Abstract
Vincristine is an integral part of the “PCV” regimen that is commonly administered to treat primary brain tumors. The efficacy of vincristine as a single agent in these tumors has been poorly studied. This study was designed to determine whether vincristine enters normal rat brain or an intracranially or subcutaneously implanted glioma and to assess the presence of the efflux pump P-glycoprotein (P-gp) on tumor and vascular endothelial cells. The 9L rat gliosarcoma was implanted intracranially and subcutaneously in three Fischer 344 rats. On day 7, [3H]vincristine (50 μCi, 4.8 μg) was injected into the carotid artery, and the animals were euthanized 10 or 20 min later. Quantitative autoradiography revealed that vincristine levels in the liver were 6- to 11-fold greater than in the i.c. tumor, and 15- to 37-fold greater than in normal brain, the reverse of the expected pattern with intra-arterial delivery. Vincristine levels in the s.c. tumor were 2-fold higher than levels in the i.c. tumor. P-gp was detected with JSB1 antibody in vascular endothelium of both normal brain and the i.c. tumor, but not in the tumor cells in either location, or in endothelial cells in the s.c. tumor. These results demonstrate that vincristine has negligible penetration of normal rat brain or i.c. 9L glioma despite intra-arterial delivery and the presence of blood-brain barrier dysfunction as demonstrated by Evan’s blue. Furthermore, this study suggests that P-gp-mediated efflux from endothelium may explain these findings. The lack of penetration of vincristine into brain tumor and the paucity of single-agent activity studies suggest that vincristine should not be used in the treatment of primary brain tumors.
A number of unique problems are associated with the use of chemotherapy in tumors of the central nervous system. Penetration of many antineo-plastic agents is restricted by the barrier function of the CNS capillaries, and nitrosoureas have become standard therapy because of their ability to cross the blood-brain barrier. Attempts to improve efficacy of nitrosoureas (such as CCNU) by combining with vincristine and procarbazine (PCV4 therapy) initially appeared promising, particularly in anaplastic tumors (Levin, 1990). During the past three decades, the PCV regimen has been used extensively in the treatment of anaplastic astrocytomas (Levin, 2002; Ron, 2002) and anaplastic oligodendrogliomas (Bauman, 2001). In addition, it is being used in patients with low-grade astrocytomas and oligodendrogliomas (Buckner, 2003; Kristof, 2002; Louis, 2003) and is commonly prescribed for patients with newly diagnosed or recurrent glioblastoma multiforme (Murphy, 2002).
Recently, questions have arisen regarding the advantage of PCV over single-agent nitrosoureas. A retrospective review of the Radiation Therapy Oncology Group database suggested that there was no survival advantage associated with the use of PCV in anaplastic astrocytomas (Prados, 1999). Furthermore, a recent randomized study of radiotherapy with or without PCV in newly diagnosed grade 3 and 4 gliomas did not demonstrate a difference in survival (Medical Research Council Brain Tumor Working Party, 2001).
Vincristine is a relatively large, lipid-soluble plant alkaloid. Its dose-limiting toxicity is peripheral neuropathy due to interference with microtubule function in axons (Hilkens, 1997). The efficacy of vincristine has not been optimally evaluated in patients with gliomas. Initial single-agent studies were performed in the 1960s, without the benefit of modern imaging techniques (Lassman, 1965, 1966; Smart, 1968) or appropriate controls for changes in steroid dose. In a subsequent study of 12 patients with relapsed glioblastoma, Fewer (1972) reported a lower rate of neurological improvement (25%) when vincristine and carmustine were given together, as compared with 30 similar patients receiving carmustine alone (53%). Peripheral neuropathy was frequent in the vincristine-treated group, necessitating withdrawal of therapy in the majority.
Central neurotoxicity is rarely reported in patients receiving vincristine for other malignancies (Gidding, 1999), whereas accidental administration into the CSF in humans is rapidly fatal due to neurotoxicity (Williams, 1983). This is consistent with animal data that demonstrates poor penetration of vincristine and vinblastine through the intact blood-brain barrier (Greig, 1990). Studies with [3H]vincristine in rats, mice, dogs, and monkeys demonstrated no accumulation in normal brain (El Dareer, 1977).
