir.12, 489–491, 1995 (French). |
| GBM | LN, L pleura | 1 | Chretien, F., et al. Arch. Anat. Cytol. Pathol.43, 342–349, 1995 (French). |
| GBM | B | 1 | Pireyre, C., et al. Rev. Rhum. Ed. Fr. 60, 827–830, 1993. |
| Low-grade astrocytoma | B | 2 | Longee, D.C., et al. Med. Pediatr. Oncol.19, 318–324, 1991. |
| GBM | B | 1 | Gamis, A.S., et al. Cancer66, 180–184, 1990. |
| GBM | B | 1 | Trattnig, S., et al. J. Comput. Assist. Tomogr.14, 294–296, 1990. |
| GBM | BM | 1 | LoRusso, P.M., et al. J. Neurooncol.6, 53–59, 1988. |
| Gliosarcoma | L | 1 | Cerame, M.A., et al. Neurosurgery17, 413–418, 1985. |
| GBM | BM, B, L | 2 | Yung, W.K., et al. Ann. Neurol.14, 581–585, 1983. |
| GBM | B, LN (supraclavicular) | 1 | Pang, D., and Ashmead, J.W. Neurosurgery10, 252–257, 1982. |
| Anaplastic astrocytoma, GBM | B, LN | 2 | Dietz, R., et al. Acta Neurochir.57, 99–105, 1981. |
| GBM | B, L, liver, LN(cervical/mediastinal) | 58 | Terheggen, H.G., and Muller, W. Eur. J. Pediatr. 124, 155–164, 1977. |
| GBM | Bone, LN (bronchial) | 1 | Hulbanni, S., and Goodman, P.A. Cancer37, 1577–1583, 1976. |
Abbreviations: B, bone; BM, bone marrow; GBM, glioblastoma multiforme; L, lung; H, heart; LN, lymph nodes.
Conclusions
While there may be little disagreement that brain tumors can spread outside the CNS an important question remains whether patients with GBM should be considered in helping bridge the need for solid organs. As is evident in this case, the implications of transmission of aggressive malignancies can be devastating in the setting of immune suppression, and multiple patients can be simultaneously affected. We believe that although large systematic studies have shown that transplanting organs from brain tumor patients is generally safe, this case calls for a second, careful, multidisciplined approach to this policy, particularly as it applies to donors with GBM. The number of reports describing the presence of extra-CNS disease is noteworthy and emphasizes the need for caution when such patients are considered. And although studies have failed to define an estimate of the risk of cancer transmission when brain tumor patients are used, this risk is real and should be considered in discussions with potential recipients during informed consent. As in our case, however, often the rapidly deteriorating condition of a potential recipient may affect clinical decisions, and risk/benefit analyses can weigh in favor of using suboptimal organs.
We conclude that careful selection is paramount if we are to continue to consider donors with primary CNS tumors to supply a lagging pool of solid organs for transplantation. Previously suggested procedures should be followed, including a careful screening history, identifying potential risk factors for dissemination, and a detailed examination of the organs at the time of harvest (Penn, 1997). If suspicious lesions are found, they should be submitted for frozen pathologic analysis and retrieval halted if results are positive for metastatic disease. However, this may not be available, and experienced clinical judgment would have to be used. In the past, the transplant community has considered the use of organs from primary brain tumor patients with history of ventriculoperitoneal shunt and stereotactic biopsies to be high risk (Fecteau et al., 1998). Additionally, patients with medulloblastoma have been excluded because of the known high incidence of extracranial disease (McComb et al., 1981). We underscore the need to have ongoing dialogue between the surgical, medical, and pathology teams in order to continue the development of safe and practical guidelines.
In 2002, 6182 deceased donors provided organs to the transplant pool. Of these, 54 were donors with CNS tumors, a total of 0.9%. While large studies have generally documented the safety of this continued practice, we would like to add a cautionary note. Although the shortage of organs is not expected to subside in the near future, there may be alternative means to minimize the risk. Given the predominance of glioblastoma among reported cases of transmission, we would propose that excluding these high-risk patients would not greatly reduce the number of organ transplant donors while minimizing the risk of transmission. In the meantime, additional studies on predictors of disease outside the CNS are needed if we are to continue considering brain tumor patients for organ donation. This would be in addition to longer and detailed follow-up of cases in existing databases.
Acknowledgments
The authors thank Drs. David Denofrio, Savant Mehta, Didier Manderblot, and Pang-Yen Fan for information regarding the status of the other recipients.
Footnotes
Abbreviations used are as follows: GBM, glioblastoma multiforme; PTLD, post-transplant lymphoproliferative disease; UNOS, United Network for Organ Sharing.
References
- American Cancer Society (2002) Cancer Facts & Figures 2002. Atlanta, Ga.: American Cancer Society. Cited May 10, 2003. Available at http://www.cancer.org/downloads/STT/CancerFacts&Figures2002TM.pdf
- Bosmans JL, Ysebaert D, De Cock AM, Hauben E, Muylle L, Schrijvers D, Van Marck E, Eyskens E, De Broe ME. Interferon-alpha and the cure of metastasis of a malignant meningioma in a kidney allograft recipient: A case report. Transplant Proc. 1997;29:838. doi: 10.1016/s0041-1345(96)00156-x. [DOI] [PubMed] [Google Scholar]
- Burger, P.C., Scheithauer, B.W., and Vogel, F.S. (2002) Surgical Pathology of the Nervous System and Its Coverings New York: Churchill Livingstone, p. 196.
