Abstract
Basic science and clinical investigations have demonstrated that 13-cis-retinoic acid (cRA) has activity against malignant gliomas. To assess its effectiveness in the setting of recurrent glioblastoma multiforme (GBM), we performed a retrospective analysis of the medical records and neuroimaging results of patients with recurrent GBM who were treated with cRA. The toxicity profile of cRA, response, and effect on progression-free survival from initiation of treatment were end points of our analysis. Eighty-two of 85 patients with a median age of 51 years received at least 1 full cycle of cRA. At the initiation of cRA treatment, the median Karnofsky performance score was 80. All patients had failed conventional radiotherapy. Seven patients were chemonaïve, whereas 75 patients had received some form of chemotherapy. Radiographic partial responses, minor responses, and stable disease were seen in 4%, 8%, and 34% of patients, respectively. Two patients were not assessable. Progression-free survival and overall survival after initiation of cRA were 10.0 and 24.6 weeks, respectively. Six-month progression-free survival was 19% for the entire group. Grade 3 or 4 toxicity developed in 14 patients (16%), one of whom developed pancreatitis and died. The results of this study demonstrate only modest efficacy for cRA therapy in this cohort of heavily pretreated patients with recurrent GBM. This data supports the use of cRA in such patients, but its further evaluation in larger, prospective, controlled studies with or without other noncytotoxic and cytotoxic agents may be warranted.
Although primary brain tumors represent fewer than 1.5% of all cancer cases reported in the United States each year, they are the third leading cause of cancer-related deaths in men and the fourth leading cause of cancer-related deaths in women between the ages of 15 and 54 years. Glioblastoma multiforme (GBM)2 is the most common and aggressive grade of glioma in adults (WHO grade IV). Despite advances in imaging, surgery, radiotherapy, and chemotherapy, these tumors usually recur, a fact that is responsible for the overall dismal prognosis of patients with GBM. The Central Brain Tumor Registry of the United States estimates 5-year survival to be 13.4% for GBM patients between 20 and 44 years, 2.1% for those between 45 and 64 years, and 0.3% for patients 65 years and older (CBTRUS, 2002). New treatment approaches are clearly needed to improve the outcomes for patients with recurrent GBM.
13-cis-Retinoic acid (cRA) is a synthetic analog of vitamin A, which binds to all three subtypes of retinoic acid receptors (RARα, β, and γ) and retinoid X receptors (RXRα, β, and γ). RAR and RXR are members of the nuclear steroid receptor family, bind as homodimers or heterodimers to specific DNA response elements, and influence the transcription of relevant genes. Retinoids have diverse biologic effects in malignant conditions, including regulating the synthesis of enzymes, growth factors, and binding proteins. Retinoids also modulate genomic and postgenomic expression, exert antiangiogenic effects, and interact with protein kinase-C pathways. The retinoic acid receptor and its ligand mediate trans- repression of activating-protein-1, which is a heterodimeric transcription factor composed of fos- and jun-related proteins (Benkoussa et al., 2002). Retinoids also protect the regulatory domain of protein kinase-C from modification induced by oxidant tumor promoters (Gundimeda et al., 1993). In hematologic and nonhematologic (skin and head and neck) tumors, treatment with retinoids has demonstrated some efficacy (Lippman and Meyskens, 1987; Lippman et al., 1988; Tallman, 1994).