The lack of penetration of normal brain by relatively large lipophilic compounds has been attributed to the multidrug resistance transporter P-glycoprotein (P-gp) on the luminal surface of brain capillaries (Shinkel, 1999), performing active transportation back into the circulation. Knockout mice lacking the gene for P-gp (mdr1) experience increased brain penetration of a related vinca alkaloid, vinblastine (Schinkel, 1994). P-gp antagonists increase penetration of the semisynthetic vinca alkaloid vinorelbine into normal brain in mice (Fine, 1996).
Penetration of vincristine might, however, be possible when the blood-brain barrier is disrupted around a high-grade glioma, with tumor vasculature becoming the limiting factor. However, tumor capillaries in human glioma biopsy samples have also been reported to express P-gp, potentially influencing entry of vincristine into tissues (Nabors, 1991). Lack of response of intracerebral xeno-graft implants to vincristine and doxorubicin has been attributed to the presence of P-gp on vessels within the tumor. P-gp was not demonstrated in blood vessels in responding s.c. implants (Takamiya, 1997).
Given recent data on the lack of efficacy of PCV chemotherapy, the individual components of this combination chemotherapy deserve to be reevaluated. If vincristine is shown to poorly penetrate into brain and brain tumor, even when there is substantial blood-brain-barrier dysfunction, its role in the treatment of CNS tumors must be questioned. This study uses a common rat gliosarcoma model (9L) to study the distribution of [3H]vincristine administered intra-arterially, comparing uptake in normal brain and in an i.c. tumor with uptake in the liver and in a subcutaneously implanted tumor. The presence of P-glycoprotein was analyzed by immunohistochemistry in i.c. and s.c. tumors.
Materials and Methods
Tumor Implantation
All animal care and experiments were performed in accordance with the guidelines set forth by the Animal Care and Use Committee of the Johns Hopkins Medical Institutions.
The 9L glioma cell line (Kimler, 1994) was grown subcutaneously in male Fischer 344 rats. The tumor was excised, and 2-mm cubes were implanted intracranially in four adult male Fischer rats (Harlan, Indianapolis, Ind.). Implantation was performed under anesthesia with 87 mg/kg ketamine (Fort Dodge Laboratories, Fort Dodge, Iowa) and 13 mg/kg xylazine (Phoenix Pharmaceuticals, St. Joseph, Mo.) administered intraperitoneally. The top of the head was shaved, and a midline incision was made under aseptic conditions. A 2-mm craniotomy was performed by using a dental drill, 4 mm lateral and just posterior to the coronal suture. The dura was pierced and the tumor cube placed in the brain. A piece of Gelfoam (Pharmacia & Upjohn Company, Kalamazoo, Mich.) was placed over the tumor, and the skin was closed with clips.
An s.c. tumor was implanted in the left flank of each animal, under the same anesthetic, with a similar cube placed into an s.c. pocket. The incision was closed with a clip, and a tumor nodule became palpable at day 5.
Preparation of [3H]Vincristine
[3H]Vincristine with a specific activity of 9.6 Ci/mM was obtained from Amersham (Arlington Heights, Ill.) and stored at −20°C. Radiochemical purity was determined by high-performance liquid chromatography to be 92.9%. In preparation for intracarotid injection, the vincristine was warmed to room temperature and diluted with saline to a final dose of 50 μCi (4.8 μg) in 0.4 ml per rat.
Intracarotid Injection
On day 6 after tumor implantation, rats were anesthetized with ketamine and xylazine. In three animals, following a midline neck incision, the right carotid artery was exposed by blunt dissection and ligated proximally to reduce flow. A 25G butterfly cannula was inserted in the carotid and held in place with a second ligature. The vincristine was injected slowly over 1 min into two animals, the cannula was flushed with saline, and the ligature was then loosened to restore flow. In the first two animals, right-sided seizures were observed briefly following injection. In the third animal, the right carotid was rudimentary and unable to be cannulated. The left carotid was cannulated, and bilateral seizures followed injection, suggesting perfusion of both hemispheres from the left carotid artery.
