Abstract
Mucolipidosis type IV is an autosomal recessive lysosomal storage disease of unknown etiology that causes severe neurological and ophthalmological abnormalities. In an attempt to obtain insight into the nature of the metabolic abnormality in this disorder, we prospectively evaluated 15 consecutive patients, aged 2 to 23 years, over a period of 22 months. The finding of iron deficiency in some of the patients led us to the discovery that all patients but one had markedly elevated blood gastrin levels. None had vitamin B12 deficiency. Gastroscopy in three patients showed normal gross appearance of the mucosa in two patients, 4 and 7 years old, and mucosal atrophy in a 22-year-old. Parietal cells were present in normal numbers and contained large cytoplasmic inclusions that were confirmed immunohistochemically to be lysosomal in nature. Other gastric epithelial cells appeared normal. Parietal cells contained very few tubulovesicular membranes, suggesting cellular activation, whereas apical canaliculi appeared relatively nonactivated. Both subunits of the parietal cell H+/K+-ATPase were present, and both partially colocalized with f-actin at the apical membrane. We conclude that patients with mucolipidosis type IV are constitutively achlorhydric and have partially activated parietal cells. We hypothesize that the defective protein in this disease is closely associated with the final stages of parietal cell activation and is critical for a specific type of cellular vacuolar trafficking between the cytoplasm and the apical membrane domain.
Mucolipidosis type IV (ML-IV) is a metabolic storage disease that causes various degrees of mental and motor retardation as well as visual impairment (1–3). It was first recognized as a distinct clinicopathological entity in 1974 (4). The mode of inheritance is autosomal recessive. To date, more than 80 patients have been diagnosed, mostly in the Ashkenazi Jewish population, but non-Jewish patients also have been described (5–7). Visual difficulties usually are progressive, even in a patient with a “static” neurological course, and are caused by a combination of corneal clouding, which is often painful, retinal degeneration, and occasionally optic atrophy (1, 8).
There are conflicting reports concerning the progressive nature of the neurological involvement. Patients with obvious motor status deterioration are well documented (9), but most patients have no apparent cognitive or motor regression (5, 8). It is likely that many patients remain undiagnosed because their neurological deficit is nonprogressive.
The brain pathology in ML-IV is characterized by pigmented cytoplasmic granules in nondistended neurons and microglial cells. These cells stain intensely with periodic-acid Schiff and Sudan black (10). The white matter of brain stains weakly with Luxol-fast blue (10). Electron microscopy reveals accumulation of lamellated membrane structures, and amorphous material in lysosomes in practically every cell type examined (10, 11). Cultured skin fibroblasts, derived from ML-IV patients, recently were found to contain autofluorescent lysosomes (12). Storage vacuoles are present in the corneal epithelium. Vacuolization was seen in many cell types, including cells in the hepatic bile duct, pancreatic acinar cells, macrophages, chondrocytes, and in the renal collecting ducts (11, 13). Although mucopolysaccharides, phospholipids, and gangliosides are elevated in tissues of ML-IV patients (12), no specific storage compound was found. In addition, the composition of the stored material varies from tissue to tissue (10). Currently, the diagnosis of ML-IV is based on identifying the characteristic intracellular inclusions in tissue biopsies or cultured skin fibroblasts by using light or electron microscopy (10, 12).
Because only isolated case reports of patients with ML-IV have been described thus far, we initiated a systematic study of patients with this disorder to try to identify biochemical abnormalities that might shed light on the molecular nature of this disease.
MATERIALS AND METHODS
Gastric Acid Analysis.
Measurement of basal acid output and maximal acid output (MAO) was performed as previously described (14). Briefly, patients were fasted overnight and maintained well hydrated with intravenous 5% dextrose in normal saline. The next morning, a nasogastric tube was inserted and the stomach was emptied by continuous aspiration for 1 hr. The gastric acid content in the aspirate was determined at 15-min intervals by titration to pH 7.0 with 0.01 N NaOH. After basal acid determination, MAO was measured by titrating gastric acid for 1 hr after administration of subcutaneous pentagastrin (6 μg/kg). MAO and basal acid output results are reported in milliequivalents of acid per hr (mEq H+/hr).
