Abstract
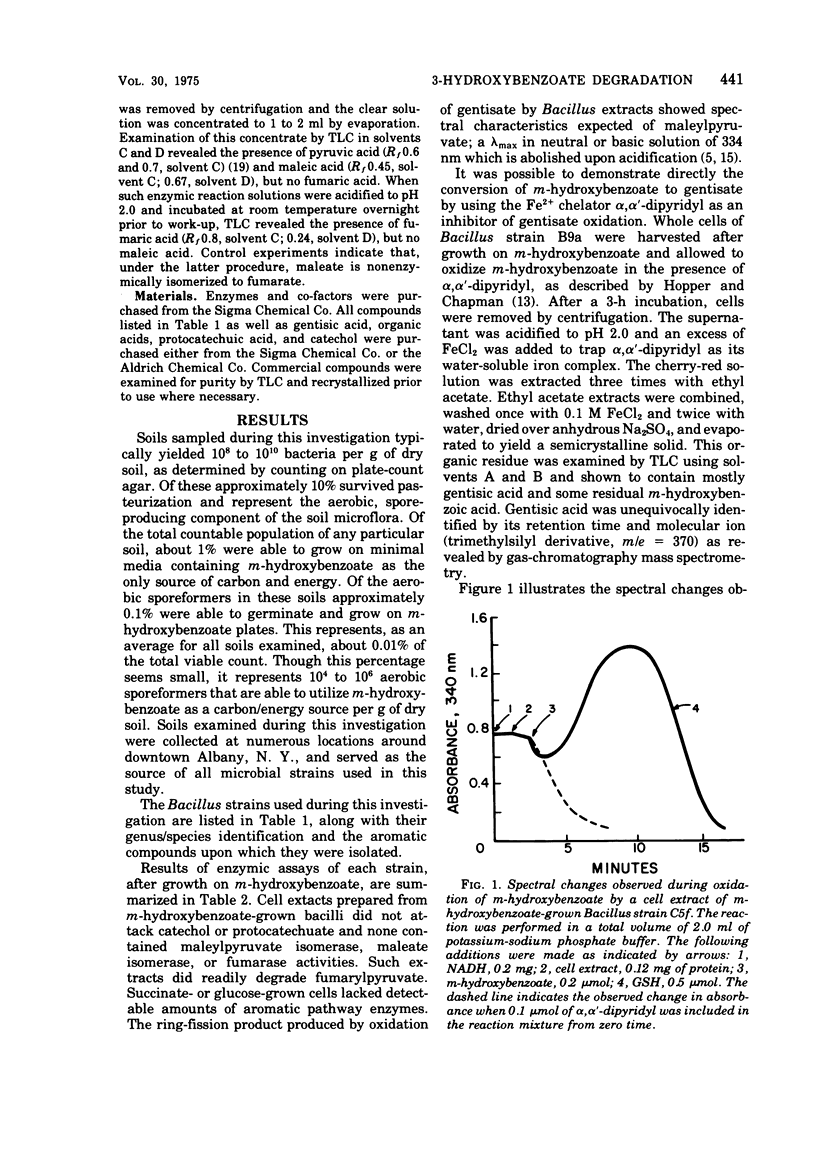
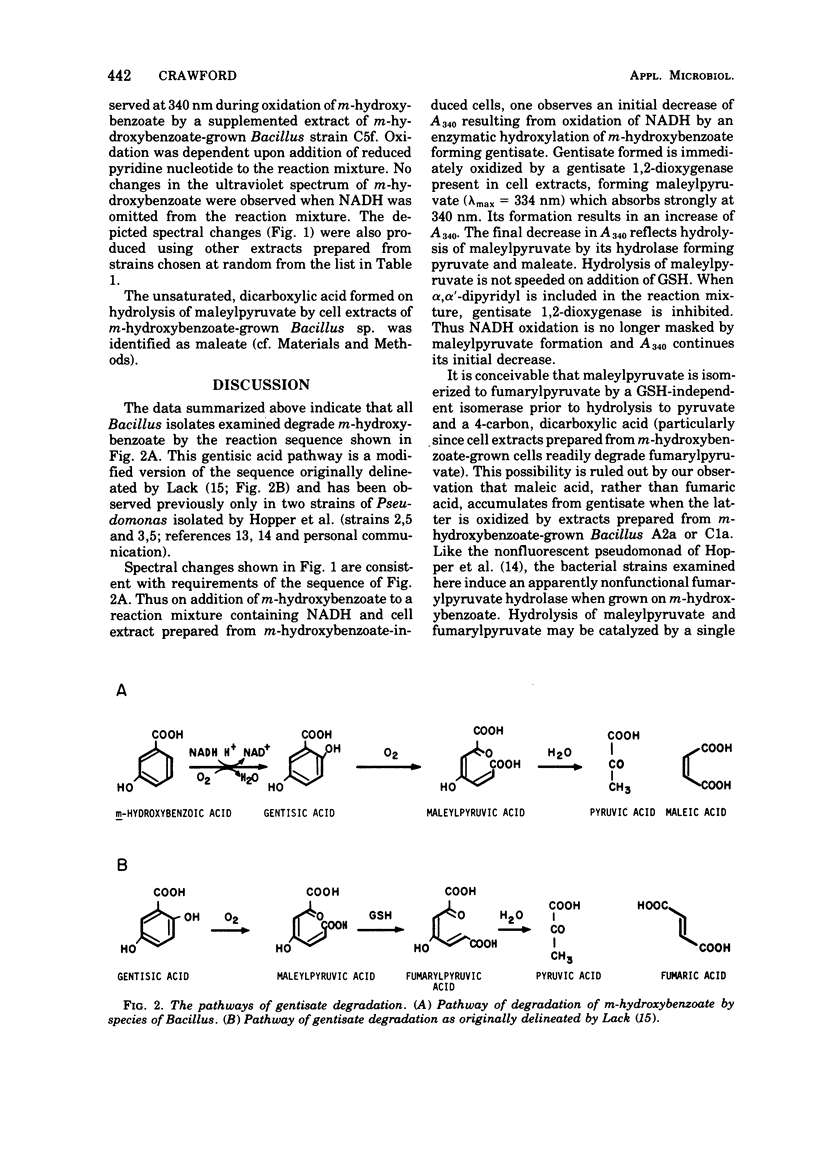
The pathway whereby certain bacterial strains of the genus Bacillus degrade m-hydroxybenzoate is delineated. Of 12 strains examined, nine were tentatively classified as representatives of the species Bacillus brevis, two of Bacillus sphaericus and one of Bacillus megaterium. All strains degraded m-hydroxybenzoate via the same pathway. m-Hydroxybenzoate was hydroxylated to 2,5-dihydroxybenzoate (gentisate), which was oxidized by a gentisate 1,2-dioxygenase yielding maleylpyruvate. Maleylpyruvate was hydrolyzed without prior cis, cis to cis, trans isomerization yielding pyruvate and maleic acid. Numerous soils were examined by plate-count procedures and found to contain 104 to 106 aerobic sporeformers able to grow on m-hydroxybenzoate per g of dry soil.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouknight R. R., Sadoff H. L. Tryptophan catabolism in Bacillus megaterium. J Bacteriol. 1975 Jan;121(1):70–76. doi: 10.1128/jb.121.1.70-76.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buswell J. A. The meta-cleavage of catechol by a thermophilic Bacillus species. Biochem Biophys Res Commun. 1974 Oct 8;60(3):934–941. doi: 10.1016/0006-291x(74)90404-5. [DOI] [PubMed] [Google Scholar]
- Collinsworth W. L., Chapman P. J., Dagley S. Stereospecific enzymes in the degradation of aromatic compounds by pseudomonas putida. J Bacteriol. 1973 Feb;113(2):922–931. doi: 10.1128/jb.113.2.922-931.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L., Hutton S. W., Chapman P. J. Purification and properties of gentisate 1,2-dioxygenase from Moraxella osloensis. J Bacteriol. 1975 Mar;121(3):794–799. doi: 10.1128/jb.121.3.794-799.