In the fourth animal, a tail vein injection of 2 ml/kg of a 2% solution of Evans blue (J.T. Baker, Phillipsburg, N.J.) was performed on day 5 to assess blood-brain-barrier integrity around the tumor. This animal was euthanized on day 6 by intracardiac injection, along with vincristine-treated animals (animals 1 and 2 at 20 min after vincristine and animal 3 at 10 min), and the brain was removed whole. A single lobe of liver was dissected, along with the s.c. tumor. Tissues were snap frozen in hexane at −40°C and stored at −20°C.
Preparation of Tissues for Quantitative Autoradiography
Frozen tissues were mounted on cryostat chucks with embedding matrix (Miles Laboratories, Inc., Elkhart, Ind.) and cut into 20-μm sections in a cryostat (Hacker Instruments, Fairfield, N.J.) at −17°C. Three pairs of consecutive samples were selected randomly from each organ or tissue. One section from each pair was mounted on a gelatin-coated slide and stained with hematoxylin and eosin for histological review; the other section was mounted on a gelatin-coated glass slide, warmed to 60°C, and used for quantitative autoradiography. Slides for autoradiography were heat fixed on a slide warmer for 15min after preparation.
3H standards (Amersham) with eight levels of activity (0.93 to 21.3 nCi/mg of tissue) and the slides containing the tissue sections for quantitative autoradiography were placed against radiation-sensitive Hyperfilm (Amersham) in an X-ray cassette and exposed for 14 days at room temperature. The films were hand developed with Kodak D19 developer (Eastman Kodak Company, Rochester, N.Y.) for 5 min, placed in a stop bath for 30 s, and finished with Photo-flo fixer (Kodak) for 5 min.
Quantitative Autoradiography Analysis
The autoradiographs and 3H standards were digitized by using an Inquiry program (Loats Assoc., Inc., Westminster, Md.). The absorbances of the standards for each animal’s results were corrected for background radioactivity and were then used to generate a standard curve, which converted absorbance data into units of disintegrations per minute per milligram of tissue. A power function using cubic regression of the absorbances of the standards yielded the highest coefficient of determination (r2 > 0.99). The specific activity of [3H]vincristine is known (9.6 Ci/mM), and hence the units of disintegrations per minute per milligram of tissue could be converted into units of concentration (fmol/mg).
For each animal, three adjacent slices of each organ were sampled. From each sample, five random pixels were measured, giving a total of 15 values to be averaged. Because the small number of animals precluded formal statistical analysis, summarized data is presented graphically with mean and confidence intervals, and fold differences between organs were calculated.
Immunohistochemistry
Frozen sections were air-dried, fixed, permeabilized in acid ethanol, and washed in phosphated-buffered saline (PBS). Blocking with 0.5% bovine serum albumin was followed by further washing in PBS. A 1:10 dilution (in PBS) of the JSB1 primary antibody was incubated for 60 min and then washed with PBS. The secondary antibody was added at a 1:50 dilution (in PBS) (using the LSAB+ kit-KO679 from Dako [Carpinteria, Calif]), incubated for 30 min, and then washed.
Streptavidin conjugated to horseradish peroxidase in a 1:200 dilution was added and incubated for 15 min. Endogenous peroxidase activity was suppressed with hydrogen peroxide prior to adding the first antibody. Slides were counterstained in Harris’s hematoxylin for 40 s, washed for 5 min, and dunked in blueing solution (1:5 dilution of saturated lithium carbonate), and then bathed in dehydration alcohols 95% and 100%, cleared in xylene, and finally mounted in mounting medium (Ultramount [Lab Vision, Fremont, Calif.]). Sections were photographed at a 10× magnification.
Whole brain and sections were photographed after snap freezing.