Tissue.
Gastric mucosal biopsies were obtained from patients with ML-IV and Zollinger–Ellison syndrome (Z-E). Tissue samples were immediately cut into 1-mm cubes and fixed in 3% paraformaldehyde/0.1% glutaraldehyde in 0.2 M Na cacodylate buffer at pH 7.4 for 1 hr for light microscopy or Karnovsky fixative for electron microscopy. For light microscopy tissue blocks were rinsed free of fixative with Na cacodylate buffer and frozen in liquid nitrogen. One-micrometer cryosections were cut at −80 to −85°C on a Reichert Ultracut E microtome (Leica, Deerfield, IL) equipped with an FC4 attachment. Sections were transferred on drops of sucrose to slides for subsequent staining and visualization. For electron microscopy, aldehyde-fixed tissue was postfixed in 2% OsO4 and embedded in resin, and ultrathin sections (<0.1 μ) were cut and stained with lead hydroxide and uranyl acetate.
Immunolabeling and Fluorescence Staining.
Cryosections were immunostained for the alpha or beta subunit of H+/K+-ATPase and a lysosomal membrane protein, LAMP2 (15) with an indirect immunolabeling procedure. Primary antibodies were mouse monoclonal IgG against pig H+/K+-ATPase alpha subunit (courtesy of A. J. Smolka, Medical University of South Carolina) and mouse monoclonal against pig H+/K+-ATPase beta subunit (Affinity Bioreagents, Golden, CO). All incubations (quench, antibody, and washes) were performed in PBS, at pH 7.4, containing goat IgG (2.5 mg/ml). Cryosections were incubated with primary antibodies for 1 hr at room temperature or overnight at 4°C, washed in PBS (3 × 10 min), incubated with fluorescein- or rhodamine-labeled secondary antibodies (Jackson ImmunoResearch) at 1:50 dilution for 1 hr, and washed (3 × 10 min). For immunocytochemical controls, cryosections were incubated in mouse IgG in buffer at the same dilution as the immune IgG and processed in an identical manner. For visualization of f-actin, cryosections were stained with fluorescein-labeled phallacidin (Molecular Probes) at 20 μg/ml PBS for 30 min. Fluorescently stained cryosections were mounted in p-phenylenediamine for imaging.
Microscopy.
Confocal light microscopy was performed on a Zeiss LSM410 confocal imaging system using a krypton/argon laser. To detect fluorescence, dichroic light 488/568 was used with BP 515–565 emission filter for fluorescein and LP590 emission filter for rhodamine or Texas red. Ultrathin resin sections on grids were viewed with a Phillips CV120 transmission electron microscope at 100 KV.
Gastric biopsies obtained from a patient with Z-E treated with omeprazole were used as controls.
RESULTS
Description of Patients.
Fifteen patients with ML-IV were examined under an Institutional Review Board-approved protocol. The diagnosis of each patient was confirmed both clinically and pathologically. The age of the patients ranged from 2 to 23 years. Iron deficiency anemia was present in four patients (aged 6, 7, 15, and 23 years). Iron deficiency without anemia was present in three other patients (aged 5, 9, and 22 years). All but one patient had elevated fasting plasma gastrin levels ranging from 450 pmol/ml to 1,800 pmol/ml (normal: 0–200 pmol/ml). Vitamin B12 in blood was normal in all patients. None of the patients had antiparietal antibodies or occult blood in the stool. Twenty-four-hour urinary iron excretion was low in all patients.
Gastric Acid Analysis.
Hydrochloric acid output in five consecutive patients is indicated in Table 1. All five patients, including the one with normal plasma gastrin and a mildly affected patient (patient 5, Table 1), were achlorhydric. There was no significant response to intravenous infusion of pentagastrin (16). As is seen in achlorhydria from other causes, there was a reduced volume of gastric juice (16).
Table 1.
