Abstract
Background
Tight glycaemic control in people with type 2 diabetes can lead to a reduction in microvascular and possibly macrovascular complications. The use of near-patient (rapid) testing offers a potential method to improve glycaemic control.
Aim
To assess the effect and costs of rapid testing for glycated haemoglobin (HbA1c) in people with type 2 diabetes.
Design of study
Pragmatic open randomised controlled trial.
Setting
Eight practices in Leicestershire, UK.
Method
Patients were randomised to receive instant results for HbA1c or to routine care. The principal outcome measure was the proportion of patients with an HbA1c <7% at 12 months. We also assessed costs for the two groups.
Results
Of the 681 patients recruited to the study 638 (94%) were included in the analysis. The mean age at baseline was 65.7 years (SD = 10.8 years) with a median (interquartile range) duration of diabetes of 4 (1–8) years. The proportion of patients with HbA1c <7% did not differ significantly between the intervention and control groups (37 versus 38%, odds ratio 0.95 [95% confidence interval = 0.69 to 1.31]) at 12 months follow up. The total cost for diabetesrelated care was £390 per patient for the control group and £370 for the intervention group. This difference was not statistically significant.
Conclusion
Near-patient testing for HbA1c alone does not lead to outcome or cost benefits in managing people with type 2 diabetes in primary care. Further research is required into the use of rapid testing as part of an optimised patient management model including arrangements for patient review and testing.
Keywords: cost, hemoglobin A glycosylated, near-patient testing, primary care, type 2 diabetes mellitus
INTRODUCTION
Diabetes is a common chronic condition managed largely in primary care. Patients with diabetes are at high risk of developing complications including cardiovascular disease, with increased associated mortality. However, tight glycaemic control can lead to a reduction in microvascular and possibly also macrovascular complications.1,2 There is evidence that direct healthcare costs are lower in patients who have either tight glycaemic control.3,4 or whose control is improving.5 Despite the evidence, there are wide variations in care between general practices in terms of glycaemic control.6,7 The National Service Framework for diabetes emphasises the importance of structured diabetes care programmes including regular recall and review.8 The traditional method of testing for glycaemic control in primary care involves sending a blood sample away for laboratory testing and waiting a number of days for the result to be returned. General practices vary in how they deal with this time delay between testing and result. In some, the patient is asked to attend for a blood test up to 2 weeks before their diabetic review involving an extra visit to the surgery. In other practices, blood is taken at the time of the review and the patient is asked to telephone for the result, or to make a second practice visit; with this arrangement, not only is an extra visit or telephone call required, but the impetus may also be lost in terms of acting on the result or the patient may not contact the practice for their result.
How this fits in
Tight glycaemic control can lead to a reduction in microvascular and possibly also macrovascular complications. Despite the evidence, there are wide and unacceptable variations in care between general practices and there is a large unmet need for tight glycaemic control. A limited number of secondary care studies have shown that near-patient testing for glycated haemoglobin can lead to improvements in glycaemic control. This study shows that near-patient testing for glycated haemoglobin alone does not lead to outcome or cost benefits in managing patients with type 2 diabetes in primary care.
The use of a rapid test offers a potential method of improving monitoring of glycated haemoglobin (HbA1c). Near-patient testing is a technology offering the facility for carrying out patient tests on site, with a rapid result obtained at the time, usually within a matter of minutes. This type of test may provide greater convenience to patients, more timely decisions on clinical management and therefore improved therapeutic control and reduced overall health costs.9 A device for rapid measurement of HbA1c is available and has been shown to perform well in a number of clinical settings.10 A randomised controlled trial of patients on insulin in a hospital centre in the US showed a significant improvement in glycaemic control in patients in the rapid test group after 6 and 12 months.11 A retrospective study comparing patients attending two hospital clinics in the UK12 found that patients attending the clinic where rapid results were obtained had better glycaemic control. The use of this method of testing for HbA1c in primary care has not, however, been prospectively investigated in terms of patient outcomes or costs. We aimed to assess the effect and costs of using this technology for testing people with type 2 diabetes in primary care but without changing other modalities of care.