Results
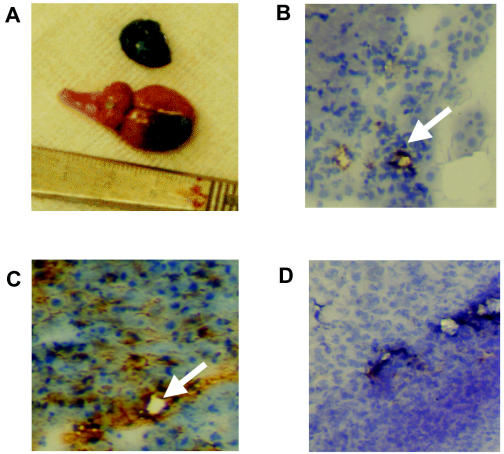
The extensive Evans blue staining within the brain tumor is shown in Fig. 1. This demonstrates that the blood-brain barrier around the tumor was open to macromolecules as large as albumin, which is bound to the Evans blue.
Fig. 1.
Evans blue staining and immunohistochemistry results. A. Albumin macromolecules, bound to Evans blue, penetrate the blood-brain barrier of the i.c. tumor and the s.c tumor, but not normal brain. B–D. JSB1 immunocytochemistry for P-gp. Staining is demonstrated in cerebral capillaries of the tumor (arrow, B) and in the liver (arrow, C), but not in the tumor cells in either location or in the s.c. capillaries (D); 40×magnification.
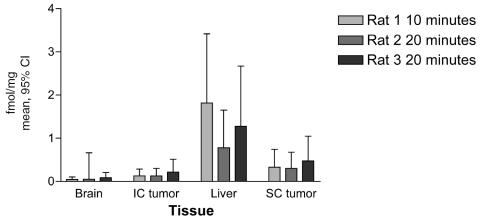
The vincristine concentrations in femtomoles per milligram (mean and 95% CI) determined by quantitative autoradiography in the brain tumor, normal brain, liver, and s.c. tumor of each animal are presented in Fig. 2. These data demonstrate that 50-μCi injections into the ipsilateral carotid resulted in concentrations of vincristine in the i.c. tumor after 20 min that were 6-fold lower than those in liver, and 2-fold lower than those in the s.c tumors. This is the reverse of the normal pattern after intra-arterial drug administration (Collins, 1984). Levels in ipsilateral normal brain were also negligible.
Fig 2.
Vincristine levels in each animal (fmol/mg, mean and 95% confidence intervals) determined by quantitative autoradiography. Levels in the liver are significantly higher than those in other organs, with normal brain being the lowest in each animal.
The 10-min time point in the third animal, which received vincristine into the dominant contralateral carotid, demonstrated no additional retention of vincristine in the brain or i.c. tumor. Liver concentrations in this animal were 11-fold higher than those in brain, consistent with the known short initial half-life, prompt tissue binding, and rapid hepatic excretion of vincristine (Castle, 1976).
Results of P-gp Immunohistochemistry
P-gp was detected in liver, a finding consistent with its role in biliary excretion of vincristine. It was not detected on tumor cells in brain or s.c. locations. However, it was detected on capillary endothelium in brain tumor and normal brain and was not detected in capillary endothelium in s.c. tumors. This is demonstrated in Figs. 1B–D.
Discussion
The findings suggest that normal brain and tumors implanted within the brain are poorly penetrated by vincristine, even when it is given as an intracarotid bolus. This occurs despite evidence that the blood-brain barrier is quite disrupted, allowing Evans blue bound to albumin, with MW 60,000, to enter the tumor. The molecular weight of vincristine is 930.
This lack of penetration is unexpected, given the lipid solubility of vincristine and its ready penetration of peripheral nerves. This is likely explained by the presence of pumps (P-gp or other related drug efflux pumps) on the cerebral capillaries but not in the peripheral nervous system. Our results do not rule out other steric factors that may hinder penetration of this large molecule. This lack of penetration explains the rarity of central neurotoxicity when vincristine is administered systemically, despite its profound neurotoxicity if administered into the CSF.