Gastric acid output in patients with ML-IV
| Patient | Age, years | Blood gastrin, pmol/ml (normal: 0–200) | BAO/volume | MAO/volume |
|---|---|---|---|---|
| 1 | 4 | 1,200 | 0.40/35 | 0.60/20 |
| 2 | 6 | 20 | 0.00/4 | 0.00/20 |
| 3 | 7 | 1,100 | 0.26/15 | 0.01/3 |
| 4 | 22 | 800 | 0.00/17 | ND |
| 5 | 23 | 700 | 0.00/24 | 0.00/18 |
BAO, basal acid output (mEq H+/hr). Normal values 3.0 ± 2.7 (adults) (33) and 0.49 ± 0.12 (children) (34). MAO, maximal acid output (mEq H+/hr). Normal values 34.16 ± 2.18 (adults) (33) and 2.96 ± 0.60 (children) (34). Volume, gastric juice excretion in ml/hr. Normal values in children: baseline, 45–70 ml/hr; maximal, 72–90 ml/hr (34). ND, not done.
Gastroscopy Results.
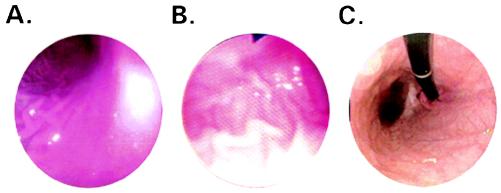
Patients 1, 3, and 4 (Table 1) underwent esophagogastroduodenoscopy. The gross appearance of the esophagus, stomach, duodenal bulb, and the second portion of the duodenum was normal in the two younger patients. Specifically, there were no abnormalities in the vascular appearance or fold thickness in the gastric fundus, body, and antrum. No ulcers, erosions, or other lesions were present. On the other hand, the 22-year-old patient had flattened, atrophic-appearing gastric folds in the gastric body and fundus (Fig. 1). The antrum, pylorus, and proximal duodenum appeared normal, as did the esophagus and duodenum. Multiple mucosal biopsies were obtained from the gastric fundus, body, and antrum of all three patients.
Figure 1.
Gastroscopic images of patients with ML-IV showing a normal mucosal surface in 4- (A) and 7-year-old (B) patients, and atrophic changes in a 22-year-old patient (C).
Pathology.
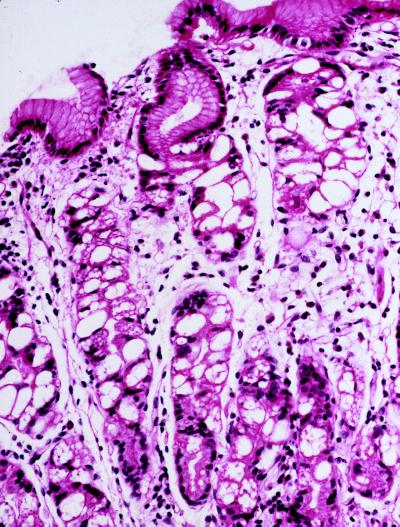
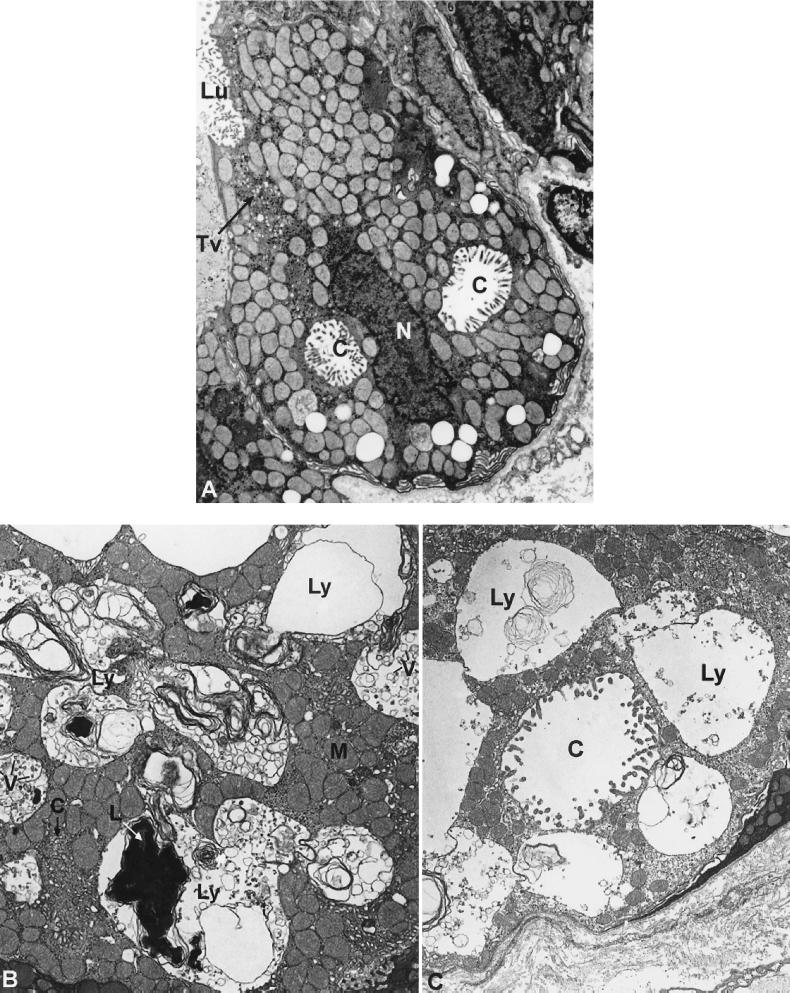