METHOD
Study design and practice recruitment
This was a prospective randomised controlled trial with randomisation at patient level within participating general practices. Approval for the study was obtained from the local ethics committee. We invited all 47 group practices with a list size of above 5000 patients to consider taking part in the study of which 20 volunteered. Eight practices were selected to give a good representation including urban and rural as well as deprived and affluent area practices. This was a pragmatic study in which the practices involved were not required to make organisational changes and the intervention was restricted to the use of a rapid test in place of traditional laboratory HbA1c testing.
Sample size
Approximately 40% of people with diabetes have a normal HbA1c in primary care.6 Our original power calculation was based on an increase in the proportion of patients in the intervention group with good glycaemic control from around 40–50%. With a significance level of 5% and power of 80%, approximately 400 patients in each arm would therefore be needed. Further power calculations were carried out at a later stage to assess the effect of lower than anticipated recruitment. Recruitment of additional practices or extension of the study was impractical due to the availability of machines and funding constraints. The new calculations suggested that with around 300 cases in each arm and an increase of 10% in the proportion of intervention group patients with good glycaemic control, the power would be reduced to approximately 70%; alternatively, the power would remain at 80% if we were able to show a 12% increase in good control.
Patient recruitment
Patients attending participating general practices for review of their diabetes care were recruited by practice nurses using information sheets provided by the research team. As the study was restricted to the management of patients attending general practices for management of type 2 diabetes, we excluded patients who were unable to attend the practice and any who were exclusively under hospital care. All patients recruited received information detailing the study aims and requirements and were asked to sign a consent form. Randomisation to the intervention group (rapid test for HbA1c) or control group (normal laboratory testing for HbA1c) was at patient level within each practice. This was carried out by practice nurses immediately following patient recruitment, using numbered sealed envelopes prepared using computer allocated block randomisation in blocks of four. The research team generated the allocation sequence but the practice nurses enrolled the patients to the study. Patients were recruited between August 2000 and May 2002. Each patient was followed up for a period of 12 months from recruitment.
Near-patient test method
The equipment used for this study was the Bayer DCA 2000. This requires a finger prick sample rather than formal venesection and the result is ready in about 6 minutes.10 The National Service Framework for diabetes8 has drawn attention to the need for quality control when using analysers for near-patient testing; during our study period, internal quality control was carried out before each diabetes clinic by the practice nurses using Bayer control kits and external quality control was carried out by the research team as part of the Wales External Quality Assessment Scheme.13
Outcome measures
The principal outcome measure was the proportion of patients with ‘good’ glycaemic control, defined as HbA1c <7% in line with the NSF8 and NICE guidelines.14 To ensure that we would be comparing like with like in our analysis, we obtained laboratory HbA1c results for all patients at baseline and after 12 months at one laboratory (Bio-Rad Variant II high performance liquid chromatography, Diabetes Control and Complications Trial aligned). During the study period, results obtained using the DCA2000 analyser were used for patient management in the intervention group.
Statistical analyses
Analyses were conducted for both intention to treat and per protocol samples. Proportions of patients achieving good control at baseline and at follow-up were estimated separately for intervention and control group patients, together with 95% confidence intervals (CIs) and the difference between proportions. To investigate the influence of potential confounding factors in analysing changes in glycaemic control, multiple logistic regression analyses were undertaken using SAS software. In these models, the outcome measure was ‘good’ glycaemic control (HbA1c <7%) at follow-up. Odds ratios (ORs) and 95% CIs were estimated for intervention group patients compared to controls. In modelling, baseline glycaemic control status (good versus poor) was always included and the potential confounding effects of sex, general practice attended, duration of diabetes, treatment at baseline, age and deprivation were also investigated. Co-linearity between variables and influential observations were assessed; overall the final models for the intention to treat and per protocol analyses were a reasonable fit to the data.
