Abstract
Protein Z (PZ) is a vitamin K-dependent plasma protein whose function has been uncertain. The structure of PZ is very similar to that of the coagulation-related factors VII, IX, and X and PC, but PZ differs from these other proteins in that it is not the zymogen of a serine protease. We have shown recently that PZ forms a calcium ion-dependent complex with activated factor X at phospholipid surfaces and that this interaction leads to the inhibition of activated factor X activity through, in part, the action of a previously unidentified plasma protein named PZ-dependent protease inhibitor. Herein, we report that the presence of PZ dampens the coagulation response in human plasma and that concomitant PZ deficiency dramatically increases the severity of the prothrombotic phenotype of factor VLeiden mice. The results indicate that PZ plays a physiologically important role in the regulation of coagulation.
Vitamin K is required for the posttranslational formation of a unique amino acid, γ-carboxyglutamic acid, which is present in a number of plasma proteins that are involved in hemostasis: prothrombin; factors VII, IX, and X; protein C (PC); and protein S (PS). γ-Carboxyglutamic acid-mediated calcium ion binding in these proteins is necessary for their association with phospholipid surfaces and is critical for their hemostatic function (1, 2). Prothrombin and factors VII, IX, and X are involved in the coagulant response leading to the generation of thrombin at sites of injury, whereas PC and PS play roles in an antithrombotic pathway that limits coagulation by inactivating the important coagulation cofactors Va and VIIIa (3). Protein Z (PZ) is a 62-kDa, vitamin K-dependent plasma protein whose structure is similar to that of factors VII, IX, and X and PC. In contrast to these other proteins, however, PZ is not the zymogen of a serine protease, because it lacks the His and Ser residues of the catalytic triad (4, 5). The rate of association (kassn = 3.36 × 10−5 s−1⋅M−1) and rate of dissociation (kdssn = 0.057 s−1) of PZ with/from phospholipids are significantly slower, and the plasma level of γ-carboxylated PZ during chronic warfarin therapy (≤1%) is dramatically lower than the rates and levels of the other vitamin K-dependent proteins (6, 7). Despite its initial isolation several years ago (8, 9), the physiological function of PZ has not been determined.
We have shown recently that PZ forms a calcium ion-dependent complex with factor Xa at phospholipid surfaces (10). This PZ–factor Xa interaction reduces the rate of factor Xa inhibition by antithrombin but dramatically enhances the inhibition of factor Xa produced by a previously unidentified plasma protein named PZ-dependent protease inhibitor (ZPI; ref. 10). ZPI is a 72-kDa member of the serpin superfamily of proteinase inhibitors that contains a tyrosine as the P1 residue at its reactive center (10, 11). In systems that use purified components, ZPI produces rapid inhibition of factor Xa (t1/2 < 10 s) that requires the presence of PZ, calcium ions, and phospholipids (10). Initial studies of the inhibitory spectrum of ZPI have shown that it does not affect the catalytic activity of thrombin, meizothrombin, factor VIIa, factor IXa, activated PC, tissue-plasminogen activator, urokinase-plasminogen activator, plasmin, trypsin, leukocyte elastase, chymotrypsin, or cathepsin G (11).
Additional in vitro and in vivo experiments were undertaken to determine the physiological relevance of PZ. The results show that the role of PZ, like that of PC or PS, is to limit the coagulation response.
Methods
Materials.
Hepes, EDTA, barium chloride, sodium oxalate, BSA, and rabbit brain cephalin were from Sigma. Affigel-10 was purchased from Bio-Rad.
Proteins.
PZ and ZPI were purified from human plasma as described (10). Prothrombin, factor X, and factor IXa were products of Enzyme Research Laboratories (South Bend, IN).
Barium-Adsorbed Plasma.