- Cerame MA, Guthikonda M, Kohli CM. Extraneural metastases in gliosarcoma: A case report and review of the literature. Neurosurgery. 1985;17:413–418. doi: 10.1227/00006123-198509000-00003. [DOI] [PubMed] [Google Scholar]
- Colquhoun SD, Robert ME, Shaked A, Rosenthal JT, Millis TM, Farmer DG, Jurim O, Busuttil RW. Transmission of CNS malignancy by organ transplantation. Transplantation. 1994;57:970–974. [PubMed] [Google Scholar]
- Fecteau AH, Penn I, Hanto DW. Peritoneal metastasis of intracranial glioblastoma via a ventriculoperitoneal shunt preventing organ retrieval: Case report and review of the literature. Clin Transplant. 1998;12:348–350. [PubMed] [Google Scholar]
- Frank S, Muller J, Bonk C, Haroske G, Schackert HK, Schackert G. Transmission of glioblastoma multiforme through liver transplantation. Lancet. 1998;352:31. doi: 10.1016/S0140-6736(98)24027-X. [DOI] [PubMed] [Google Scholar]
- Frappaz D, Jouvet A, Pierre GS, Giammarile F, Guyotat J, Deruty R, Jouanneau E, Ranchere-Vince D. Lack of evidence of osteo-medullary metastases at diagnosis in patients with high grade gliomas. J Neurooncol. 2001;52:249–252. doi: 10.1023/a:1010616417169. [DOI] [PubMed] [Google Scholar]
- Fuller CE, Schmidt RE, Roth KA, Burger PC, Scheithauer BW, Banerjee R, Trinkaus K, Lytle R, Perry A. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol. 2003;62:1118–1128. doi: 10.1093/jnen/62.11.1118. [DOI] [PubMed] [Google Scholar]
- Jonas S, Bechstein WO, Lemmens HP, Neuhaus R, Thalmann U, Neuhaus P. Liver graft-transmitted glioblastoma multiforme. A case report and experience with 13 multiorgan donors suffering from primary cerebral neoplasia. Transplant Int. 1996;9:426–429. doi: 10.1007/BF00335707. [DOI] [PubMed] [Google Scholar]
- Kauffman HM, McBride HM, Delmonico FL. First report of the United Network for Organ Sharing Transplant Tumor Registry: Donors with a history of cancer. Transplantation. 2000;70:1747–1751. doi: 10.1097/00007890-200012270-00014. [DOI] [PubMed] [Google Scholar]
- Kauffman HM, McBride MA, Cherikh WS, Spain PC, Delmonico FL. Transplant tumor registry: Donors with central nervous system tumors. Transplantation. 2002;73:579–582. doi: 10.1097/00007890-200202270-00017. [DOI] [PubMed] [Google Scholar]
- Konigsrainer A, Steurer W, Schumer J, Geissler D, Mourad M, Squifflet JP, Margreiter R. Transmission of non-Hodgkin’s lymphoma through renal allografts—disastrous result of false diagnosis and inadequate information. Transplant Proc. 1993;25:3075–3076. [PubMed] [Google Scholar]
- Lefrancois N, Touraine JL, Cantarovich D, Cantarovich F, Faure JL, Dubernard JM, Dureau G, Colpart JJ, Bouvier R, Traeger J. Transmission of medulloblastoma from cadaver donor to three organ transplant recipients. Transplant Proc. 1987;19:2242. [PubMed] [Google Scholar]
- McComb JG, Davis RL, Isaacs H., Jr Extraneural metastatic medulloblastoma during childhood. Neurosurgery. 1981;9:548–551. doi: 10.1227/00006123-198111000-00010. [DOI] [PubMed] [Google Scholar]
- Morse JH, Turcotte JG, Merion RM, Campbell DA, Jr, Burtch GD, Lucey MR. Development of a malignant tumor in a liver transplant graft procured from a donor with a cerebral neoplasm. Transplantation. 1990;50:875–877. doi: 10.1097/00007890-199011000-00026. [DOI] [PubMed] [Google Scholar]
- Penn I. Overview of the problem of cancer in organ transplant recipients. Ann Transplant. 1997;2:5–6. [PubMed] [Google Scholar]
- Ruiz JC, Cotorruelo JG, Tudela V, Ullate PG, Val-Bernal F, de Francisco AL, Zubimendi JA, Prieto M, Canga E, Arias M. Transmission of glioblastoma multiforme to two kidney transplant recipients from the same donor in the absence of ventricular shunt. Transplantation. 1993;55:682–683. [PubMed] [Google Scholar]
- UNOS. United Network for Organ Sharing (2003) OPTN. The Organ Procurement and Transplantation Network (2003) Data files. Richmond, Va.: United Network for Organ Sharing. Cited May 15, 2003. Available at www.OPTN.org
- Val-Bernal F, Ruiz JC, Cotorruelo JG, Arias M. Glioblastoma multiforme of donor origin after renal transplantation: Report of a case. Hum Pathol. 1993;24:1256–1259. doi: 10.1016/0046-8177(93)90224-5. [DOI] [PubMed] [Google Scholar]
- Weill D. Donor criteria in lung transplantation: An issue revisited. Chest. 2002;121:2029–2031. doi: 10.1378/chest.121.6.2029. [DOI] [PubMed] [Google Scholar]