Costs
For this study we focused solely on a comparison of actual costs between the two groups. We also measured patient-borne costs of attending the general practice as the number of visits required could potentially be changed by a regime involving instant results. As we were estimating the costs that accrued in a single year, costs were not discounted. Costs in the intervention and control groups were compared for statistical significance using independent sample t-tests.
The cost of a laboratory test for HbA1c (£12) was obtained from University Hospitals of Leicester NHS Trust. The information used to estimate the cost of the intervention test method was obtained from Bayer Diagnostics; to calculate average costs, the total costs of providing the tests were divided by the number of rapid tests performed in each practice. Information relating to prescribing, consultations with practice staff and use of secondary care services was collected from GP records by a trained data collector using a standardised data collection form. Information was also collected on the use of urine and blood test strips, lancets and sundries such as needles. For estimating usage of diabetes-related medication, the data collected were the name of the drug, dosage and duration of prescribing. All prescribing was costed by means of information obtained from the British National Formulary.15
The costs of GP and nurse-related practice and telephone consultations were obtained from a published source.16 The duration of nurse reviews in the practice were assumed at 20 minutes and with GPs at 12.6 minutes. The duration of telephone consultations with both GPs and practice nurses was estimated at 10.8 minutes.16 The cost of visiting a phlebotomist was obtained from the NHS reference costs.17 Use of hospital inpatient and outpatient services was costed using prices obtained from University Hospitals of Leicester NHS Trust. For inpatient stays a cost per day was used.
At recruitment, all patients were asked to complete a questionnaire relating to patient-borne costs, which included information on mode of travel, mileage, fares, parking fees incurred, travelling time and time spent at the practice. The questionnaire also asked about employment status and whether anyone had accompanied the responder to the surgery. The cost per mile of car travel was taken from the Automobile Association website.18 The cost of time spent travelling and receiving care was estimated using information from the Department of Transport19 on the value of both working and non-working time. These costs were inflated to 2002–2003 prices by means of the Retail Price Index. The values used were £15.13 per hour for working time and £4.89 for non-working time. The value of any accompanying adults' time was assumed to be at the non-working rate.
RESULTS
Characteristics of participating practices
The median list size of practices recruited was 10 650 (range = 6000–12 800) and the median number of partners was 5 (range = 4–7). The median number of people with Type 2 diabetes in these practices was 215 (range = 140–450). The median Jarman score of the practices taken as an indicator of deprivation was −7.18 (range = −13.97 to 22.51). Three practices were training practices.
Patient recruitment and characteristics of patients recruited
Figure 1 shows the CONSORT diagram describing the flow of participants through the trial. A total of 681 patients were recruited to the study. From records kept by nurses, it was estimated that this sample represented approximately 90% of those invited to participate. Of those recruited, 10 were identified as having Type 1 diabetes and two had impaired glucose tolerance and these were excluded from the analysis. Two patients asked to be withdrawn from the study due to ill health. A further 68 patients did not fulfil the ‘per protocol’ study requirements in that we were unable to identify both a baseline and 12-month follow up HbA1c laboratory test result, because the patient had left the practice, died or failed to attend appointments. For 29 of these patients, no further HbA1c result was available after recruitment and these cases were therefore excluded from the analysis. The other 39 cases were included in the intention-to-treat analysis using either rapid results or interim results from tests carried out at any time between recruitment and 12 months. The final sample for the intention-to-treat analysis therefore comprised a total of 638 patients with a range of 44 to 115 per practice.
Figure 1.