Results from in vitro studies of retinoids demonstrating growth-inhibitory effects and differentiation in neuroblastoma and glioma cells support the use of retinoids for the treatment of malignant brain tumors. The underlying mechanism of growth inhibition in glioma cells may be partially mediated by a decreased affinity for binding between ligand epidermal growth factor and epidermal growth factor receptor (Yung et al., 1989). Hepatocyte growth factor/scatter factor and its receptor, MET (a transmembrane tyrosine kinase), are expressed at abnormally high levels in various human malignant gliomas and exert a strong proliferative action in an autocrine fashion (Lamszus et al., 1999). Retinoids, acting via the RARs and RXRs, can inhibit the secretion and expression of hepatocyte growth factor (Chattopadhyay et al., 2001), which could be another pathway for the antitumor effects of retinoids. In gliomas, migratory and invasive behavior could be antagonized by retinoids by inhibiting the expression of tenascin-C, an extracellular matrix protein that promotes growth, invasion, and angiogenic activity (Alvarez-Dolado et al., 1999). Retinoic acid induces an increase in the mRNA and protein levels of p55 tumor necrosis factor α receptor, which is involved in apoptotic signaling triggered by tumor necrosis factor α(Chambaut-Guerin et al., 2000). It is also possible that antitumor immunity is modulated by the increased expression of intercellular cell adhesion molecule-1 mRNA and protein levels in glioma cells treated with retinoic acid (Bouillon et al., 1991). A phase 2 pilot study of cRA given to patients with progressive or recurrent glioma after failing radiation and conventional chemotherapy demonstrated encouraging results (Yung et al., 1996). This panoply of molecular activity and promising experimental results led to our review of the treatment efficacy and toxicity profile for patients with recurrent GBM treated on a practice protocol with cRA in The University of Texas M.D. Anderson Cancer Center between July 1991 and March 1999.
Materials and Methods
Study eligibility required (1) a prior diagnosis of supra-tentorial GBM (histologically proven) that had recurred or progressed despite radiation therapy and (2) treatment with cRA as a monotherapy for recurrent or progressive disease. Patients were defined as having progression when they developed a new focus of tumor or when their existing tumor increased in size by 25% in bidimensional area when measured on contrast-enhanced axial imaging scans. Patients must have been on stable or increasing doses of steroids. Patients were treated at least 2 weeks following the completion of radiotherapy and at appropriate intervals following cytotoxic chemotherapy and at least 1 week after completing noncytotoxic chemotherapy. All patients were treated at M.D. Anderson in Houston, Texas, all were at least 18 years old, and all signed an informed consent for treatment with cRA. The first 15 patients were enrolled under an Institutional Review Board–approved, phase 2 protocol, the results of which have been previously reported (Yung et al., 1996). The remainder of the patients we report here were treated with the same treatment regimen, but were not enrolled into the formal phase 2 protocol. Patients who were treated after the protocol was closed signed generic Institutional Review Board consent forms.
Treatment
cRA was administered orally as Accutane (Roche Pharmaceuticals, Nutley, N.J.) at a dose of 100 mg/m2 daily in divided doses for 21 consecutive days, followed by 7 drug-free days. Of the first three patients, one was treated with 60 mg/m2/day, and two were treated with 80 mg/m2/day in divided doses for 21 consecutive days, followed by 7 drug-free days. The remainder of the patients were treated at 100 mg/m2/day. Doses were rounded down rather than up, depending on body surface area and available Accutane capsule sizes. The 21-days-on and 7-days-off regimen constitutes 1 course. The regimen was repeated until treatment failure or the development of significant toxicity. Treatment failure was defined as progressive disease (PD; see below) or death. Medications were used as needed to control hypercholesterolemia and hypertriglyceridemia.
Evaluation and Outcome Measures
A determination of each patient’s response was made every 8 weeks by using neurologic assessment and MRI of the brain with and without gadolinium. Each patient’s best response is reported here. Response to therapy was assessed by radiographic criteria and extent of corticosteroid use. Complete response was defined as disappearance of all enhancing tumor on the contrast-enhanced MRI scan, with the patient on a stable or decreased dose of corticosteroids. Partial response (PR) referred to at least a 50% reduction in the size (the product of the greatest perpendicular diameters) of enhancing tumor, with the patient receiving a stable or reduced dosage of corticosteroids. Minor response (MR) consisted of a decrease in enhancing tumor size (the product of the greatest perpendicular diameters) by less than 50% with the patient on a stable or decreasing steroid dosage. We defined PD as at least a 25% increase in the size (the product of the greatest perpendicular diameters) of enhancing tumor or any new tumor, as assessed by MRI, with the patient receiving a stable or increased dosage of corticosteroids. Stable disease (SD) is any condition other than complete response, PR, MR, or PD.
Laboratory tests, including complete blood count, serum electrolytes, serum amylase, liver function, and serum cholesterol and triglyceride levels, were assessed every 8 weeks or at closer intervals depending on results. Toxicity was graded according to National Cancer Institute Common Toxicity Criteria (version 2.0).