Light microscopic examination of gastric mucosa biopsies stained with hematoxylin-eosin showed marked chronic inflammation in all three patients. Gastric mucosa atrophy was mild in the 4-year-old patient, moderate in the 7-year-old patient, and severe in the 22-year-old patient. Parietal cells in the gastric body and fundus were distended by large vacuoles (Fig. 2). In all three patients, the enterochromaffin-like cells showed diffuse or nodular hyperplasia on Grimelius silver stain (not shown). These cells did not stain with antigastrin antibodies. The antral mucosa biopsies showed no abnormalities. The number of parietal cells appeared to be normal in all patients. Electron microscopic examination showed that the parietal cells were markedly distended by large lysosomes containing lamellar, concentric, microgranular inclusions, as well as vacuoles (Fig. 3 B and C). The apical membrane of affected parietal cells did not appear expanded as in activated parietal cells, and they contained very few cytoplasmic tubulovesicular membrane structures. Other cell types in the gastric body and fundus appeared similar to those in normal tissue without enlarged vacuoles and cytoplasmic inclusions.
Figure 2.
Biopsy of the gastric mucosa of a patient with ML-IV stained with hematoxylin and eosin. Vacuolated parietal cells are evident, whereas the mucus-secreting cells at the mucosal surface appear normal. (Magnification: ×40.)
Figure 3.
(A) Electron micrograph of a parietal cell from stomach of a patient with Z-E showing secretory canaliculi (C) and tubulovesicles (TV); Lu, lumen. (Magnification: ×6,500.) (B) Electron micrograph of a parietal cell from the stomach of a patient with ML-IV. The parietal cell lysosomes (Ly) are large and contain structurally heterogeneous material including vesicles (V) and masses of lamellar structures (L). M, mitochondria. (Magnification: ×6,500.) (C) A similar electron micrograph showing an abnormally distended canaliculus that is identified by the cell surface microvilli. (Magnification: ×6,500.)
Immunohistochemistry.
