Abstract
Telomerase is a ribonucleoprotein complex that elongates telomeric DNA and appears to play an important role in cellular immortalization of cancers. Because telomerase is expressed in the vast majority of malignant gliomas but not in normal brain tissues, it is a logical target for glioma-specific therapy. The telomerase inhibitor GRN163, a 13-mer oligonucleotide N3′→P5′ thio-phosphoramidate (Geron Corporation, Menlo Park, Calif.), is complementary to the template region of the human telomerase RNA subunit hTR. When athymic mice bearing U-251 MG human brain tumor xenografts in their flanks were treated intratumorally with GRN163, a significant growth delay in tumor size was observed (P < 0.01 in all groups) as compared to the tumor size in mice receiving a mismatched oligonucleotide or the carrier alone. We also investigated biodistribution of the drug in vivo in an intracerebral rat brain-tumor model. Fluorescein-labeled GRN163 was loaded into an osmotic minipump and infused directly into U-251 MG brain tumors over 7 days. Examination of the brains revealed that GRN163 was present in tumor cells at all time points studied. When GRN163 was infused into intracerebral U-251 MG tumors shortly after their implantation, it prevented their establishment and growth. Lastly, when rats with larger intracerebral tumors were treated with the inhibitor, GRN163 increased animal survival times. Our results demonstrate that the antitelomerase agent GRN163 inhibits growth of glioblastoma in vivo, exhibits favorable intracerebral tumor uptake properties, and prevents the growth of intracerebral tumors. These findings support further development of this compound as a potential anticancer agent.
Malignant gliomas, including anaplastic astrocytoma and glioblastoma, are the most common malignant primary brain tumors in adults. Patients with these tumors typically undergo surgery followed by radiation therapy and chemotherapy (Levin et al., 1990; Recht et al., 1990). Despite aggressive therapy, patients with glioblastoma survive only about one year after diagnosis (Burger et al., 1990; Miller et al., 1990; Shibamoto et al., 1990), and patients with anaplastic astrocytoma usually survive only a few years (Burger et al., 1990). Clearly, there is a great need for effective therapies that will preferentially target these tumors and spare normal tissues.
Telomerase is a specialized ribonucleoprotein polymerase that contains the integral RNA component hTR with a short template segment that directs the synthesis of telomeric repeats at the ends of chromosomes (Greider and Blackburn, 1985; Yu et al., 1990). Human chromosomes terminate with several kilobases of the simple telomere hexanucleotide repeat d(TTAGGG)n. Telomerase elongates telomeric DNA by these repeats and plays an important role in cellular immortalization. The vast majority of normal somatic cells either do not express telomerase activity or express it at very low levels. Therefore, gradual telomeric shortening occurs at each cell division, and this eventually leads to death of the cell. In contrast, telomerase is expressed and functionally active in a wide variety of human tumors, including bladder, breast, prostate, and head and neck tumors (Hiyama et al., 1995; Kim et al., 1994; Lin et al., 1996; Mao et al., 1996; Sommerfeld et al., 1996; Sugino et al., 1996). Thus, cancer cell DNA is continuously extended or maintained by telomerase to compensate for the loss of telomeric repeats (Counter et al., 1992), and as a result the cells become immortalized.
Telomerase is also expressed in the vast majority of primary brain tumors but not in normal brain tissues (Chong et al., 1998; Langford et al., 1995; Le et al., 1998; Mori et al., 1997; Sano et al., 1998). For example, Le et al. (1998) found telomerase activity in 89% of glioblastomas and 45% of anaplastic astrocytomas, but it was absent in normal brain tissues. Therefore, inhibition of telomerase provides a therapeutic strategy for selectively targeting malignant gliomas and sparing normal brain tissue. It is important to note that a group of gliomas with exceptionally long telomeres, >17 kb, have been identified (Hakin-Smith et al., 2003). These tumors are thought to maintain their telomeres by a mechanism termed alternative lengthening of telomeres, and these tumors can be either telomerase positive or telomerase negative. Thus telomere length, by itself, is not a definitive indication of telomerase activity.
The 13-mer oligonucleotide N3′→ P5′ thio-phosphoramidate GRN163 (Geron Corporation, Menlo Park, Calif.), which binds to the telomerase RNA subunit hTR at the template region (Gryaznov et al., 2001; Herbert et al., 2002), has shown potent inhibitory activity against human telomerase in several biochemical assays. In vitro, telomerase inhibition by GRN163 induces cellular senescence and apoptosis in several human tumor cell lines, and these effects are accompanied by telomere shortening (Herbert et al., 2002). We report experimental evidence showing that GRN163 inhibited the growth of s.c. human U-251 MG glioblastoma xenografts in mice. The compound also exhibited favorable i.c. uptake and biodistribution properties, prevented the growth of U-251 MG cells in rats, and increased the survival times of rats bearing i.c. tumors.