Pooled normal citrated plasma (George King Biomedical, Overland Park, KS) was barium-salt-adsorbed by adding 1.0 M BaCl2 to a final concentration of 0.1 M, stirring on ice for 30 min, and centrifuging at 10,000 × g for 20 min at 4°C. The plasma supernatant was dialyzed against 0.1 M NaCl/0.02 M Hepes, pH 7.4/0.01 M sodium oxalate overnight at 4°C. Barium sulfate (100 mg/ml) was added with mixing (4°C); the mixture was centrifuged at 10,000 × g for 20 min; and the supernatant plasma was frozen (−70°C) in small aliquots. Based on immunoassay, the concentration of PZ in the barium adsorbed plasma was <3% (<1.2 nM) of the starting plasma.
Coagulation Assays.
Thrombin and factor Xa functional assays were performed as described (12). Samples were obtained from the soluble phase after vortexing of reactions containing fibrin clots, diluted in 0.1 M NaCl/0.02 M Hepes, pH 7.4 with 1.0 mg/ml BSA (factor Xa assay) or the same buffer with 5 mM EDTA (thrombin assay), and tested immediately.
Factor VLeiden (FVLeiden) and PZ Gene-Deleted Mice.
The production of FVLeiden mice will be described in a separate publicaiton (unpublished work). To produce PZ gene-deleted mice, the full-length murine PZ cDNA was isolated from a mouse liver cDNA library (lambda ZAP, Stratagene) with a PCR-generated probe corresponding to codons 59–472 of the human PZ cDNA sequence. Based on the intron–exon boundaries in the human PZ gene, a cDNA fragment predicted to encompass exon 2 of the mouse PZ gene was used to screen a P1 genomic DNA library made from the 129/Sv mouse strain (Genome Systems, St. Louis). A 10.5-kilobase (kb) KpnI fragment of the isolated murine PZ gene that spanned exons 2–6 of the gene was subcloned for restriction enzyme mapping, partial DNA sequencing, and construction of the targeting vector (see Fig. 2). A 1.3-kb fragment of PZ DNA extending from the KpnI site in intron 1 to the BglI site in exon 2 was inserted into the multiple cloning site of pBluescript II between KpnI and BglI. A 1.5-kb neomycin phosphotransferase (neo) cassette containing the phosphoglycerate kinase promoter, was introduced at the XhoI site of pBluescript II in the same orientation as the PZ DNA. A 1.8-kb herpes simplex virus–thymidine kinase cassette was added at the XbaI site of pBluescript II for negative selection. The targeting vector was completed by inserting a 5.9-kb AatII–BglI fragment of PZ DNA containing a portion of exon 2 and exons 3–6 at the SalI site of pBluescript II. The structure of the targeting construct was confirmed by PCR, restriction mapping, and sequencing of ligation junctions. The targeting vector was linearized and introduced into 129/Sv-derived RW4 embryonic stem cells by electroporation, and stable transfectants were selected as described (13). Of 480 clones, 11 contained the expected allele by Southern analysis. Two independent stem cell clones were injected into C57BL/6 blastocysts as described (13), and the resulting chimeric males were bred to C57BL/6 females to generate initial F1 PZ(+/−) offspring. To produce FV(λ/+)/PZ(+/−) mice, PZ(−/−) mice derived from F1 PZ(+/−) intercrosses were mated with homozygous FVLeiden [FV(λ/λ)] mice on a mixed C57BL/6 × 129/Sv genetic background. Animals from subsequent FV(λ/+)/PZ(+/−) crosses were then used in various mating strategies to determine the effect of the PZ genotype on the survival of FVLeiden mice. Because of the effect of genetic background on the phenotype of FV(λ/λ) mice (unpublished work), only the phenotypes of littermate mice were compared in these studies.