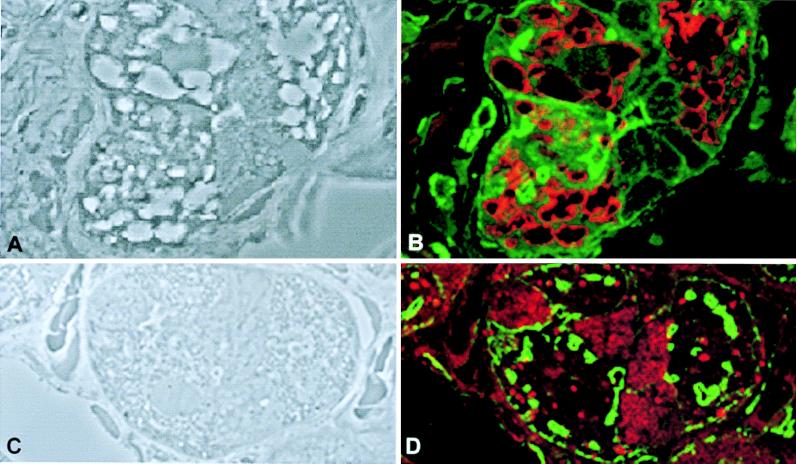
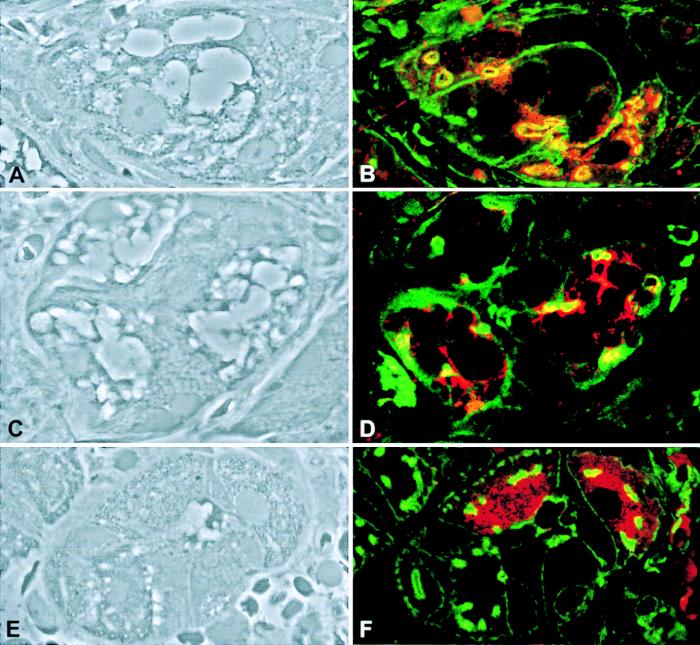
Vacuolar membranes were stained with anti-LAMP2 antibody (Fig. 4), confirming that these large cytoplasmic vacuoles are lysosomes (Fig. 4B). For comparison, the anti-LAMP2 staining in parietal cells of a Z-E patient indicated the presence of punctate lysosomes (Fig. 4D). The distribution of f-actin in both the patient and the Z-E control was similar. Staining for the beta (Fig. 5B) and alpha (Fig. 5D) subunit of the H+/K+-ATPase showed that this ion pump was present in parietal cells. In certain areas on the cell, it colocalized with the f-actin, suggesting the presence of the ion pump around canaliculi in the apical membrane of the parietal cells of the ML-IV patient. Parietal cells from the patient with Z-E showed a diffuse cytoplasmic distribution of the H+/K+-ATPase with no marked colocalization with the f-actin, suggesting a nonactivated state (Fig. 5F). Immunostaining demonstrated the presence of intrinsic factor in the patients’ parietal cells (data not shown).
Figure 4.
Lysosome distribution in parietal cells from a patient with ML-IV and a patient with Z-E. (A and C) Phase microscopy view. (B and D) The same sections as in A and C, respectively stained with fluorescein phallacidin (green) and LAMP2 antibodies (red). (Magnification: ×1,000.)
Figure 5.
H+/K+-ATPase beta (B) and alpha (D and F) subunits distribution in parietal cells from a patient with ML-IV (A–D) and patient with Z-E (E and F). Phase microscopy view (A, C, and E) and the corresponding parietal cells stained with fluorescein phallacidin in green, and anti-H+/K+-ATPase beta and alpha subunits in red (B, D, and F). (Magnification: ×1,000.)