All patients were analyzed for response, progression-free survival (PFS), overall survival (OS), and response. Patients were divided according to the prognostic factors of age, Karnofsky performance score, and number of prior chemotherapies. Fisher’s exact test was used for comparing the response (PR + MR) between the patient prognostic groups. Kaplan-Meier estimates were calculated for PFS at 6 months and OS at 12 months after initiation of cRA. Hazard ratios for progression and death were computed for the patient groups by using the Cox proportional hazards regression model.
Results
Patient Characteristics
Eighty-five patients (53 men and 32 women) with recurrent GBM were treated with cRA. Fifteen of the 85 patients were reported previously as part of a phase 2 study of malignant gliomas (Yung et al., 1996). All patients were assessed for toxicity. Three of the patients from the initial 15-patient cohort had received a lower dose of cRA (60–80 mg/m2/day) and are included in this review. The median number of 4-week cycles of cRA administered was 2 (range, 0.125–34). Eighty-two patients received at least 1 full cycle (4 weeks) of cRA. Three patients failed to complete 1 cycle of treatment because of rapid neurologic deterioration. Medical records of all patients were available for review. At initial presentation, 32 patients underwent gross total resection, 47 underwent subtotal resection, and 6 had a biopsy. Following the first surgery, all patients received conventional radiotherapy, which was administered at a median dose of 60.0 Gy. Table 1 contains additional patient demographic and treatment information.
Table 1.
Patient demographic and treatment data
| Patient Characteristics | Value |
|---|---|
| Age, years | |
| Median [S.D.] | 51 [13.5] |
| Range | 19–90 |
| Male:Female ratio | 53:32 |
| KPS at beginning of therapy, n (%) | |
| Median [S.D.] | 80 [13.5] |
| 100 | 8 (9) |
| 90 | 22 (26) |
| 80 | 20 (24) |
| 70 | 18 (21) |
| 60 | 15 (18) |
| 50 | 1 (1) |
| 40 | 1 (1) |
| Extent of original surgery, n (%) | |
| Gross total resection | 32 (38) |
| Subtotal resection | 47 (55) |
| Biopsy | 6 (7) |
| Radiotherapy dose (Gy) | |
| Median | 60 |
| Range | 35–65.25 |
| Additional surgery prior to cRA, n (%) | 52 (61) |
| Within 4 weeks of cRA | 14 (16) |
| Gross total resection | 9 (11) |
| Subtotal resection | 5 (6) |
| cRA as first chemotherapy, n (%) | 8 (9) |
| Failed 1 prior chemotherapy | 45 (53) |
| Failed 2 or more prior regimens | 32 (38) |
| Received prior nitrosourea | 64 (75) |
| Time to tumor recurrence from diagnosis, weeks | |
| Median [S.D.] | 47 [53.5] |
| Range | 9–378 |
Toxicity
Grade 3 or 4 toxicity developed in 14 patients (16.5%), including 5 patients with hypercholesterolemia or hyper-triglyceridemia (1 died of acute pancreatitis), 2 with rash, 7 with neutropenia or leukopenia, and 1 with retinal changes.