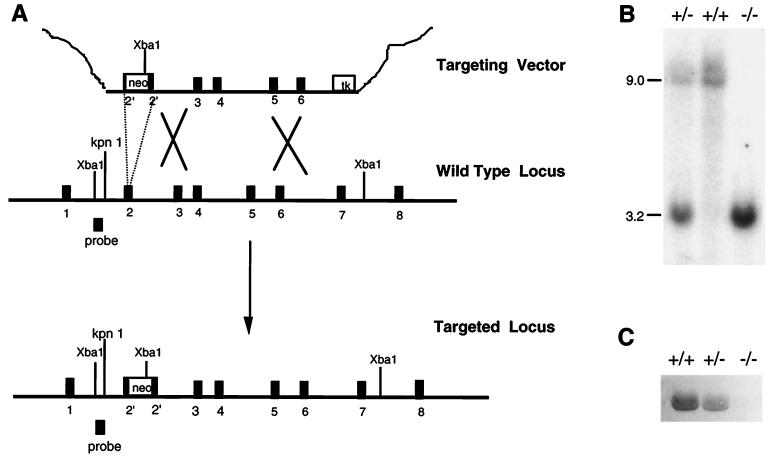
Figure 2.
Generation of PZ(−/−) mice. (A) Gene targeting. The targeting construct (Top) contains a neo cassette (neo) that replaces a 139-bp portion of exon 2 and induces a frameshift mutation. A herpes simplex virus–thymidine kinase cassette (tk) was added at the 3′ end of the mouse PZ DNA to permit negative selection. The predicted product of homologous recombination is shown (Bottom). The position of the ≈500-bp hybridization probe used to detect successful gene targeting is also depicted. (B) Southern blot analysis. Genomic DNA prepared from tail biopsies was analyzed by restriction digestion with XbaI and hybridization with the probe. Expected DNA fragment sizes are 9.0 kb for the wild-type allele and 3.2 kb for the disrupted allele. (C) Western blot analysis of mouse plasma with rabbit anti-human PZ polyclonal antibodies.
Genotyping of Mice.
DNA prepared from tail biopsies was used for Southern and PCR analysis. For Southern blotting, the hybridization probe was a ≈0.5-kb XbaI–KpnI fragment of PZ genomic DNA just 5′ of the region of homologous recombination (see Fig. 2). The PCR assay for the PZ gene used three primers derived from (i) a portion of exon 2 deleted in the mutant allele (5′-AAACAACGTTCTGCGGAGGTGGA-3′); (ii) intron 2 (5′-AACGAACTAGTTAGTCCTGAGACA-3′); and (iii) neo cassette (5′-ATTGCTGAAGAGCTTGGCGG-3′). The PCR product derived from the wild-type PZ allele is 214 bp; that derived from the mutant PZ allele is 449 bp. The PCR assay for the FVLeiden genotype used two primers: (i) intron 10, upstream of an inserted lox P sequence (5′-CCTCTGGACTCTGACTGCAG-3′); and (ii) intron 10, downstream of the lox P (5′-TATTCTGGACTACAAGAGTGA-3′). The PCR product derived from the wild-type FV allele is 400 bp; that derived from the FVLeiden allele is 544 bp.
Other Methods.
Mouse blood for platelet count and fibrinogen assay was obtained from the retro-orbital plexus with a heparinized capillary tube. Fibrinogen levels were determined with a modification of the microturbidimetric method (14). Pooled plasma from PZ(+/+) mice (n = 12) was used as standard and assigned a fibrinogen level of 100%. Western blotting for mouse plasma PZ was performed as described with 1 μl of murine plasma and rabbit polyclonal anti-human PZ antibodies (13). FV(λ/λ) embryos with PZ(+/+), PZ(+/−), and PZ(−/−) genotypes (four each) were fixed in paraformaldehyde, embedded in paraffin, and sectioned at 6 μm. Three serial sections were mounted per slide. Every fifth slide was stained with hematoxylin and eosin, and the following slide was analyzed after antifibrin(ogen) immunohistochemical staining as described (13).
Results
Effect of PZ on in Vitro Coagulation.