Materials and Methods
Cell Cultures
In our laboratory, we routinely use both U-251 MG and U-87 MG human glioblastoma cells because they are well characterized and they form both i.c. and s.c. tumors in athymic rodents (Ozawa et al., 1998, 2002). Both cell lines have relatively short telomeres; U-251 MG cells have ≈3.9 kilobases, and U-87 MG cells have ≈3.3 kilobases. However, the relative telomerase activity is approximately 13-fold higher in U-251 MG cells than in U-87 MG cells. Therefore, we chose to study U-251 MG cells for these studies.
The human glioblastoma cell line U-251 MG was obtained from the Department of Neurological Surgery Tissue Bank at the University of California San Francisco (UCSF).3 Cells were maintained as exponentially growing monolayers in complete minimal essential medium (CMEM) consisting of Eagle’s minimal essential medium supplemented with 10% fetal calf serum and nonessential amino acids. Cells were cultured at 37°C in a humidified atmosphere containing 95% air and 5% CO2.
Oligonucleotides
GRN163 is complementary to a part of the template region of hTR and has the nucleotide sequence 5′-TAGGGTTA-GACAA-3′. The mismatch control thio-phosphoramidate oligonucleotide (MM Control) has the same nucleotide composition and the sequence 5′-TAGGTGTAAGCAA-3′, in which the 4 mismatched nucleotides are underlined (Gryaznov et al., 2001; Herbert et al., 2002). The thio-phosphoramidate oligonucleotides were prepared as described previously (Pongracz and Gryaznov, 1999). Fluorescein-labeled GRN163 was prepared according to the published procedure (Gryaznov and Letsinger, 1992).
For tumor-distribution studies, stock solutions of GRN163 were made that contained 500 nmol of GRN163 per milliliter of phosphate-buffered saline (PBS), and a stock solution of 5000 nmol of GRN163 per milliliter of PBS was used for the i.c. tumor-prevention and efficacy studies.
In Vitro Telomerase-Activity Inhibition Study
Cells (2.5 × 105) in log-phase growth were seeded into T-25 plastic flasks containing CMEM, and 24 h later GRN163 or MM Control was added to final concentrations of 1, 4, or 7 μM. Cells were exposed to the oligonucleotides for 3 days at 37°C and then were trypsinized, counted, and washed twice with PBS (Ca2+, Mg2+ free). After the cells were washed with PBS, cell lysates were prepared with lysis buffer consisting of 10 mM Tris-HCl, pH 7.5, 1 mM MgCl2, 1 mM ethylene glycol tetraacetic acid, 0.1 mM benzamide, 5 mM β-mercaptoethanol, 0.5% 3-[(3-cholamidopropyl) dimethylammonio]-1-propane sulfonate (CHAPS), and 10% glycerol. The CHAPS extracts with equivalent protein concentrations were prepared and evaluated with the telomeric repeat amplification protocol assay to measure telomerase activity (Kim et al., 1994).
Animals
Five-week-old female athymic mice (nu/nu genotype, BALB/c background) were purchased from Simonsen Laboratories (Gilroy, Calif.), and 6- to 8-week-old male athymic rats (rnu/rnu, homozygous) were purchased from Harlan (Indianapolis, Ind.). Animals were housed under aseptic conditions, which included filtered air and sterilized food, water, bedding, and cages. The UCSF Institutional Animal Care and Use Committee approved all animal protocols.
Tumor-Cell Implantation into Flanks of Athymic Mice
Implantation of tumor cells into the flanks of athymic mice was done as previously described (Ozawa et al., 1998). Mice were anesthetized by isoflurane inhalation, and 5 × 106 U-251 MG cells in 0.1 ml of CMEM were injected s.c. into the right flank. Tumor size was measured periodically with calipers, and tumor volume (V) was calculated as V = (L × W2) × π/6, in which L is the greatest length (mm) and W is the width of a perpendicular axis.
Guide-Screw Method for Implanting Intracerebral Tumors in Athymic Rats
Implantation of tumor cells into the brains of athymic rats was done as previously described (Ozawa et al., 2002). Rats were anesthetized with intraperitoneal injections of 60 mg/kg of ketamine hydrochloride and 7.5 mg/kg of xylazine hydrochloride and were positioned in a stereotaxic device with ear bars. The scalp was cleaned with 2% chlorhexidine, and a skin incision approximately 15 mm in length was made electrosurgically over the middle frontal bone. The surface of the skull was exposed so that a small hole could be drilled with a Number 6 round dental burr. A hole through the skull was drilled 3.0 mm to the right of the midline and just behind the bregma. The dura was pierced with a sharp 25-gauge needle, and a Teflon screw with a hole drilled through its center was screwed into the skull hole. Animals were removed from the stereotaxic device, and a blunt 25-gauge needle attached to a Hamilton syringe (Hamilton Co., Reno, Nev.) was inserted into the hole in the screw. The needle had a metal sleeve that limits the depth of injection to 4.0 to 4.5 mm from the bottom of the skull. U-251 MG cells (2 × 106) in 10 μl Hanks’ balanced salt solution were injected very slowly (over approximately 1 min) by free hand, and then the needle was removed. The skull and screw were swabbed with hydrogen peroxide before the screw hole was sealed with bone wax to prevent reflux. The scalp was closed with surgical staples.