Outcomes
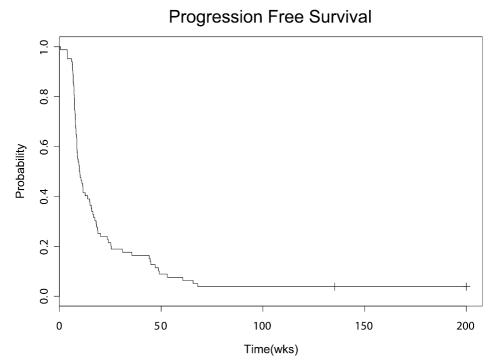
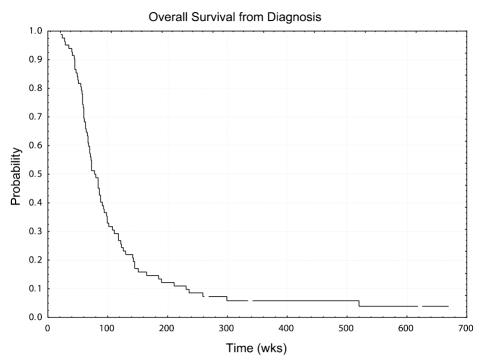
There were 3 patients (4%) with radiographic PR, 7 patients (8%) with MR, and 29 patients (34%) with SD. There were no patients with a complete response. Information from 2 patients was not adequate for evaluation. Median PFS and OS for all patients after initiation of cRA were 10.0 weeks and 24.6 weeks, respectively. Six-month PFS and 12-month OS after initiation of cRA for the entire group were 0.19 (95% CI, 0.12 – 0.30) and 0.21 (95% CI, 0.14–0.32), respectively. Median overall survival for all patients reported is 78 weeks with a range of 22 to 672 weeks, with four patients being censored. Figures 1 and 2 represent the Kaplan-Meier survival curves of patients reported in this study. For the 3 patients with PR, time to response from initiation of cRA was 8, 9, and 29 weeks; duration of response was 66 and 53 weeks in 2 patients; the third patient remained free of disease progression at 510 weeks of follow-up. Table 2 shows the characteristics and outcomes for the 10 patients with PR or MR. Of these 10 patients, 3 (1, 4, and 6) had histological evidence of recurrent tumor in addition to MRI evidence.
Fig. 1.
Kaplan-Meier curve for progression-free survival of patients with recurrent GBM from initiation of cRA therapy
Fig 2.
Kaplan-Meier curve for overall survival of patients with recurrent GBM from diagnosis
Table 2.
Characteristics of 10 patients with recurrent GBM with partial or minor responses to cRA
| No. | Age (years) | Gender | Number of prior chemotherapies | Number of cycles of cRA administered | Response | PFS (weeks) |
|---|---|---|---|---|---|---|
| 1 | 57 | F | 1 | 15 | PR | 65.7 |
| 2 | 42 | F | 2 | 6 | PR | 53.0 |
| 3 | 35 | M | 1 | 10 | PR | No progression at 414 weeks of follow-up |
| 4 | 42 | M | 1 | 16 | MR | 68.0 |
| 5 | 56 | M | 4 | 11 | MR | 47.1 |
| 6 | 47 | M | 3 | 34 | MR | No progression at 135.3 weeks of follow-up |
| 7 | 43 | F | 1 | 5 | MR | 14.9 |
| 8 | 61 | M | 1 | 6 | MR | 24.0 |
| 9 | 50 | M | 1 | 12 | MR | 49.1 |
| 10 | 48 | F | 1 | 12 | MR | No progression at 510.9 weeks of follow-up |
Abbreviations: cRA, 13-cis-retinoic acid; GBM, glioblastoma multiforme; MR, minor response; PFS, progression-free survival; PR, partial response.
Prognostic Factors
Patients were divided into prognostic groups according to their age (<50, ≥50), Karnofsky performance score (80–100, <80) and number of prior chemotherapies before initiation of cRA (<2 ≥2). Age and KPS cutoffs of 50 years and 80, respectively, were chosen, as these were close to the median value and provided an approximately even split of the patients. Table 3 summarizes the outcomes according to the prognostic groups. Similar results were obtained when these variables were treated as continuous covariates. Dichotomized results are shown to facilitate presentation. There was no significant difference in PFS between the patient groups. Poorer survival was seen in the older age group (hazard ratio 1.31; 95% CI, 1.04–1.65). There was no difference in OS between the other patient groups. There was no significant difference in the response rate between the prognostic groups.
Table 3.