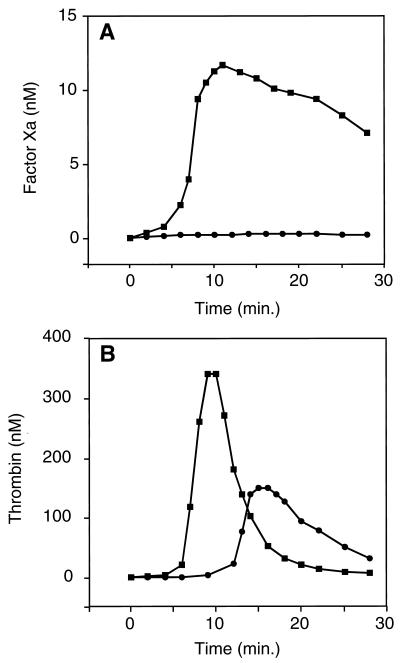
In in vitro studies, the effect of PZ on factor Xa activity and thrombin generation during coagulation was determined by using pooled normal human plasma that had been depleted of PZ (<3%) and other vitamin K-dependent factors by barium salt adsorption (see Methods). In this artificial plasma replenished with factor X but not prothrombin (to prevent significant thrombin generation), the factor Xa activity produced after the induction of coagulation by factor IXa was reduced dramatically in the presence of PZ (Fig. 1A). Thrombin generation was examined in the same system by the addition of prothrombin as well as factor X to the plasma. In the presence of PZ, thrombin generation was delayed significantly, and the peak thrombin concentration was reduced >50% (Fig. 1B). These in vitro results suggested that PZ could play a role in limiting coagulation.
Figure 1.
Regulation of coagulation by PZ. (A) Factor Xa activity. Factor IXa (0.5 nM) was used to induce coagulation in mixtures containing rabbit brain phospholipids (cephalin, 15 μM); CaCl2 (10 mM); barium-adsorbed, oxalated, pooled normal plasma (50% vol/vol); and factor X (100 nM) with or without PZ (30 nM). Phospholipids and CaCl2 with or without PZ were incubated 2 min before the addition of the remaining reagents, and the reaction was initiated by the addition of the plasma. (B) Thrombin generation. Reactions were the same as described for A, except prothrombin (700 nM) was also added to each mixture. ■, −PZ; ●, +PZ.
PZ Gene Disruption in Mice.
To investigate the in vivo consequences of PZ deficiency, the PZ gene was disrupted in mice. Successful gene targeting resulted in replacement of a 139-bp fragment of exon 2, encoding a portion of the signal sequence and the N terminus of PZ, by a neo cassette (Fig. 2A). The expected structure of the targeted PZ locus was confirmed by Southern blot analysis, and PZ was not detectable in the plasma of homozygous gene-disrupted mice by Western blotting (Fig. 2 B and C).
DNA analysis of 303 progeny derived from PZ(+/−) intercrosses showed that PZ(−/−) mice were born in expected numbers: 23% PZ(+/+), 54% PZ(+/−), and 23% PZ(−/−). Further, the growth and development of PZ null mice were indistinguishable from those of their PZ(+/−) and PZ(+/+) littermates. Blood platelet counts (×103) were 1,115 ± 210, 1,120 ± 281, and 1,116 ± 98, and fibrinogen levels (percentages) were 100 ± 28, 102 ± 33, and 93 ± 29 in PZ(+/+) (n = 15), PZ(+/−) (n = 14), and PZ(−/−) (n = 14) mice, respectively. Immunohistological analysis of the tissues from PZ(−/−) mice did not demonstrate vascular thrombosis or hepatic fibrin deposition (not shown). Thus, in the absence of an additional challenge, murine PZ deficiency produces a grossly normal phenotype. To evaluate further the potential prothrombotic risk associated with PZ deficiency, PZ gene-disrupted mice were crossbred with mice carrying the FVLeiden mutation.
Effect of PZ Deficiency on the FVLeiden Phenotype.