Treatment of Flank Tumors in Athymic Mice with GRN163
Treatment was initiated when tumors reached approximate volumes of 50, 85, or 135 mm3. Tumor-bearing mice were anesthetized with isoflurane, the skin overlying the tumors was disinfected with 70% alcohol, and 3 nmol GRN163 in a PBS/Lipofectamine (Life Technologies, Gaithersburg, Md.) solution was injected into the tumor. The PBS/Lipofectamine solution was prepared by adding 6 μl of GRN163 stock solution to 1 μl Lipofectamine and 13 μl PBS (Mukai et al., 2000). Control animals were similarly injected with MM Control prepared in the same PBS/Lipofectamine solution. Other control animals were injected either with PBS alone, or with only the PBS/Lipofectamine solution. All solutions were administered by direct injection into the tumor mass with a Hamilton syringe with a sharp 25-gauge needle. Three independent experiments were conducted. In the first experiment, mice with an average tumor size of approximately 85 mm3 were treated 3 times the first week (Monday, Wednesday, and Friday) and once the following Monday with 3 nmol GRN163, 3 nmol MM Control, or one of the control solutions (4 mice received PBS, 4 mice received PBS and Lipofectamine, 5 mice received MM Control, and 6 mice received GRN163). In the second (beginning on day 17 after tumor implantation, tumor size ≈ 135 mm3) and third (beginning on day 10 after tumor implantation, tumor size ≈ 50 mm3) experiments, mice were treated 3 times a week for 3 weeks with 3 nmol GRN163 or MM Control (in 7 μl per injection, 9 injections total). (In the second experiment, 4 mice received PBS, 4 mice received PBS and Lipofectamine, 4 mice received MM Control, and 9 mice received GRN163. In the third experiment, 8 mice received MM Control, and 8 mice received GRN163.) We reduced the volume of injection from 20 μl to 7 μl in the second and third experiments because there was minor reflux of the drug solution in the first experiment. The PBS/Lipofectamine solution contained 6 μl PBS and 1 μl Lipofectamine. The second and third experiments were fully blinded. All mice were monitored at least twice a week, and tumor volumes were measured until they reached approximately 2000 mm3 or until significant skin breakdown or tumor ulceration occurred, at which time mice were euthanized by cervical dislocation.
Distribution Study of GRN163 in Athymic Rats
Eight rats were used for the distribution study. On day 18 after implantation of U-251 MG cells, when the i.c. tumors were approximately 20 mg as determined from earlier growth studies (Ozawa et al., 2002), 100 μl of 3′-fluorescein isothiocyanate (FITC)–labeled GRN163 (50 nmol/pump) was loaded into Alzet osmotic minipumps (Durect Corporation, Cupertino, Calif.), and the compound was infused into the tumors with a flow rate of 0.5 μl/h over a 7-day period. We did not include Lipofectamine in the solution because in early studies we noted that a precipitate formed in solutions containing Lipofectamine within a few hours, and we wanted to prevent this from occurring during the 7-day delivery period required by the minipumps. The minipump was implanted s.c. into the midscapular region of the rats, and a catheter connected the minipump to a brain infusion cannula that was inserted into the center of the U-251 MG cell–derived tumors. After 7 days of infusion, 2 rats each were euthanized at 0, 1, 3, and 4 days after treatment, and their brains were dissected and frozen in ethanol and dry ice. Brains were sectioned axially, and 10-μm-thick sections were placed on microscope slides. Mounting medium (10–15 μl) containing 2 μg/ml of 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI, Boehringer Mannheim Biochemica, Mannheim, Germany) was added over the sections, and a cover glass was placed over the samples. The resulting slides were immediately viewed and photographed with a fluorescent microscope (Carl Zeiss MicroImaging, Thornwood, N.Y.). An ultraviolet-light transmission filter was used for DAPI to visualize the nuclei of cells. A blue-light transmission filter was used for FITC to visualize the green signal that indicates cells containing oligonucleotides.
Prevention of Intracerebral Tumors in Athymic Rats by GRN163
One to two hours after i.c. implantation of U-251 MG cells, 100 μl of PBS containing 500 nmol of GRN163 or a control solution of PBS alone was infused into the original site of the cell injection by using an Alzet osmotic minipump at a flow rate of either 0.5 μl/h over 7 days or 0.25 μl/h over 14 days. Two rats received PBS and 3 rats received GRN163 on the 7-day regimen; 2 rats received PBS and 4 rats received GRN163 on the 14-day regimen. As above, Lipofectamine was not included in these solutions. Rats were monitored every day and were euthanized when animals exhibited neurological symptoms indicative of impending death (Ozawa et al., 2002). After euthanasia, brains were collected and examined for the presence of i.c. tumor.