The poor penetration of vincristine into brain tumors suggests that it is unlikely that this drug adds substantially to the efficacy of brain tumor regimens. Vincristine is also associated with significant toxicities. Peripheral and autonomic neuropathy is an additional burden to a group of patients already at risk of problems with mobility and sensation due to the tumor itself and to other drugs, including steroids. In addition, the pharmacology of vincristine in patients with gliomas might be significantly affected by the coadministration of cytochrome P450–inducing anticonvulsant drugs. As with other chemotherapy agents, this drug interaction would be expected to reduce the systemic levels of vincristine, further diminishing the chances that this drug contributes to the therapy of this patient population (Fetell, 1997; Gilbert, 2003; Grossman, 1998.)
The limitations of this study include the small number of animals and the lack of plasma space correction for the different organs. This may lead to an underestimate of levels in the normal brain, since it is less vascular than the i.c. tumor, liver, and s.c. tumor. Penetration of drugs may also be affected by regional differences in vascularity of the 9L model, although this is a feature of human glioblastoma also, as demonstrated by regional difference in perfusion with MRI. It is also possible that seizures may have affected permeability, although these would be expected to increase rather than reduce observed drug levels.
Nevertheless, these findings provide experimental evidence that leads us to question the routine inclusion of vincristine in regimens for glioma treatment, given the lack of single-agent data in clinical trials and its recognized toxicity. Furthermore, its use in combination therapy for other CNS tumors, such as lymphomas and pediatric malignancies, should also be reevaluated.
Footnotes
The studies presented in this report were supported in part by the New South Wales Cancer Council and the Bill Walsh Cancer Research Trust.
This data has been presented in part as Boyle, F.M., Eller, S., and Grossman, S.A., The emperor’s new clothes: Vincristine distribution in experimental glioma, at the Fourth Annual Meeting of the Society for Neuro-Oncology, Scottsdale, Ariz., USA, November 17–21, 1999.
Abbreviations used are as follows: PBS, phosphated-buffered saline; PCV, procarbazine, CCNU, and vincristine; P-gp, P-glycoprotein.
References
- Bauman GS, Cairncross JG. Multidisciplinary management of adult anaplastic oligodendrogliomas and anaplastic mixed oligo-astrocytomas. Semin Radiat Oncol. 2001;11:170–180. doi: 10.1053/srao.2001.21429. [DOI] [PubMed] [Google Scholar]
- Buckner JC, Gesme D, Jr, O’Fallon JR, Hammack JE, Stafford S, Brown PD, Hawkins R, Scheithauer BW, Erickson BJ, Levitt R, Shaw EG, Jenkins R. Phase II trial of procarbazine, lomustine, and vincristine as initial therapy for patients with low-grade oligodendroglioma or oligoastrocytoma: Efficacy and associations with chromosomal abnormalities. J Clin Oncol. 2003;21:251–255. doi: 10.1200/JCO.2003.06.023. [DOI] [PubMed] [Google Scholar]
- Castle MC, Margileth DA, Oliverio VT. Distribution and excretion of (3H)vincristine in the rat and the dog. Cancer Res. 1976;36:3684–3689. [PubMed] [Google Scholar]
- Collins JM. Pharmacological rationale for regional drug delivery. J Clin Oncol. 1984;2:498–504. doi: 10.1200/JCO.1984.2.5.498. [DOI] [PubMed] [Google Scholar]