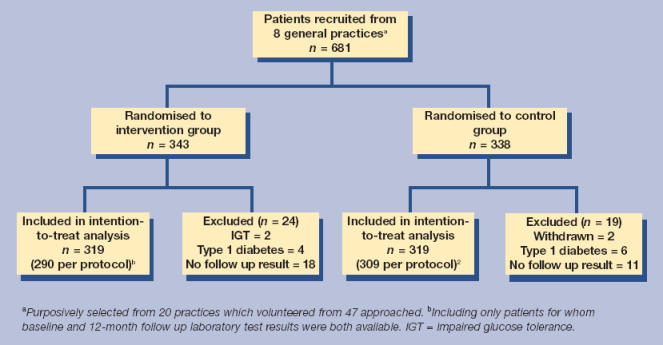
Flow of participants through the trial.
Baseline characteristics of the 638 patients are shown in Table 1. The two groups were well matched overall, but the difference of 7% (95% CI = −1 to 15%) for good glycaemic control was of borderline statistical significance at the 5% level.
Table 1.
Baseline characteristics of 638 patients included in the analysis. n (%) unless indicated.
| Intervention group (n = 319) | Control group (n = 319) | |
|---|---|---|
| Sex | ||
| Female | 138 (43) | 132 (41) |
| Male | 181 (57) | 187 (59) |
| Care arrangements:a | ||
| GP | 277 (88) | 283 (90) |
| Shared | 39 (12) | 33 (10) |
| Glycaemic control: | ||
| Good (HbA1c <7%) | 140 (44) | 119 (37) |
| Poor (HbA1c <7%) | 179 (56) | 200 (63) |
| Mean (SD) HbA1c | 7.5(1.6) | 7.7 (1.6) |
| Blood pressure:a | ||
| Systolic <140 mmHg | 115 (36) | 112 (35) |
| ≥140 mmHg | 203 (64) | 204 (65) |
| Diastolic <80mmHg | 150 (47) | 127 (40) |
| 80mmHg | 168 (53) | 189 (60) |
| Treatment: | ||
| Insulin | 33 (10) | 33 (10) |
| Oral hypoglycaemic agents | 208 (65) | 186 (58) |
| Diet/lifestyle only | 78 (24) | 100 (31) |
| Mean age in years at recruitment (SD) | 65.96 (10.85) | 65.42 (10.67) |
| Diabetes duration (years) at recruitmenta | 4 | 4 |
| Median (IQR) | (2 to 8) | (1 to 8) |
| Deprivation scorea | 11.51 | 11.33 |
| Median (IQR) | (6.50 to 16.71) | (6.50 to 16.71) |
Missing data: care arrangements = 6; blood pressure = 4; diabetes duration = 3; deprivation score = 21. SD = standard deviation. IQR = interquartile range.
Principal outcome measure
Proportions of patients in the intervention and control groups achieving good metabolic control (HbA1c <7.0%) at follow up were very similar (Table 2): intervention 0.37 (95% CI = 0.32 to 0.42) versus controls 0.38 (95% CI = 0.33 to 0.43). The unadjusted odds ratio (OR) for intervention group patients compared to controls achieving good glycaemic control at follow up was 0.95 (95% CI = 0.69 to 1.31). The OR for the intervention versus control group was 0.80 (95% CI = 0.56 to 1.15) when adjusted for baseline HbA1c status. We also conducted multiple logistic regression modelling giving the best fit to the data. The final model included baseline HbA1c status (good or poor control), sex, duration of diabetes treatment at baseline and general practice attended, OR = 0.84 (95% CI = 0.58 to 1.22). Restricting the analysis to those patients (n = 599) who completed the study per protocol, the unadjusted OR was 0.97 (95%CI = 0.70 to 1.36) and the adjusted OR was 0.88 (95% CI = 0.60 to 1.29).
Table 2.