In humans, an altered form of coagulation FV, termed FVLeiden, is caused by a missense mutation that replaces Arg-506 in the FV molecule with a glutamine residue (R506Q; ref. 15). In contrast to normal FV, the activated form of FVLeiden is resistant to inactivation by activated PC, and FVLeiden functions poorly as a cofactor for activated PC-mediated inactivation of factor VIIIa (15–20). These abnormalities lead to increased thrombin production and a prothrombotic phenotype. Genetic heterozygosity for FVLeiden [FV(λ/+)] is common in humans of European descent (≈5%), and the FV(λ/+) and FV(λ/λ) genotypes have been shown to increase the risk of thrombosis by ≈5- and ≈25-fold, respectively (21, 22). Mice homozygous for the FVLeiden mutation (R504Q, equivalent to the human R506Q mutation) express a strain-specific thrombotic phenotype (unpublished work). In the mixed C57BL/6 × 129 genetic background present in these experiments, ≈20% of the FV(λ/λ) mice die during the neonatal period with microvascular thrombosis.
The genotypes of 6-week-old mice derived from a variety of FVLeiden/PZ mating strategies are shown in Table 1. The PZ(−/−) genotype markedly increases the mortality of FV(λ/λ) mice, and only a single FV(λ/λ)/PZ(−/−) animal has survived to adulthood thus far. The PZ(+/−) genotype also increases mortality in FV(λ/λ) mice, because the ratio of FV(λ/λ)/PZ(+/+) to FV(λ/λ)/PZ(+/−) progeny derived from FV(λ/λ)/PZ(+/−) intercrosses is 1:1, instead of the anticipated 1:2 (P < 0.01). Platelet counts (×103) and fibrinogen levels (percentages) were similar in FV(λ/λ)/PZ(+/+) (832 ± 183 and 87 ± 24, respectively; n = 19) and FV(λ/λ)/PZ(+/−) (891 ± 212 and 89 ± 21, respectively; n = 19) animals. The survival of FV(λ/+)/PZ(−/−) mice is significantly less than that of FV(λ/+)/PZ(+/−) animals (P < 0.01). Thus, the level of PZ affects the phenotype of heterozygous FVLeiden mice as well.
Table 1.
Progeny of FVLeiden/PZ matings
| Mating pairs | Observed (%) | Expected, % | P* |
|---|---|---|---|
| FV(λ/λ)/PZ(+/−) × FV(λ/λ)/PZ(+/−) | |||
| At 6 weeks | |||
| FV(λ/λ)/PZ(+/+) | 40 (52) | 25 | |
| FV(λ/λ)/PZ(+/−) | 37 (48) | 50 | <0.01 |
| FV(λ/λ)/PZ(−/−) | 0 (0) | 25 | <0.01 |
| At E17.5–E18.5† | |||
| FV(λ/λ)/PZ(+/+) | 7 (21) | 25 | |
| FV(λ/λ)/PZ(+/−) | 18 (53) | 50 | |
| FV(λ/λ)/PZ(−/−) | 9 (26) | 25 | |
| FV(λ/λ)/PZ(+/−) × FV(+/+)/PZ(−/−) (6 weeks) | |||
| FV(λ/+)/PZ(+/−) | 28 (74) | 50 | |
| FV(λ/+)/PZ(−/−) | 10 (26) | 50 | <0.01 |
| FV(λ/λ)/PZ(−/−)‡ × FV(λ/λ)/PZ(+/−) (6 weeks) | |||
| FV(λ/λ)/PZ(+/−) | 16 (100) | 50 | |
| FV(λ/λ)/PZ(−/−) | 0 (0) | 50 | <0.01 |
Difference between observed and expected frequencies derived by the confidence interval for binomial distributions.
En, embryonic day n.
Single FV(λ/λ)/PZ(−/−) male, derived from a F(λ/+)/PZ(+/−) intercross, mated with five females.