DISCUSSION
Five patients with ML-IV were constitutively achlorhydric. The other 10 patients were considered to be achlorhydric as well in view of their marked hypergastrinemia. Although achlorhydria has always been associated with hypergastrinemia (17), the patient with the lowest gastric acid output had normal blood gastrin levels. This finding may be the result of the extreme severity of the metabolic defect in this patient affecting the function of gastrin-secreting cells. Achlorhydria in the ML-IV patients was associated with lysosomal inclusions found specifically in the parietal cells of the stomach. Lysosomes in the parietal cells were larger than those seen in most cell types in ML-IV patients but were similar in structure. Clinical and morphological findings indicated that lack of hydrochloric acid secretion was selective. It spared the viability of the parietal cells and their ability to differentiate and secrete intrinsic factor. The hyperplasia of enterochromaffin-like cells indicated long-standing hypergastrinemia (16, 18). The iron deficiency in some of our patients is likely to be secondary to decreased dietary iron absorption. The severity of the mucosal inflammation and atrophy found on stomach biopsies increased with age and can be considered secondary to long-standing achlorhydria (16).
We describe constitutive achlorhydria in a lysosomal storage disease. The only other known genetic disease that frequently is associated with achlorhydria is a form of congenital agammaglobulinemia (19). The deficiency of gastric acid secretion documents a defective cellular metabolic pathway in patients with ML-IV. Ganglioside GM3 sialidase deficiency previously was thought to be the cause of ML-IV, but its activity was later found to be normal (20). Achlorhydria can be used as a biochemical marker to assist in the diagnosis of ML-IV and to characterize these patients as a homogenous pathogenetic group. The pathogenesis of the achlorhydria in this disorder is unclear, but is likely to be closely related to the primary metabolic defect in ML-IV. Activation of parietal cells to secrete hydrochloric acid is associated with expansion of the apical cellular membrane with concomitant decrease in the tubulovesicular cytoplasmic structures (21). The combination of a few tubulovesicular membranes and the impression of nonexpansion of the apical membranes in our patients suggest that the parietal cells in ML-IV may be only partially activated. For that reason, and because some of the H+/K+-ATPase colocalizes with f-actin at the cellular apical membrane, dysfunction of signal transduction seems unlikely. Because expression of H+/K+-ATPase proteins is restricted to parietal cells (22), whereas the pathology of ML-IV is widespread, a defect in an associated ubiquitous protein that is crucial for the mechanism of gastric acid secretion is a likely cause of achlorhydria in these patients.
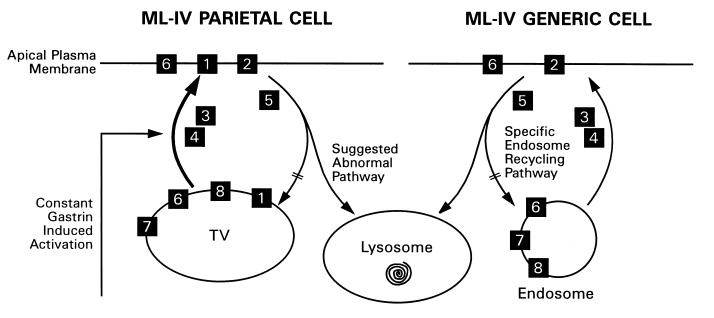
For gastric acid secretion to occur, other proteins such as a chloride channel called ClC-2 (23) and a potassium channel (19) need to function in concert with H+/K+-ATPase. Other proteins associated with H+/K+-ATPase in the tubulovesicular membrane elements have been described. These proteins include ezrin, myosin light chain kinase, syntaxin 3, VAMP-2 (vesicle-associated membrane protein 2), SCAMPs (secretory carrier membrane proteins), and synaptobrevin (24–26). Calcium channels also are likely to play an important role in regulating vesicular targeting and fusion (27). These proteins also are expressed elsewhere, including neuronal synaptic vesicles (16, 24), where they participate in membrane trafficking events such as vesicle transport, docking, and fusion with the cellular membrane (27, 28). Therefore, we hypothesize that the defect in ML-IV is caused by an abnormality in one of the above-mentioned proteins or in an unknown protein that is crucial to these cellular processes, as well as to hydrochloric acid secretion (Fig. 6). As a result of this specific protein dysfunction, protein-membrane complexes that fail to recycle to the plasma membrane instead are diverted to the lysosomes, causing their enlargement (Fig. 6). According to our hypothesis, lysosomal storage is a secondary phenomenon.
Figure 6.