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L. Novel pathway for degradation of protocatechuic acid in Bacillus species. J Bacteriol. 1975 Feb;121(2):531–536. doi: 10.1128/jb.121.2.531-536.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENSIGN J. C., RITTENBERG S. C. THE PATHWAY OF NICOTINIC ACID OXIDATION BY A BACILLUS SPECIES. J Biol Chem. 1964 Jul;239:2285–2291. [PubMed] [Google Scholar]
- Hegeman G. D., Rosenberg E. Y., Kenyon G. L. Mandelic acid racemase from Pseudomonas putida. Purification and properties of the enzyme. Biochemistry. 1970 Oct 13;9(21):4029–4036. doi: 10.1021/bi00823a001. [DOI] [PubMed] [Google Scholar]
- Hirschberg R., Ensign J. C. Oxidation of nicotinic acid by a Bacillus species: purification and properties of nicotinic acid and 6-hydroxynicotinic acid hydroxylases. J Bacteriol. 1971 Nov;108(2):751–756. doi: 10.1128/jb.108.2.751-756.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg R., Ensign J. C. Oxidation of nicotinic acid by a Bacillus species: regulation of nicotinic acid and 6-hydroxynicotinic acid hydroxylases. J Bacteriol. 1972 Oct;112(1):392–397. doi: 10.1128/jb.112.1.392-397.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg R., Ensign J. C. Oxidation of nicotinic acid by a Bacillus species: source of oxygen atoms for the hydroxylation of nicotinic acid and 6-hydroxynicotinic acid. J Bacteriol. 1971 Nov;108(2):757–759. doi: 10.1128/jb.108.2.757-759.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J., Chapman P. J., Dagley S. Enzymic formation of D-malate. Biochem J. 1968 Dec;110(4):798–800. doi: 10.1042/bj1100798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J., Chapman P. J. Gentisic acid and its 3- and 4-methyl-substituted homologoues as intermediates