Treatment of Intracerebral Tumors in Athymic Rats with GRN163
Fourteen days after i.c. implantation of U-251 MG cells, 100 μl of PBS containing 500 nmol of the MM Control or 150 or 500 nmol of GRN163 was infused directly into the tumor site by using Alzet osmotic minipumps at a flow rate of 0.5 μl/h over 7 days. Four rats received MM Control, 8 rats received 150 nmol GRN163, and 8 rats received 500 nmol GRN163. No Lipofectamine was added to these solutions. Rats were monitored every day and were euthanized when they exhibited neurological symptoms indicative of impending death (Ozawa et al., 2002). Brains were collected and examined for the presence of i.c. tumor.
Statistical Analysis
For all tumor studies, tests for differences in treatment effect were based on differences in time from tumor implantation to euthanasia between the treatment groups. For the in vivo flank-tumor studies, a Wilcoxon rank sum test was used to compare these times for the GRN163 group with those for each of the control groups in the specified experiment. Because the requirement was that the GRN163 group perform better, in terms of inhibited tumor growth and improved animal survival, than all control groups, no adjustment was made for multiple comparisons. For the comparison of i.c. tumors, we tested whether increasing the dose of GRN163 (from 0 to 150 to 500 nmol) tended to increase the lifespan of the rats. This was done by using an exact Jonckheere Terpstra test (Hollander and Wolf, 1973), which is conducted by ranking the survival times from shortest to longest and then testing whether the times tend to be longer in the treatment groups receiving the higher doses. To minimize the use of animals, we used fewer animals in the control groups of some experiments. Nonetheless, the unequal number of animals in this preplanned design does not invalidate the statistical analysis.
Results
In Vitro Telomerase-Activity Inhibition Study
In U-251 MG cell cultures, GRN163 showed a dose-dependent inhibition of telomerase activity; exposure of cells to 1, 4, or 7 μM of GRN163 for 3 days inhibited telomerase activity by 18%, 48%, and 68%, respectively, relative to the activity seen in untreated U-251 MG cells.
Treatment of Flank Tumors in Athymic Mice with GRN163
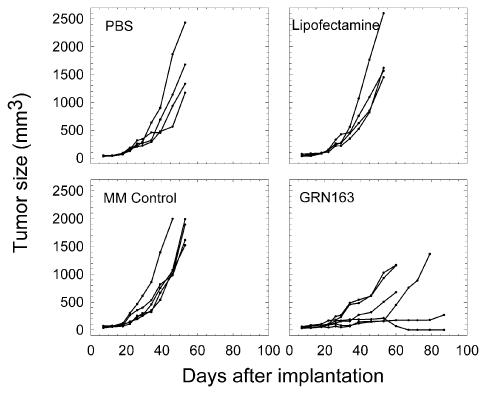
In the first experiment, in which mice with U-251 MG tumors were treated with 4 intratumoral injections of GRN163, growth rates of the individual tumors in the mice treated with PBS, Lipofectamine, or MM Control were higher, and in general more uniform, than the growth rates of the individual tumors in mice treated with GRN163 (Fig. 1). For mice treated with GRN163, tumor volumes on day 46 ranged from 146 to 620 mm3 (average of 340 mm3). In contrast, tumor volumes on day 46 for PBS, Lipofectamine, and MM Control treated mice ranged from 565 to 1865 mm3 (average of 1126 mm3), from 820 to 1764 mm3 (average of 1132 mm3), and from 983 to 1997 mm3 (average of 1226 mm3), respectively. With the exception of 1 mouse in the MM Control group, which was euthanized on day 46 because of tumor burden, all animals from the 3 control groups were euthanized on day 53 because of tumor burden. The 6 animals that received GRN163 had survival times ranging from 60 to 87 days.
Fig. 1.
Growth curves for individual subcutaneous tumors in the first experiment. In vivo efficacy study in which s.c. U-251 MG cell–derived tumors (≈85-mm3 average volume) were injected 4 times with GRN163. Mice received direct intratumoral injections of PBS, Lipofectamine, MM Control, or GRN163 on days 18, 20, 22, and 25 after implantation of the tumor cells. GRN163 and MM Control were given at a dose of 3 nmol per injection in 20 μl total volume.
The efficacy of GRN163 in this tumor model was also demonstrated in the second and third in vivo experiments, in which flank tumors were treated with 9 injections over a period of 3 weeks (Figs. 2 and 3). GRN163 inhibited tumor growth in both studies, when treatment of the mice was initiated either on day 17 or day 10 after tumor implantation. Tumors in all control groups grew at a similar rate, whereas GRN163 significantly suppressed the growth of tumors as compared with tumor growth in each control treatment group. Because tumors in the various control groups grew similarly in the first 2 experiments, in the third experiment we deleted the PBS and Lipofectamine controls and compared tumor growth between only MM Control-treated and GRN163-treated mice (Fig. 3). Again, tumors in the MM Control-treated mice grew similarly to the control groups in the first two experiments, whereas GRN163 treatment inhibited tumor growth. In all cases, GRN163-treated tumors grew significantly slower, which resulted in a longer time to euthanasia compared to that for the control mice (P < 0.01). There was no apparent difference in the degree of inhibition when either 4 or 9 doses of the compound were administered. No systemic toxicity was observed in any of these studies.