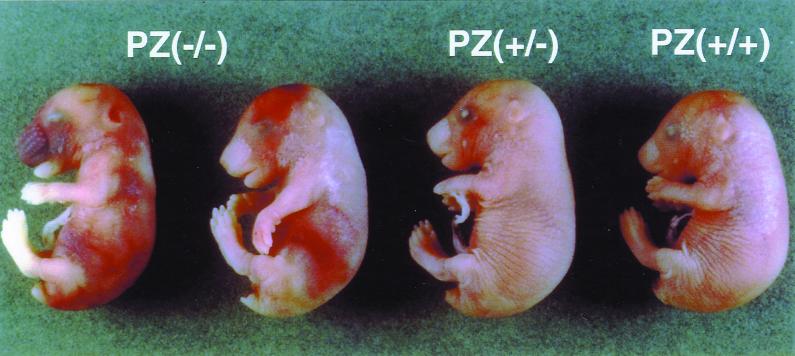
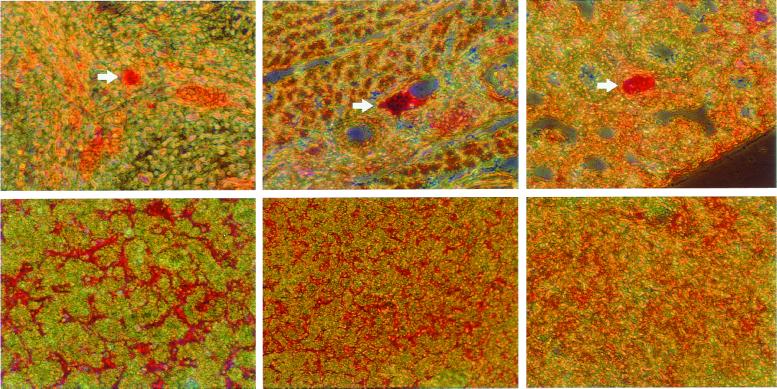
Of the nine pups found dead during the 6 weeks after birth, one had the FV(λ/λ)/PZ(−/−) genotype; three had the FV(λ/λ)/PZ(+/−) genotype; and five had the FV(λ/λ)/PZ(+/+) genotype, implying that many of the FV(λ/λ)/PZ(−/−) and FV(λ/λ)/PZ(+/−) mice were lost in utero or during the perinatal period. To determine whether the PZ genotype affected the intrauterine survival of FV(λ/λ) mice and to obtain appropriate tissues for histological analysis, embryos derived from FV(λ/λ)/PZ(+/−) intercrosses were examined at late gestation. FV(λ/λ)/PZ(−/−) and FV(λ/λ)/PZ(+/−) embryos were present at E17.5–E18.5 in expected numbers, but eight of the nine FV(λ/λ)/PZ(−/−) embryos showed obvious signs of hemorrhage during a visual inspection (Table 1 and Fig. 3). Antifibrin(ogen) immunohistochemical analysis of the FV(λ/λ) embryos showed vascular thrombosis and hepatic fibrin deposition, the scope of which was related directly to the PZ genotype. [PZ(−/−) > PZ(+/−) > PZ(+/+); Fig. 4].
Figure 3.
E17.5 FV(λ/λ) embryos with PZ(−/−), PZ(+/−), and PZ(+/+) genotypes.
Figure 4.
Antifibrin(ogen) staining of tissues from E17.5 embryos. (Upper) Thrombosis (arrows) of spinal (Left), chest wall (Center), and pulmonary (Right) vessels in a FV(λ/λ)/PZ(−/−) embryo. (Lower) Hepatic fibrin deposition in FV(λ/λ) embryos with PZ(−/−) (Left), PZ(+/−) (Center), and PZ(+/+) (Right) genotypes.