Outcome in different patient prognostic groups divided according to age, KPS, and number of prior chemotherapies
| Patient group | Number of patients | Median PFS (weeks) | Hazard ratio (progression) (95% CI) | Median OS (weeks) | Hazard ratio (death) (95% CI) |
|---|---|---|---|---|---|
| Age (years) | |||||
| ≥50 | 44 | 9.6 | 1.14 (0.91–1.44) | 22.6 | 1.31 (1.01–1.65) |
| vs. | |||||
| <50 | 37 | 11.6 | 27.7 | ||
| KPS | |||||
| 80 –100 | 50 | 11.6 | 0.87 (0.69–1.09) | 27.9 | 1.10 (0.88–1.38) |
| vs. | |||||
| <80 | 33 | 8.6 | 20.1 | ||
| Number of prior chemotherapies | |||||
| ≥2 | 33 | 9.6 | 1.03 (0.82–1.30) | 24.6 | 1.04 (0.83–1.31) |
| vs. | |||||
| <2 | 50 | 10.0 | 24.6 | ||
Abbreviations: KPS, Karnofsky performance score; OS, overall survival; PFS, progression-free survival.
Discussion
Treatment of patients with recurrent GBM after radiotherapy remains unsatisfactory, and no efficacious standard of care has been established. Additional radiotherapy has a limited effect on tumor growth and is associated with a significant risk of neurologic compromise, including necrosis. Repeated surgical resection at recurrence is of limited benefit and may not be an option at all if the tumor is diffusely infiltrating or is located in eloquent brain. Conventional chemotherapy continues to demonstrate limited efficacy in recurrent GBM. The responses to cRA reported in this retrospective review (PR or MR, 12%; PR, MR, or SD, 46%) are modest, but compare well with responses from conventional cytotoxic agents used to treat patients with recurrent GBM (Friedman et al., 1999; Yung et al., 1991, 2000). Since radiation-induced enhancement can mimic progressive tumor, one caveat to consider in evaluating the cRA responders is the time period between the end of radiotherapy and the initiation of treatment with cRA. The average time from the end of radiotherapy and the initiation of treatment with cRA was 12 months in the 10 responders. Three of the 10 responders began treatment with cRA within 3 months after radiotherapy, one each at 2, 2.25, and 3 months. A significant proportion of patients in this report were heavily pretreated with chemotherapy prior to initiation of cRA: 38% of patients had prior treatment with 2 or more chemotherapeutic regimens. Notably, outcome measures were similar between patients with 1 or no prior chemotherapies and those with 2 or more prior chemotherapies. This finding differs from results in several earlier chemotherapy trials, where patients with a first recurrence of GBM demonstrated longer PFS when treated with chemotherapy than patients with second or third recurrences who received the same treatment (Fulton et al., 1996; Prados et al., 1996; Rodriguez et al., 1989; Warnick et al., 1994; Yung et al., 2000).
Previously, 15 patients were reported as part of a phase 2 study of cRA. In that report there were 2 MRs (defined as a decrease of 25%–50% in tumor size) and 6 SDs (MR/SD 53%), and median PFS and OS were 19 weeks and 43 weeks, respectively (Yung et al., 1996). Updating this experience in the treatment of 85 patients with recurrent GBM, this review demonstrates the efficacy and durability of cRA for some patients but does not confirm the better results reported in the phase 2 study, a discrepancy that could be due to the small number of patients enrolled in that study.
When considering the limited chemotherapeutic options for patients with recurrent GBM, it is crucial for the side effects from whatever treatment is selected to be mild and not significantly impair quality of life. In this respect, cRA has advantages over conventional cytotoxic agents. It is orally available and well tolerated, with side effects consisting mainly of hypercholesterolemia, hyper-triglyceridemia, and skin rash. Hyperlipidemia requires close monitoring and therapy with lipid-lowering agents as needed.
This report is encumbered by the usual limitations of a retrospective study. In addition, not all patients had a biopsy or resection to confirm tumor recurrence and rule out tumor necrosis. Quality of life was not routinely assessed in this group of patients. However, studies have shown that improvement or maintenance of health-related quality of life issues is associated with objectively assessed responses and PFS in patients with malignant gliomas (Brada et al., 2001; Osoba et al., 2000), which is a further impetus to study cRA.
In summary, this report provides additional supportive data for the use of cRA (100 mg/m2/day in 2 divided doses) in the treatment of patients with recurrent GBM who have failed radiation therapy and chemotherapy. Efficacy is demonstrated in patients despite prior heavy exposure to other chemotherapeutic regimens. Responses seen in this retrospective review are similar to those reported with conventional cytotoxic chemotherapy regimens. The side effects are tolerable, although close monitoring is required for hyperlipidemia.