Fig. 2.
Growth curves for individual subcutaneous tumors in the second experiment. In vivo efficacy study in which s.c. U-251 MG cell–derived tumors (≈135-mm3 average volume) were injected 9 times with GRN163. Mice received direct intratumoral injection of PBS, Lipofectamine, MM Control, or GRN163 on days 17, 20, 22, 24, 27, 29, 31, 34, and 36 after implantation of tumor cells. GRN163 and MM Control were given at a dose of 3 nmol per injection in 7 μl total volume.
Fig. 3.
Growth curves for individual subcutaneous tumors in the third experiment. In vivo efficacy study in which s.c. U-251 MG cell–derived tumors (≈50-mm3 average volume) were injected 9 times with GRN163. Mice received direct intratumoral injection of MM Control or GRN163 on days 10, 12, 14, 17, 19, 21, 24, 26, and 28 after implantation of tumor cells. GRN163 and MM Control were given at a dose of 3 nmol per injection in 7 μl total volume.
Distribution Study of GRN163 in Athymic Rats
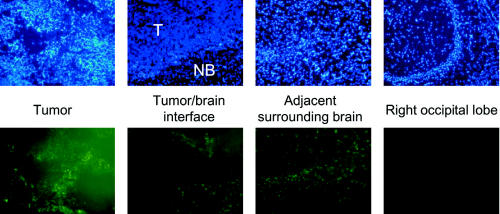
We observed fluorescence produced by the oligonucleotides in the tumor immediately after infusion on day 0, and no significant reduction in fluorescence was observed up to day 4, which was the longest time point used in the experiment. Images obtained on day 3 are shown in Fig. 4. Fluorescein-labeled GRN163 was also detected in cells at the tumor/brain interface and in normal brain cells in the adjacent brain (Fig. 4). Very little fluorescence was detected in ipsilateral brain tissue in the right occipital lobe, which is distant from the infusion site (Fig. 4), and no fluorescence at all was detected in the contralateral hemisphere (not shown). No apparent toxicity or behavioral abnormalities were observed during the course of this experiment in any of the rats receiving fluorescein-labeled GRN163 i.c.
Fig. 4.
Distribution of fluorescein-labeled GRN163 into brain tumor and normal brain in athymic rats on day 3 after completion of a 7-day infusion at the same relative depth in each rat brain. Upper panels show DAPI fluorescence, and lower panels show FITC fluorescence. In the tumor/brain interface panels, the tumor is at the top of each image. All magnifications are 10×.
Prevention of Intracerebral Tumors in Athymic Rats by GRN163
Control rats required euthanization beginning on day 41, and all 4 control rats were euthanized by day 43 (Table 1). In contrast, only 2 of 7 GRN163-treated animals required euthanasia during the experiment. All euthanized rats had large tumors (approximately 7 mm in diameter) at the implantation site. The remaining 5 GRN163-treated rats were alive and well, gaining weight and exhibiting no neurological symptoms on day 103, at which time they were euthanized and their brains were examined histopathologically. There was no evidence of tumor at the original implantation site in any of these animals.
Table 1.
Days post–tumor implantation to euthanization for chemopreventive study*
| PBS Control Group | GRN163 (500 nmol) Group** | ||
|---|---|---|---|
| 7-day pump (n = 2) | 14-day pump (n = 2) | 7-day pump (n = 3) | 14-day pump (n = 4) |
| day 41 | day 43 | cured* | day 43 |
| day 43 | day 43 | cured* | day 57 |
| cured* | cured* | ||
| cured* | |||
Abbreviation: PBS, phosphate-buffered saline.
Rats with symptoms of tumor were euthanized on days 41–57. Rats showing no symptoms (those that were cured) by day 103 were euthanized, and no evidence of tumor was observed histopathologically.
Rats received 100 μl of GRN163 (500 nmol) from an Alzet pump over either a 7-day or a 14-day period beginning 1 to 2 h after tumor-cell implantation.
Treatment of Intracerebral Tumors in Athymic Rats with GRN163
Table 2 shows the survival times for athymic rats receiving either 150 or 500 nmol of GRN163 through an Alzet minipump over a 7-day period. MM-Control rats received 500 nmol of mismatch oligonucleotides because our preliminary data showed that the rats’ survival was not affected by mismatch oligonucleotide doses from 50 to 1500 nmol (data not shown). Survival times in control animals ranged from 37 to 43 days; median survival was 37.5 days. Median survival times were 45 and 54 days, respectively, for the 150- and 500-nmol GRN163-treatment groups. Differences between the MM-Control group and either GRN163-treated group were statistically significant (P < 0.05). Difference between the two GRN163-treated groups was minimal. At the time of euthanasia, all 12 GRN163-treated rats that were euthanized before day 94 had neurological symptoms and large tumors at the site of implantation. However, the 4 GRN163-treated rats that were euthanized on day 94 did not show any adverse neurological symptoms, and there was no evidence of tumor at the original implantation site in any of these rats.