Glycaemic control in intervention and control group patients (intention to treat analysis, n = 638 patients).
| Intervention group (near-patient testing) n = 319 (%) | Control group (laboratory testing) n = 319 (%) | |||
|---|---|---|---|---|
| Proportion of patients achieving good control (HbA1c <7%) | 95% CI | Proportion of patients achieving good control (HbA1c <7%) | 95%CI | |
| Baseline (recruitment) data | 0.44 | 0.39 to 0.49 | 0.37 | 0.32 to 0.42 |
| Follow-up (12 month) data | 0.37 | 0.32 to 0.42 | 0.38 | 0.33 to 0.43 |
Costs
For eight of the 638 patients included in the intention-to-treat analysis, insufficient data were available and our comparison of costs is therefore based on 630 patients (315 in each group). Costs relating to diabetes care provided by the NHS are summarised in Table 3. The only cost items for which a statistically significant difference between the two groups was detected when carrying out detailed comparisons were that of the HbA1c tests and practice nurse telephone contacts. The number of HbA1c tests performed was similar in the two groups; the difference in total cost was due to the higher unit cost of the rapid test (£20.88 compared to £12.00 for the laboratory test). The total cost for diabetes-related care was £390 per patient for the control group and £370 for the intervention group. This comparison was not statistically significant and the average total number of GP visits for any reason during the study year was also similar at 12.4 and 12.7 for intervention and control group patients, respectively. The absolute number of surgery contacts for diabetes was lower in the intervention group (1598 contacts, mean 5.1 per patient) than in the control group (1765 contacts, mean 5.6 per patient) but this difference was not statistically significant (P = 0.11). Patients included in the intention-to-treat analysis returned 529/638 (83%) questionnaires to estimate patient-borne costs of visiting the GP. These costs were similar in the intervention and control groups. (Table 4).
Table 3.
Costs (£) of diabetes related care for the study year.
| Control group patients n = 315 | Intervention group patients n = 315 | |||
|---|---|---|---|---|
| Total cost | Mean cost per patient | Total cost | Mean cost per patient | |
| Diabetic medication | 34 360 | 109.08 | 34 467 | 109.42 |
| Consumables (blood and urine strips, lancets and sundries) | 10 086 | 32.02 | 11 627 | 36.91 |
| General practice contacts (with nurse, GP, phlebotomist or chiropodist at surgery and GP or nurse home visits) | 22 681 | 72.00 | 20 287 | 64.40 |
| Contacts with hospital | 47 071 | 149.43 | 35 153 | 111.60 |
| HbA1c tests | 8520 | 27.05* | 15 162 | 48.13a |
| Total diabetes-related costs | 122 718 | 389.58 | 116 696 | 370.46 |
All costs are for the year 2002–2003 except for those of the HbA1c test which are for 2003.
Denotes costs where there is a statistically significant difference at the 5% level between the control and intervention group.
Table 4.
Patient-borne costs of diabetes related visits to general practice.
| Mean value for control group/patients (n = 260) | Mean value for intervention group patients (n = 269) | |
|---|---|---|
| Cost of average visit (travel expenses, £) | 1.53 | 1.32 |
| Cost of average visit (time costs, £) | 8.05 | 8.60 |
| Total cost of visit (£) | 9.58 | 9.92 |
| Total number of visits to general practicea | 5.48 | 5.07 |
| Total responder-borne cost of all visits (£) | 52.47 | 50.31 |
There were no statistically significant differences between any values for intervention and control groups.
Home visits and telephone contacts excluded from analysis.
DISCUSSION
Summary of main findings
This study adds to the evidence relating to the use of near-patient testing in a general practice setting in the UK. It indicates that use of a rapid test to measure HbA1c in patients with type 2 diabetes in primary care is unlikely on its own to lead to improvements in metabolic control or to cost savings.
Strengths and limitations of the study
Little rigorous research exists on potential uses of near-patient testing in primary care.20 Although it could be argued that our study was based on a naïve premise that near-patient testing alone would be likely to lead to improvements in glycaemic control in our study population, evidence from secondary care studies11,12 offered good reason to surmise that this might be the case. As might be expected, mean HbA1c levels at baseline (Table 1) suggested that the patients in our study had relatively good glycaemic control compared to those in the secondary care studies.11,12 We conducted qualitative interviews which showed that practice nurses and patients had generally high satisfaction within their diabetes clinics prior to the intervention including their usual arrangement for carrying out HbA1c testing and obtaining results. This suggests that there may have been limited scope for improvement. Nevertheless, baseline HbA1c levels were far from optimal in our study population, with well over half in each group having levels over 7%.