Future Directions
As our understanding of the biologic pathways underlying the tumorigenesis of malignant gliomas increases, future treatments for GBM will include attempts to manipulate aberrant biologic pathways to improve patient outcomes with tolerable adverse effects. Retinoids, RARs, and RXRs play diverse roles in malignant conditions. We are hopeful that phase 2 studies combining retinoids with biologic and cytotoxic agents, as well as radiation, will uncover the potential for synergism and tolerability of multiple agents with different antitumor mechanisms. cRA is an excellent candidate for future studies.
Acknowledgment
The authors acknowledge the assistance of Sandra Ictech in gathering relevant data, Joann Aaron in editorial review, and Lei Feng with statistical analysis.
Footnotes
Abbreviations used are as follows: cRA, 13-cis-retinoic acid, isotretinoin; GBM, glioblastoma multiforme; MR, minor response; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; RAR, retinoic acid receptor; RXR, retinoid X receptor; SD, stable disease; TTP, time to progression.
References
- Alvarez-Dolado M, Gonzalez-Sancho JM, Navarro-Yubero C, Garcia-Fernandez LF, Munoz A. Retinoic acid and 1,25-dihydroxy-vitamin D3 inhibit tenascin-C expression in rat glioma C6 cells. J Neurosci Res. 1999;58:293–300. doi: 10.1002/(sici)1097-4547(19991015)58:2<293::aid-jnr9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Benkoussa M, Brand C, Delmotte MH, Formstecher P, Lefebvre P. Retinoic acid receptors inhibit AP1 activation by regulating extra-cellular signal-regulated kinase and CBP recruitment to an AP1-responsive promoter. Mol Cell Biol. 2002;22:4522–4534. doi: 10.1128/MCB.22.13.4522-4534.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillon M, Tessier P, Boulianne R, Destrempe R, Audette M. Regulation by retinoic acid of ICAM-1 expression on human tumor cell lines. Biochim Biophys Acta. 1991;1097:95–102. doi: 10.1016/0925-4439(91)90091-m. [DOI] [PubMed] [Google Scholar]
- Brada M, Hoang-Xuan K, Rampling R, Dietrich PY, Dirix LY, Macdonald D, Heimans JJ, Zonnenberg BA, Bravo-Marques JM, Henriksson R, Stupp R, Yue N, Bruner J, Dugan M, Rao S, Zaknoen S. Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol. 2001;12:259–266. doi: 10.1023/a:1008382516636. [DOI] [PubMed] [Google Scholar]
- CBTRUS. Central Brain Tumor Registry of the United States (2002–2003). Primary Brain Tumors in the United States: Statistical Report, 1995–1999 (available at http://cbtrus.org/page2t.htm).
- Chambaut-Guerin AM, Costa SL, Lefrancois T, Fages C, Gauthereau X, Tardy M. Effects of retinoic acid and tumor necrosis factor alpha on GL-15 glioblastoma cells. Neuroreport. 2000;11:389–393. doi: 10.1097/00001756-200002070-00033. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay N, Butters RR, Brown EM. Agonists of the retinoic acid- and retinoid X-receptors inhibit hepatocyte growth factor secretion and expression in U87 human astrocytoma cells. Brain Res Mol Brain Res. 2001;87:100–108. doi: 10.1016/s0165-3806(00)00154-1. [DOI] [PubMed] [Google Scholar]
- Friedman HS, Petros WP, Friedman AH, Schaaf LJ, Kerby T, Lawyer J, Parry M, Houghton PJ, Lovell S, Rasheed K, Cloughesy T, Stewart ES, Colvin OM, Provenzale JM, McLendon RE, Bigner DD, Cokgor I, Haglund M, Rich J, Ashley D, Malczyn J, Elfring GL, Miller LL. Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol. 1999;17:1516–1525. doi: 10.1200/JCO.1999.17.5.1516. [DOI] [PubMed] [Google Scholar]