Table 2.
| MM Control Group | GRN163 Group | ||
|---|---|---|---|
| 500 nmol (n = 4) | 150 nmol (n = 8) | 500 nmol (n = 8) | |
| day 37 | day 37 | day 37 | |
| day 37 | day 38 | day 41 | |
| day 38 | day 41 | day 42 | |
| day 43 | day 42 | day 52 | |
| day 48 | day 56 | ||
| day 59 | day 56 | ||
| cured* | cured* | ||
| cured* | cured* | ||
| Median survival | day 37.5 | day 45 | day 54 |
Rats with symptoms of tumor were euthanized on days 37 to 59. Rats showing no symptoms (those that were cured) by day 94 were euthanized, and no evidence of tumor was observed histopathologically. Differences in growth among the groups was assessed on the basis of time to euthanasia by using the Jonckheere Terpstra test, with P < 0.05. For this test, animals that were cured were assigned a survival time of 94 days.
Rats received a 7-day infusion of MM Control or 100 μl GRN163 in PBS beginning 14 days after tumor-cell implantation.
Discussion
Recent studies have shown that GRN163, which belongs to a new class of modified N3′→P5′ thio-phosphoramidates, has potent human telomerase inhibitory activity in several biochemical assays with 50% inhibitory concentration values less than 1 nM (Gryaznov et al., 2001; Herbert et al., 2002). This oligonucleotide was designed to bind by formation of a complementary duplex with part of the template region of hTR. Also, it appears that the thio-phosphoramidate backbone of GRN163 is capable of forming—in addition to Watson-Crick base pairing—a stabilizing interaction with telomerase protein subunit hTERT upon the duplex formation with hTR (Gryazov et al., 2001). A recent study also revealed that GRN163 effectively inhibits telomerase activity in spontaneously immortalized human breast epithelial HME50-5E cells, with recorded 50% inhibitory concentration values of approximately 0.5 to 5 nM and 0.5 to 1 μM with and without lipid uptake enhancers, respectively (Herbert et al., 2002). Moreover, inhibition of telomerase activity by GRN163 in HME50-5E cells produced a gradual reduction of telomere length, followed by the onset of cellular senescence and apoptosis (Herbert et al., 2002).
In the present study we tested the telomerase inhibitor GRN163 as a potential therapeutic agent in experimental models of human malignant glioma. We hypothesized that the specific inhibition of telomerase activity in malignant gliomas might be a promising therapeutic approach because of the relatively high telomerase activity detected in these tumors, but not in normal brain tissue (Chong et al., 1998; Langford et al., 1995; Le et al., 1998; Mori et al., 1997; Sano et al., 1998). Our initial in vitro studies showed that a 3-day exposure of U-251 MG cells to GRN163 inhibited telomerase activity by up to 68% as compared to levels in untreated cells.
The results of our in vivo studies show that treatment of s.c. tumors with GRN163 inhibited their growth. Tumor-growth inhibition occurred in all three independently conducted experiments regardless of frequency of injection (4 or 9 injections) or tumor size at the time of initiation of the treatment. Failure to observe differences in outcome between the two treatment regimens may indicate that both were equally effective, or it may simply mean that the animal numbers used were too small to reveal differences in outcome. Further experimentation would be needed to determine which of these possibilities is correct. Comparisons of tumor growth for the individual mice (Figs. 1–3) suggest that animals with smaller tumors at the time of treatment might respond better to treatment with GRN163; however, there was too much variability in tumor growth patterns in each group of GRN163-treated animals to make a definitive statement.
Our results are in general agreement with the findings of Kondo et al. (1998), who also used the U251-MG cell line for their in vitro and in vivo studies of antitelomerase 2-5A- oligonucleotide conjugates directed to the site of hTR. Although this site is different from the template region for GRN163, administration of that oligonucleotide in vivo also inhibited flank-tumor growth.
Our studies show that direct i.c. administration by slow infusion of fluorescein-labeled GRN163 formulated in PBS without any cellular-uptake enhancers produces efficient uptake of the oligonucleotide into human brain-tumor cells. Moreover, a significant amount of this compound was retained by the tumor cells for up to 4 days after termination of the infusion, as shown by our distribution study. These data indicate there is good bio-availability and hydrolytic stability of GRN163 in the lipid-rich environment of the brain and suggest the possibility of intermittent dosing. Although GRN163 was found in normal brain cells that were adjacent to tumor cells, no abnormal behavior was detected in any animals, and there were no pathological indications of toxicity. This finding was expected because normal brain cells do not express telomerase activity.
When animals were infused with GRN163 shortly after implantation of tumor cells, tumor growth was prevented in the majority of animals (Table 1). This result appears to be specific to the antitelomerase activity of GRN163 because tumor formation and growth was not impeded by the administration of MM Control at the time of tumor implantation. Our finding suggests that GRN163 might have applicability as a chemopreventive agent, and while it is unlikely to be used for that purpose, it might be useful for treating patients with minimal residual tumors or micrometastatic disease.