Schematic representation of a hypothesis regarding the cellular lesion in ML-IV. Lack of hydrochloric acid secretion by parietal cells that are constantly stimulated by gastrin is accompanied by diversion of the tubulovesicular membranes (TV) into lysosomes. On the right, we show the effect of the lesion on other cell types in ML-IV. Recycling endosomes involved in a deficient plasma membrane-specific function are diverted into lysosomes. 1, H+/K+-ATPase; 2, ion channels; 3, cytoskeletal elements; 4, kinases involved in plasma membrane activation; 5, kinases and phosphatases involved in the deactivation process; 6, SNAREs; 7, G-proteins; and 8, annexins.
Our findings are also compatible with recent evidence that H+/K+-ATPase translocation to the apical membrane during parietal cell activation is separate from tubulovesicular membrane fusion (29) and that membranous structures accumulate in lysosomes in ML-IV (30). The abnormalities in parietal cells from ML-IV patients are similar to those described in the parietal cells of animals treated with omeprazole, a well-known H+/K+-ATPase inhibitor (31, 32). These latter cells showed lysosomal proliferation and enlargement, and it was hypothesized that the “unused” tubulovesicular membranes were discarded in the lysosomes.
We conclude that patients with ML-IV are constitutively achlorhydric as a result of a distinct block in gastric acid secretion. It is likely that additional exploration of the specific blocking mechanism present in these cells will shed light on the primary defect in this disorder.
Acknowledgments
We thank Devera G. Schoenberg, M.S., for editorial assistance and Dr. R. Levenson and R. T. Jensen for helpful discussions. The work was supported in part by the Mucolipidosis IV Foundation.
ABBREVIATIONS
- Z-E
Zollinger–Ellison syndrome
- ML-IV
mucolipidosis type IV
References
- 1.Chitayat D, Meunier C M, Hodgkinson K A, Silver K, Flanders M, Anderson I J, Little J M, Whiteman D A, Carpenter S. Am J Med Genet. 1991;41:313–318. doi: 10.1002/ajmg.1320410310. [DOI] [PubMed] [Google Scholar]
- 2.Amir N, Zlotogora J, Bach G. Pediatrics. 1987;79:953–959. [PubMed] [Google Scholar]
- 3.Crandall B F, Philippart M, Brown W J, Bluestone D A. Am J Med Genet. 1982;12:301–308. doi: 10.1002/ajmg.1320120308. [DOI] [PubMed] [Google Scholar]
- 4.Berman E R, Livni N, Shapira E, Merin S, Levij I S. J Pediatr. 1974;84:519–526. doi: 10.1016/s0022-3476(74)80671-2. [DOI] [PubMed] [Google Scholar]
- 5.Zeigler M, Bach G. Clin Chim Acta. 1986;157:183–189. doi: 10.1016/0009-8981(86)90224-x. [DOI] [PubMed] [Google Scholar]
- 6.Lake B D, Milla P J, Taylor D S, Young E P. Birth Defects. 1982;18:391–404. [PubMed] [Google Scholar]
- 7.Zwaan J, Kenyon K R. Birth Defects. 1982;18:381–390. [PubMed] [Google Scholar]
- 8.Newman N J, Starck T, Kenyon K R, Lessell S, Fish I, Kolodny E H. Arch Ophthalmol. 1990;108:251–254. doi: 10.1001/archopht.1990.01070040103041. [DOI] [PubMed] [Google Scholar]
- 9.von Figura K. Curr Opin Cell Biol. 1991;3:642–646. doi: 10.1016/0955-0674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- 10.Folkerth R D, Alroy J, Lomakina I, Skutelsky E, Raghavan S S, Kolodny E H. J Neuropathol Exp Neurol. 1995;54:154–164. [PubMed] [Google Scholar]