- Fulton D, Urtasun R, Forsyth P. Phase II study of prolonged oral therapy with etoposide (VP16) for patients with recurrent malignant glioma. J Neurooncol. 1996;27:149–155. doi: 10.1007/BF00177478. [DOI] [PubMed] [Google Scholar]
- Gundimeda U, Hara SK, Anderson WB, Gopalakrishna R. Retinoids inhibit the oxidative modification of protein kinase C induced by oxidant tumor promoters. Arch Biochem Biophys. 1993;300:526–530. doi: 10.1006/abbi.1993.1072. [DOI] [PubMed] [Google Scholar]
- Lamszus K, Laterra J, Westphal M, Rosen EM. Scatter factor/hepatocyte growth factor (SF/HGF) content and function in human gliomas. Int J Dev Neurosci. 1999;17:517–530. doi: 10.1016/s0736-5748(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Lippman SM, Meyskens FL., Jr Treatment of advanced squamous cell carcinoma of the skin with isotretinoin. Ann Intern Med. 1987;107:499–502. doi: 10.7326/0003-4819-107-4-499. [DOI] [PubMed] [Google Scholar]
- Lippman SM, Kessler JF, Al-Sarraf M, Alberts DS, Itri LM, Mattox D, von Hoff DD, Loescher L, Meyskens FL., Jr Treatment of advanced squamous cell carcinoma of the head and neck with isotretinoin: A phase II randomized trial. Invest New Drugs. 1988;6:13–17. doi: 10.1007/BF00170781. [DOI] [PubMed] [Google Scholar]
- Osoba D, Brada M, Yung WK, Prados MD. Health-related quality of life in patients with anaplastic astrocytoma during treatment with temozolomide. Eur J Cancer. 2000;36:1788–1795. doi: 10.1016/s0959-8049(00)00165-9. [DOI] [PubMed] [Google Scholar]
- Prados MD, Schold C, Spence AM, Berger MS, McAlister LD, Mehta MP, Gilbert MR, Fulton D, Kuhn J, Lamborn K, Rector DJ, Chang SM. Phase II study of paclitaxel in patients with recurrent malignant glioma. J Clin Oncol. 1996;14:2316–2321. doi: 10.1200/JCO.1996.14.8.2316. [DOI] [PubMed] [Google Scholar]
- Rodriguez LA, Prados MD, Silver P, Levin VA. Reevaluation of procarbazine for the treatment of recurrent malignant central nervous system tumors. Cancer. 1989;64:2420–2423. doi: 10.1002/1097-0142(19891215)64:12<2420::aid-cncr2820641204>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Tallman MS. All-trans-retinoic acid in acute promyelocytic leukemia and its potential in other hematologic malignancies. Semin Hematol. 1994;31 (4 Suppl. 5):38–48. [PubMed] [Google Scholar]
- Warnick RE, Prados MD, Mack EE, Chandler KL, Doz F, Rabbitt JE, Malec MK. A phase II study of intravenous carboplatin for the treatment of recurrent gliomas. J Neurooncol. 1994;19:69–74. doi: 10.1007/BF01051050. [DOI] [PubMed] [Google Scholar]
- Yung WKA, Lotan R, Lee P, Lotan D, Steck PA. Modulation of growth and epidermal growth factor receptor activity by retinoic acid in human glioma cells. Cancer Res. 1989;49:1014–1019. [PubMed] [Google Scholar]
- Yung WKA, Mechtler L, Gleason MJ. Intravenous carboplatin for recurrent malignant glioma: A phase II study. J Clin Oncol. 1991;9:860–864. doi: 10.1200/JCO.1991.9.5.860. [DOI] [PubMed] [Google Scholar]
- Yung WK, Kyritsis AP, Gleason MJ, Levin VA. Treatment of recurrent malignant gliomas with high-dose 13-cis-retinoic acid. Clin Cancer Res. 1996;2:1931–1935. [PubMed] [Google Scholar]
- Yung WKA, Albright RE, Olson J, Fredericks R, Fink K, Prados MD, Brada M, Spence A, Hohl RJ, Shapiro W, Glantz M, Greenberg H, Selker RG, Vick NA, Rampling R, Friedman H, Phillips P, Bruner J, Yue N, Osoba D, Zaknoen S, Levin VA. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]