The results of our i.c. tumor efficacy study, in which rats were treated with a 7-day infusion of either 150 or 500 nmol of GRN163, showed that both doses of GRN163 prolonged survival of the rats as compared to survival of rats treated with MM Control; however, there was minimal, if any, difference between the 150- and 500-nmol GRN163 groups. We have not yet studied doses between 500 and 1500 nmol to see whether a dose-response relationship occurs; however, a preliminary experiment revealed that 50 nmol of GRN163 did not prolong animal survival and 1500 nmol of GRN163 was toxic to animals.
The inhibition of growth observed in GRN163-treated flank tumors, as well as the prolonged survival of GRN163-treated animals with i.c. tumors, provides evidence that GRN163 was active in these tumor models. Although we cannot say that this activity was due strictly to its antitelomerase activity, because we did not measure it, GRN163 is not known to exhibit any other biological activity within the experimental time periods used in our experiments. Moreover, it did not exhibit any adverse effects when injected into normal brains, which for the most part lack telomerase activity. Finally, results from our in vitro studies clearly showed that GRN163 could inhibit telomerase activity. Thus, we think it is reasonable to presume that the biological effects produced by GRN163 are attributable to its antitelomerase activity.
The results of our in vivo studies confirm that agents that target telomerase show promise as potential antitumor drugs for malignant glioma. They also suggest that these agents may have potential for treating patients with minimal residual disease or micrometastatic disease. The findings support further investigations of GRN163 and other antitelomerase agents for their possible use in brain-tumor therapy.
Acknowledgments
The work at UCSF was supported by Geron Corporation. We thank Jingli Wang, Hangjun Ruan, and David Karpf for helpful discussions and Eileen Kong for technical assistance. We also thank Sharon Reynolds for editorial assistance.
Footnotes
The work performed at the University of California San Francisco was supported by Geron Corporation.
Abbreviations used are as follows: CHAPS, 3-[(3-cholamidopropyl) dimethylammonio]-1-propane sulfonate; CMEM, complete minimal essential medium; DAPI, 4′6-diamidine-2′-phenylindole dihydrochloride; FITC, 3′-fluorescein isothiocyanate; MM Control, mismatch control; PBS, phosphate-buffered saline; UCSF, University of California San Francisco.
References
- Burger, P.C., Scheithauer, B.W., and Vogel, F.S. (1990) Brain: Tumors. In: Surgical Pathology of the Nervous System and its Coverings New York: Churchill Livingstone, pp. 193–196.
- Chong EY, Lam PY, Poon WS, Ng HK. Telomerase expression in gliomas including the nonastrocytic tumors. Hum Pathol. 1998;29:599–603. doi: 10.1016/s0046-8177(98)80009-9. [DOI] [PubMed] [Google Scholar]
- Counter CC, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Gryaznov SM, Letsinger RL. Synthesis and properties of oligonucleotides containing aminodeoxythymidine units. Nucleic Acids Res. 1992;20:3403–4309. doi: 10.1093/nar/20.13.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryaznov SM, Pongracz K, Matray T, Schultz R, Pruzan R, Aimi J, Chin A, Harley C, Shea-Herbert B, Shay J, Oshima Y, Asai A, Yamashita Y. Telomerase inhibitors—oligonucleotide phosphoramidates as potential therapeutic agents. Nucleosides Nucleotides Nucleic Acids. 2001;20:401–410. doi: 10.1081/NCN-100002314. [DOI] [PubMed] [Google Scholar]
- Hakin-Smith V, Jellinek DA, Levy D, Carroll T, Teo M, Timperley WR, McKay MJ, Reddel RR, Royds JA. Alternative lengthening of telomeres and survival in patients with glioblastoma multiforme. Lancet. 2003;361:836–838. doi: 10.1016/s0140-6736(03)12681-5. [DOI] [PubMed] [Google Scholar]
- Herbert B-S, Pongracz K, Shay JW, Gryaznov SM. Oligonucleotide N3′→P5′ phosphoramidates as efficient telomerase inhibitors. Oncogene. 2002;21:638–642. doi: 10.1038/sj.onc.1205064. [DOI] [PubMed] [Google Scholar]
- Hiyama E, Hiyama K, Yokoyama T, Matsuura Y, Piatyszek MA, Shay JW. Correlating telomerase activity levels with human neuroblastoma outcomes. Nature Med. 1995;1:249–255. doi: 10.1038/nm0395-249. [DOI] [PubMed] [Google Scholar]
- Hollander, M., and Wolfe, D.A. (1973) Nonparametric Statistical Methods. New York: John Wiley & Sons, pp. 120–123.