in the bacterial degradation of m-cresol, 3,5-xylenol and 2,5-xylenol. Biochem J. 1971 Mar;122(1):19–28. doi: 10.1042/bj1220019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACK L. The enzymic oxidation of gentisic acid. Biochim Biophys Acta. 1959 Jul;34:117–123. doi: 10.1016/0006-3002(59)90239-2. [DOI] [PubMed] [Google Scholar]
- Leung P. T., Chapman P. J., Dagley S. Purification and properties of 4-hydroxy-2-ketopimelate aldolase from Acinetobacter. J Bacteriol. 1974 Oct;120(1):168–172. doi: 10.1128/jb.120.1.168-172.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad C., Srinivasan V. R. Tryptophan catabolism during sporulation in Bacillus cereus. Biochem J. 1970 Sep;119(2):343–349. doi: 10.1042/bj1190343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher W., Jakoby W. B. Maleate isomerase. J Biol Chem. 1969 Apr 10;244(7):1878–1882. [PubMed] [Google Scholar]
- Spokes J. R., Walker N. Chlorophenol and chlorobenzoic acid co-metabolism by different genera of soil bacteria. Arch Mikrobiol. 1974 Mar 4;96(2):125–134. doi: 10.1007/BF00590169. [DOI] [PubMed] [Google Scholar]
- Wallnöfer P., Engelhardt G. [The degradation of phenylamides by Bacillus sphaericus]. Arch Mikrobiol. 1971;80(4):315–323. [PubMed] [Google Scholar]
- Willetts A. J., Cain R. B. Microbial metabolism of alkylbenzene sulphonates. Bacterial metabolism of undecylbenzene-p-sulphonate and dodecylbenzene-p-sulphonate. Biochem J. 1972 Sep;129(2):389–402. doi: 10.1042/bj1290389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts A. J. Microbial metabolism of alkylbenzene sulphonates. The oxidation of key aromatic compounds by a Bacillus. Antonie Van Leeuwenhoek. 1974;40(4):547–559. doi: 10.1007/BF00403819. [DOI] [PubMed] [Google Scholar]