Due to funding constraints, randomisation in this study was at patient level within each participating practice; randomisation at practice level would have required a much larger, more costly study. In practice, it proved difficult for surgeries to organise their management of patients with diabetes in such a way as to maximise the benefits of using the rapid test for intervention group patients. Practices therefore often continued their follow-up in the usual way. This also explains our failure to show any cost savings in terms of a reduction in appointments as the practices did not re-organise their clinics and the patients in many instances continued to attend twice.
The patients participated voluntarily and may therefore have been atypical but a high proportion of patients approached were recruited to the trial and followed up for 1 year suggesting that our results are not limited to a very select group. The nurses and the GPs were not blind to the intervention and may have changed the care given to patients in both arms of the study. Due to the study design, it was not possible to blind the data collector. With the exception of baseline HbA1c the distribution of patient characteristics was similar in the intervention and control groups.
Participating practices were volunteers so may also have been atypical, but the proportion of patients with a normal HbA1c in our study was similar to other published studies.6 From our baseline comparison of intervention and control group patients, it was noted that there was a borderline statistically significant difference between the proportion of patients in each group with good glycaemic control. Randomisation had therefore worked sub-optimally in terms of achieving balanced groups, but we addressed this by including baseline glycaemic control status as a covariate in our logistic regression modelling.
Comparison with existing literature
Two previous studies of near-patient testing showed improved glycaemic control.11,12 However, both these studies were carried out in secondary care and patients in these studies had poorer diabetes control than patients in our study. Only one of these was a randomised controlled trial and this study was restricted to patients with type 1 and type 2 patients treated with insulin.11 In the other group of studies,12 the comparison of HbA1c levels was provided by a retrospective cohort study involving patients attending clinics in two different hospitals. Differences in methodology, setting and baseline levels of glycaemic control may therefore help to explain the difference between our findings and those of previous studies.
In other evaluations of HbA1c target achievement, structured and intensive management regimes have been used; in our study no effort was made to change the overall management strategy. We used a simple intervention; although it could be argued that a multifactorial approach would have had more likelihood of success, this approach may lead to difficulties with identifying which elements of the intervention have led to effectiveness.
Implications for future research and clinical practice
Our results indicate that near-patient testing for HbA1c alone does not lead to improvements in outcomes or reduction in costs in managing patients with type 2 diabetes in primary care. Further research is required into the use of the rapid test as part of an overall diabetes management strategy including arrangements for reviewing and testing patients. The evaluation of this type of intervention would need to be conducted with randomisation at practice rather than patient level to facilitate the effective adoption of a suitable model of care for all eligible patients in intervention surgeries. If future research is able to suggest that a multifactorial intervention of this type would be effective, our study would indicate that it is not simply the near-patient testing technology that led to this positive outcome. In the meantime, our results suggest that general practices should not rush to adopt of the use of in-house testing for HbA1c on the assumption that this alone will lead to better glycaemic control within their patient population.
Acknowledgments
We wish to acknowledge the contribution made by the participating general practices, particularly the practice nurses. We would like to thank Alison Dunkley for the data collection and data entry.