- El Dareer SM, White VM, Chen FP, Mellet LB, Hill DL. Distribution and metabolism of vincristine in mice, rats, dogs, and monkeys. Cancer Treatment Rep. 1977;61:1269–1277. [PubMed] [Google Scholar]
- Fetell MR, Grossman SA, Fisher JD, Erlanger B, Rowinsky E, Stockel J, Piantadosi S. Preirradiation paclitaxel in glioblastoma multiforme: Efficacy, pharmacology, and drug interactions. J Clin Oncol. 1997;15:3121–3128. doi: 10.1200/JCO.1997.15.9.3121. [DOI] [PubMed] [Google Scholar]
- Fewer D, Wilson CB, Boldrey EB, Enot KJ, Powell MR. The chemotherapy of brain tumors: Clinical experience with carmustine (BCNU) and vincristine. JAMA. 1972;222:549–552. [PubMed] [Google Scholar]
- Fine RL, Morgan PF, Hsieh A-L, Boncek V, Durcan MJ. Inhibition of P-glycoprotein (Pgp) in the intact blood-brain barrier (BBB) leads to increased penetration of Navelbine (vinorelbine) into the brain. Proc Am Assoc Cancer Res. 1996;37:290. Abstract #1970. [Google Scholar]
- Gidding CE, Kellie SJ, Kamps WA, de Graaf SS. Vincristine revisited. Crit Rev Oncol Hematol. 1999;29:267–287. doi: 10.1016/s1040-8428(98)00023-7. [DOI] [PubMed] [Google Scholar]
- Gilbert MR, Supko JG, Batchelor T, Lesser G, Fisher JD, Piantadosi S, Grossman S. Phase I clinical and pharmacokinetic study of irinotecan in adults with recurrent malignant glioma. Clin Cancer Res. 2003;9 :2940–2949. [PubMed] [Google Scholar]
- Greig NH, Soncrant TT, Shetty HU, Momma S, Smith QR, Rapoport SI. Brain uptake and anticancer activities of vincristine and vinblastine are restricted by their low cerebrovascular permeability and binding to plasma constituents in rat. Cancer Chemother Pharmacol. 1990;26:263–268. doi: 10.1007/BF02897227. [DOI] [PubMed] [Google Scholar]
- Grossman SA, Hochberg F, Fisher J, Chen TL, Kim L, Gregory R, Grochow LB, Piantadosi S. Increased 9-aminocamptothecin dose requirements in patients on anticonvulsants. NABTT CCNS Consortium The New Approaches to Brain Tumor Therapy. Cancer Chemother Pharmacol. 1998;42:118–126. doi: 10.1007/s002800050794. [DOI] [PubMed] [Google Scholar]
- Hilkens PHE, van den Bent MJ. Chemotherapy-induced peripheral neuropathy. J Peripher Nerv Syst. 1997;2:350–361. [PubMed] [Google Scholar]
- Kimmler BF. The 9L rat brain tumor model for pre-clinical investigation of radiation-chemotherapy interactions. J Neurooncol. 1994;20:103–109. doi: 10.1007/BF01052721. [DOI] [PubMed] [Google Scholar]
- Kristof RA, Neuloh G, Hans V, Dickert M, Urbach H, Schlegel U, Simon M, Schramm J. Combined surgery, radiation, and PCV chemotherapy for astrocytomas compared to oligodendrogliomas and oligoastrocytomas WHO grade III. J Neurooncol. 2002;59:231–237. doi: 10.1023/a:1019987116596. [DOI] [PubMed] [Google Scholar]
- Lassman LP, Pearce GW, Gang J. Sensitivity of intracranial gliomas to vincristine sulphate. Lancet, Feb. 1965;6:296–298. doi: 10.1016/s0140-6736(65)91029-9. [DOI] [PubMed] [Google Scholar]
- Lassman LP, Pearce GW, Gang J. Effect of vincristine sulphate on the intracranial gliomata of childhood. Br J Surg. 1966;53:774–777. doi: 10.1002/bjs.1800530910. [DOI] [PubMed] [Google Scholar]
- Levin VA, Silver P, Hannigan J, Wara WM, Gutin PH, Davis RL, Wilson CB. Superiority of post-radiotherapy adjuvant chemotherapy with CCNU, procarbazine, and vincristine (PCV) over BCNU for anaplastic gliomas: NCOG 6G61 final report. Int J Radiat Oncol Biol Phys. 1990;18:321–324. doi: 10.1016/0360-3016(90)90096-3. [DOI] [PubMed] [Google Scholar]