- 11.Tellez-Nagel I, Rapin I, Iwamoto T, Johnson A B, Norton W T, Nitowsky H. Arch Neurol. 1976;33:828–835. doi: 10.1001/archneur.1976.00500120032005. [DOI] [PubMed] [Google Scholar]
- 12.Goldin E, Blanchette-Mackie E J, Dwyer N K, Pentchev P G, Brady R O. Pediatr Res. 1995;37:687–692. doi: 10.1203/00006450-199506000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Livni N, Merin S. Arch Pathol Lab Med. 1978;102:600–604. [PubMed] [Google Scholar]
- 14.Pisegna J R, Norton J A, Slimak G G, Metz D C, Maton P M, Gardner J D, Jensen R T. Gastroenterology. 1992;102:767–778. doi: 10.1016/0016-5085(92)90157-t. [DOI] [PubMed] [Google Scholar]
- 15.Mane S M, Marzella L, Bainton D F, Holt V K, Cha Y, Hildreth J E K, August J T. Arch Biochim Biophys. 1989;268:360–378. doi: 10.1016/0003-9861(89)90597-3. [DOI] [PubMed] [Google Scholar]
- 16.Modlin I M, Goldenring J R, Lawton G P, Hunt R. Am J Gastroenterol. 1994;89:308–318. [PubMed] [Google Scholar]
- 17.Magee D F. J Gastroenterol. 1996;31:758–763. doi: 10.1007/BF02347632. [DOI] [PubMed] [Google Scholar]
- 18.Creutzfeldt W. Yale J Biol Med. 1994;67:181–194. [PMC free article] [PubMed] [Google Scholar]
- 19.DelValle J, Lucey M R, Yamada T. In: Textbook of Gastroenterology. Yamada T, editor. Philadelphia: Lippincott; 1995. pp. 295–326. [Google Scholar]
- 20.Lieser M, Harms E, Kern H, Bach G, Cantz M. Biochem J. 1989;260:69–74. doi: 10.1042/bj2600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forte T M, Machen T E, Forte J G. Gastroenterology. 1977;73:941–955. [PubMed] [Google Scholar]
- 22.van Driel I R, Callaghan J M. Clin Exp Pharmacol Physiol. 1995;22:952–960. doi: 10.1111/j.1440-1681.1995.tb02332.x. [DOI] [PubMed] [Google Scholar]
- 23.Jordt S E, Jentsch T J. EMBO J. 1997;16:1582–1592. doi: 10.1093/emboj/16.7.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calhoun B C, Goldenring J R. Biochem J. 1997;325:559–564. doi: 10.1042/bj3250559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsukita S, Yonemura S. Curr Opin Cell Biol. 1997;9:70–75. doi: 10.1016/s0955-0674(97)80154-8. [DOI] [PubMed] [Google Scholar]
- 26.Peng X R, Yao X, Chow D C, Forte J G, Bennett M K. Mol Biol Cell. 1997;8:399–407. doi: 10.1091/mbc.8.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett M K. Curr Opin Neurobiol. 1997;7:316–322. doi: 10.1016/s0959-4388(97)80058-x. [DOI] [PubMed] [Google Scholar]
- 28.Hanson P I, Heuser J E, Jahn R. Curr Opin Neurobiol. 1997;7:310–315. doi: 10.1016/s0959-4388(97)80057-8. [DOI] [PubMed] [Google Scholar]
- 29.Courtois-Coutry N, Roush D, Rajendran V, McCarthy J B, Geibel J, Kashgarian M, Caplan M J. Cell. 1997;90:501–510. doi: 10.1016/s0092-8674(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 30.Bargal R, Bach G. J Inherit Metab Dis. 1997;20:625–632. doi: 10.1023/a:1005362123443. [DOI] [PubMed] [Google Scholar]
- 31.Karasawa H, Tani N, Miwa T. Gastroenterol Jpn. 1988;23:1–8. doi: 10.1007/BF02918848. [DOI] [PubMed] [Google Scholar]
- 32.Scott D R, Besancon M, Sachs G, Helander H. Dig Dis Sci. 1994;39:2118–2126. doi: 10.1007/BF02090359. [DOI] [PubMed] [Google Scholar]
- 33.Collen M J, Ciarleglio C A, Stanczak V J, Treem W R, Lewis J H. Am J Gastroenterol. 1988;83:923–926. [PubMed] [Google Scholar]
- 34.Euler A R, Byrne W J, Campbell M F. J Pediatr. 1983;103:766–768. doi: 10.1016/s0022-3476(83)80482-x. [DOI] [PubMed] [Google Scholar]