Funding body
This work was funded by the NHS Executive (Trent). Near-patient testing equipment and associated sundries were loaned or provided by Bayer Diagnostics
Ethics committee
The study was approved by the Leicestershire Ethics Committee (reference 5102)
Competing interests
As stated above, DCA2000 analysers were loaned and cartridges and quality control kits were provided by Bayer Diagnostics. There was no other conflict of interest
REFERENCES
- 1.Turner RC, Holman RR, Cull CA, et al. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 2.Shamoon H, Duffy H, Fleischer N, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Gilmer TP, O'Connor PJ, Manning WG, Rush WA. The cost to health plans of poor glycemic control. Diabetes Care. 1997;20:1847–1853. doi: 10.2337/diacare.20.12.1847. [DOI] [PubMed] [Google Scholar]
- 4.Menzin JP, Langley-Hawthorne CML, Friedman MM, et al. Potential short-term economic benefits of improved glycemic control: a managed care perspective. Diabetes Care. 2001;24:51–55. doi: 10.2337/diacare.24.1.51. [DOI] [PubMed] [Google Scholar]
- 5.Wagner EH, Sandhu N, Newton KM, et al. Effect of improved glycemic control on health care costs and utilization. JAMA. 2001;285:182–189. doi: 10.1001/jama.285.2.182. [DOI] [PubMed] [Google Scholar]
- 6.Khunti K, Ganguli S, Baker R, Lowy A. Features of primary care associated with variations in process and outcome of care of people with diabetes. Br J Gen Pract. 2001;51:356–360. [PMC free article] [PubMed] [Google Scholar]
- 7.Guthrie B, Emslie-Smith A, Morris A, et al. Quality measurement of care for people with type 2 diabetes in Tayside, Scotland: Implications for the new UK general practice contract. Br J Gen Pract. 2003;53:709–713. [PMC free article] [PubMed] [Google Scholar]
- 8.Department of Health. National Service Framework for Diabetes: Standards. London: Department of Health; 2001. 2001/026. [Google Scholar]
- 9.Hobbs R. Near patient testing in primary care—offers better patient management but needs proper evaluation and quality control. BMJ. 1996;312:263–264. doi: 10.1136/bmj.312.7026.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pope RM, Apps JM, Page MD, et al. A novel device for the rapid in-clinic measurement of haemoglobin A(1c) Diabet Med. 1993;10:260–263. doi: 10.1111/j.1464-5491.1993.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 11.Cagliero E, Levina EV, Nathan DM. Immediate feedback of HbA(1c) levels improves glycemic control in type 1 and insulin-treated type 2 diabetic patients. Diabetes Care. 1999;22:1785–1789. doi: 10.2337/diacare.22.11.1785. [DOI] [PubMed] [Google Scholar]
- 12.Grieve R, Beech R, Vincent J, Mazurkiewicz J. Near patient testing in diabetes clinics: Appraising the costs and outcomes. Health Technol Assess. 1999;3:1–74. [PubMed] [Google Scholar]
- 13.WEQAS. Wales External Quality Assessment Scheme. http://www.weqas.co.uk/dsp_frame.cfm?link=schemes (accessed 1 Jun 2006)
- 14.National Institute for Health and Clinical Excellence. Inherited clinical guideline G. Management of type 2 diabetes: management of blood glucose. London: NICE; 2002. [Google Scholar]
- 15.British Medical Association and Royal Pharmaceutical Society of Great Britain. British National Formulary. Vol. 44. London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2002. [Google Scholar]
- 16.Netten J, Curtis J. Unit costs of health and social care. Cantebury: Personal Social Services Research Unit, University of Kent; 2003. [Google Scholar]
- 17.NHS Executive. The new NHS—reference costs 2003. London: NHS Executive; 2004. [Google Scholar]
- 18.Automobile Association. http://www.theaa.com/allaboutcars/advice/advice_rcosts_petrol_table.jsp. (accessed 22 May 2006)
- 19.Department of Transport. Transport Economics Note 1999. London: Department of Transport; 2004. [Google Scholar]
- 20.Delaney BC, Hyde CJ, McManus RJ, et al. Systematic review of near patient test evaluations in primary care. BMJ. 1999;319:824–827. doi: 10.1136/bmj.319.7213.824. [DOI] [PMC free article] [PubMed] [Google Scholar]