- Levin VA, Yung WK, Bruner J, Kyritsis A, Leeds N, Gleason MJ, Hess KR, Meyers CA. Phase II study of accelerated fractionation radiation therapy with carboplatin followed by PCV chemotherapy for the treatment of anaplastic gliomas. Int J Radiat Oncol Biol Phys. 2002;53 :58–66. doi: 10.1016/s0360-3016(01)02819-x. [DOI] [PubMed] [Google Scholar]
- Louis E, Keime-Guibert F, Delattre J-Y, Sanson M. Dramatic response to chemotherapy in oligodendroglial gliomatosis cerebri. Neurology. 2003;60:151. doi: 10.1212/wnl.60.1.151. [DOI] [PubMed] [Google Scholar]
- Medical Research Council Brain Tumor Working Party. Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: A Medical Research Council trial. J Clin Oncol. 2001;19:509–518. doi: 10.1200/JCO.2001.19.2.509. [DOI] [PubMed] [Google Scholar]
- Murphy C, Pickles T, Knowling M, Thiesse B. Concurrent modified PCV chemotherapy and radiotherapy in newly diagnosed grade IV astrocytoma. J Neurooncol. 2002;57:215–220. doi: 10.1023/a:1015797713149. [DOI] [PubMed] [Google Scholar]
- Nabors MW, Griffin CA, Zehnbauer BA, Hruban RH, Phillips PC, Grossman SA, Brem H, Colvin OM. Multidrug resistance gene (MDR1) expression in human brain tumors. J Neurosurg. 1991;75:941–946. doi: 10.3171/jns.1991.75.6.0941. [DOI] [PubMed] [Google Scholar]
- Prados MD, Scott C, Curran WJ, Jr, Nelson DF, Leibel S, Kramer S. Procarbazine, lomustine, and vincristine (PCV) chemotherapy for anaplastic astrocytoma: A retrospective review of Radiation Therapy Oncology Group protocols comparing survival with carmustine or PCV adjuvant chemotherapy. J Clin Oncol. 1999;17:3389–3395. doi: 10.1200/JCO.1999.17.11.3389. [DOI] [PubMed] [Google Scholar]
- Ron IG, Gal O, Vishne TH, Kovner F. Long-term follow-up in managing anaplastic astrocytoma by multimodality approach with surgery followed by postoperative radiotherapy and PCV-chemotherapy: Phase II trial. Am J Clin Oncol. 2002;25:296–302. doi: 10.1097/00000421-200206000-00020. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, Mol CAAM, van der Valk MA, Robanus-Maandag EC, te Riele HPJ, Berns AJM, Borst P. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Schinkel AH. P-glycoprotein, a gatekeeper in the blood-brain barrier. Adv Drug Deliv Rev. 1999;36:179–194. doi: 10.1016/s0169-409x(98)00085-4. [DOI] [PubMed] [Google Scholar]
- Smart CR, Ottoman RE, Rochlin DB, Hornes J, Silva AR, Goepfert H. Clinical experience with vincristine (NSC-67574) in tumors of the central nervous system and other malignant diseases. Cancer Chemother Rep Part 1. 1968;52:733–741. [PubMed] [Google Scholar]
- Takamiya Y, Abe Y, Tanaka Y, Tsugu A, Kazuno M, Oshika Y, Maruo K, Ohnishi Y, Sato O, Yamazaki H, Kijima H, Ueyama Y, Tamaoki N, Nakamura M. Murine P-glycoprotein on stromal vessels mediates multidrug resistance in intracerebral human glioma xenografts. Br J Cancer. 1997;76:445–450. doi: 10.1038/bjc.1997.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ME, Walker AN, Bracikowski JP, Garner L, Wilson KD, Carpenter JT. Ascending myeloencephalopathy due to intrathecal vincristine sulfate. A fatal chemotherapeutic error. Cancer. 1983;51:2041–2047. doi: 10.1002/1097-0142(19830601)51:11<2041::aid-cncr2820511114>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]