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Kondo S, Kondo Y, Li G, Silverman RH, Cowell JK. Targeted therapy of human malignant glioma in a mouse model by 2-5A antisense directed against telomerase RNA. Oncogene. 1998;16:3323–3330. doi: 10.1038/sj.onc.1201885. [DOI] [PubMed] [Google Scholar]
- Langford LA, Piatyszek MA, Xu R, Schold SC, Jr, Shay JW. Telomerase activity in human brain tumours. Lancet. 1995;346:1267–1268. doi: 10.1016/s0140-6736(95)91865-5. [DOI] [PubMed] [Google Scholar]
- Le S, Zhu JJ, Anthony DC, Greider CW, Black PM. Telomerase activity in human gliomas. Neurosurgery. 1998;42:1120–1125. doi: 10.1097/00006123-199805000-00099. [DOI] [PubMed] [Google Scholar]
- Levin VA, Silver P, Hannigan J, Wara WM, Gutin PH, Davis RL, Wilson CB. Superiority of post-radiotherapy adjuvant chemotherapy with CCNU, procarbazine, and vincristine (PCV) over BCNU for anaplastic gliomas: NCOG 6G61 final report. Int J Radiat Oncol Biol Phys. 1990;18:321–324. doi: 10.1016/0360-3016(90)90096-3. [DOI] [PubMed] [Google Scholar]
- Lin Y, Miyamoto H, Fujinami K, Uemura H, Hosaka M, Iwasaki Y, Kubota Y. Telomerase activity in human bladder cancer. Clin Cancer Res. 1996;2:929–932. [PubMed] [Google Scholar]
- Mao L, El-Naggar AK, Fan YH, Lee JS, Lippman SM, Kayser S, Lotan R, Hong WK. Telomerase activity in head and neck squamous cell carcinoma and adjacent tissues. Cancer Res. 1996;56:5600–5604. [PubMed] [Google Scholar]
- Miller PJ, Hassanein RS, Giri PG, Kimler BF, O’Boynick P, Evans RG. Univariate and multivariate statistical analysis of high-grade gliomas: The relationship of radiation dose and other prognostic factors. Int Radiat Oncol Biol Phys. 1990;19:275–280. doi: 10.1016/0360-3016(90)90534-q. [DOI] [PubMed] [Google Scholar]
- Mori K, Tanaka R, Onda K, Tsumanuma I, Yoshimura J. Expression of telomerase RNA, telomerase activity, and telomere length in human gliomas. Biochem Biophys Res Commun. 1997;239:830–834. doi: 10.1006/bbrc.1997.7562. [DOI] [PubMed] [Google Scholar]
- Mukai S, Kondo Y, Koga S, Komata T, Barna BP, Kondo S. 2-5A antisense telomerase RNA therapy for intracranial malignant gliomas. Cancer Res. 2000;60:4461–4467. [PubMed] [Google Scholar]
- Ozawa T, Wang J, Hu LJ, Lamborn KR, Bollen AW, Deen DF. Characterization of human glioblastoma xenograft growth in athymic mice. In Vivo. 1998;12:369–374. [PubMed] [Google Scholar]
- Ozawa T, Wang J, Hu LJ, Bollen AW, Lamborn KR, Deen DF. Growth of human glioblastomas as xenografts in the brains of athymic rats. In Vivo. 2002;16:55–60. [PubMed] [Google Scholar]
- Pongracz K, Gryaznov SM. Oligonucleotide N3′→P5′ thio-phosphoramidates: Synthesis and properties. Tetrahedron Lett. 1999;40:7661–7664. [Google Scholar]
- Recht L, Fram RJ, Strauss G, Fitzgerald TJ, Liepman M, Lew R, Kadish S, Sherman D, Wilson J, Greenberger J, Egan P, Silver D. Preirradiation chemotherapy of supratentorial malignant primary brain tumors with intracarotid cis-platinum (CDDP) and i.v BCNU A phase II trial. Am J Clin Oncol. 1990;13:125–131. doi: 10.1097/00000421-199004000-00008. [DOI] [PubMed] [Google Scholar]
- Sano T, Asai A, Mishima K, Fujimaki T, Kirino T. Telomerase activity in 144 brain tumours. Br J Cancer. 1998;77:1633–1637. doi: 10.1038/bjc.1998.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibamoto Y, Yamashita J, Takahashi M, Yamasaki T, Kikuchi H, Abe M. Supratentorial malignant glioma: An analysis of radiation therapy in 178 cases. Radiother Oncol. 1990;18:9–17. doi: 10.1016/0167-8140(90)90018-r. [DOI] [PubMed] [Google Scholar]
- Sommerfeld HJ, Meeker AK, Piatyszek MA, Bova GS, Shay JW, Coffey DS. Telomerase activity: A prevalent marker of malignant human prostate tissue. Cancer Res. 1996;56:218–222. [PubMed] [Google Scholar]
- Sugino T, Yoshida K, Bolodeoku J, Tahara H, Buley I, Manek S, Wells C, Goodison S, Ide T, Suzuki T, Tahara E, Tarin D. Telomerase activity in human breast cancer and benign breast lesions: Diagnostic applications in clinical specimens, including fine needle aspirates. Int J Cancer. 1996;69:301–306. doi: 10.1002/(SICI)1097-0215(19960822)69:4<301::AID-IJC11>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Yu GL, Bradley JD, Attardi ML, Blackburn EH. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature. 1990;344:126–132. doi: 10.1038/344126a0. [DOI] [PubMed] [Google Scholar]