Discussion
These in vitro and in vivo studies suggest that PZ plays an important role in dampening coagulation. The cofactor effect of PZ for the inactivation of factor Xa by ZPI is presumably an important part of this regulatory action of PZ, but additional, ZPI-independent effects of PZ have not been excluded. Isolated PZ deficiency is apparently compatible with normal survival in mice. When combined with the homozygous FVLeiden genotype, however, PZ deficiency causes intrauterine and perinatal thrombosis and an apparent consumptive coagulopathy that leads to near absolute mortality. The genetic combinations FV(λ/λ)/PZ(+/−) and FV(λ/+)/PZ(−/−) produce smaller, although significant reductions in survival. The intensification of the thrombotic phenotype in FVLeiden mice produced by PZ deficiency is consistent with recent human data showing that a combination of prothrombotic traits significantly increases the risk of thrombosis (23) and underscores the multigenic nature of thrombophilia.
In contrast to tissue factor pathway inhibitor (TFPI) null and PC null mice (13, 24), which develop lethal disseminated intravascular coagulation, homozygous PZ-deficient mice have an apparently normal phenotype, at least in the absence of a thrombotic challenge. In this regard, they are similar to FV(λ/+), TFPI(+/−), and PC(+/−) mice, which are also asymptomatic in the unchallenged state (refs. 13 and 24; unpublished work). Like the PZ(−/−) genotype, the TFPI(+/−) genotype in combination with the FV(λ/λ) genotype produces near absolute mortality (unpublished work). Thus, the thrombotic risk associated with homozygous PZ deficiency seems similar to that of heterozygous TFPI deficiency in the mouse. The risk of thrombosis associated with PZ deficiency in humans remains to be determined. Based on the data from the murine gene-deletion models and the very broad range of PZ plasma levels present in apparently normal blood donors (7), this risk is likely to be modest.
The oral anticoagulant warfarin interferes with the action of vitamin K, leading to the production of incompletely γ-carboxylated, nonfunctional proteins. The reduction in functional prothrombin and factor X levels seems to be responsible for the antithrombotic effects of warfarin therapy (25). Levels of PC and PS, however, are also reduced by warfarin treatment, and the microvascular thrombosis that occurs with warfarin-induced skin necrosis is thought to be due to a relative imbalance between procoagulant and anticoagulant forces during the initiation of warfarin therapy (26). PZ deficiency, like PC and PS deficiency, may increase the risk of this infrequent but serious complication of warfarin therapy (27, 28). Finally, the levels of vitamin K-dependent plasma proteins are low during the perinatal period in comparison to adult levels (29). It is conceivable that relative deficiencies of endogenous PC (24), PS, and/or PZ may contribute to the partial neonatal mortality observed in mice that carry the FV(λ/λ) genotype as their sole defect.
Acknowledgments
We thank Terri Lewis for secretarial assistance in the preparation of this manuscript. This work was supported in part by National Institutes of Health Grant HL-60782.
Abbreviations
- PZ
PC, and PS, proteins Z, C, and S
- factor na
activated factor n
- ZPI
PZ-dependent protease inhibitor
- FV
factor V
- kb
kilobase
- En
embryonic day n
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.120081897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.120081897
References
- 1.Stenflo J, Fernlund P, Egan W, Roepstorff P. Proc Natl Acad Sci USA. 1974;71:2730–2733. doi: 10.1073/pnas.71.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelsestuen G L, Zytkovicz T H, Howard J B. J Biol Chem. 1974;249:6347–6350. [PubMed] [Google Scholar]
- 3.Esmon C T. Arterioscler Thromb. 1992;12:135–145. doi: 10.1161/01.atv.12.2.135. [DOI] [PubMed] [Google Scholar]
- 4.Sejima H, Hayashi T, Deyashiki Y, Nishioka J, Suzuki K. Biochem Biophys Res Commun. 1990;171:661–668. doi: 10.1016/0006-291x(90)91197-z. [DOI] [PubMed] [Google Scholar]
- 5.Ichinose A, Takeya H, Espling E, Iwanaga S, Kisiel W, Davie E W. Biochem Biophys Res Commun. 1990;172:1139–1144. doi: 10.1016/0006-291x(90)91566-b. [DOI] [PubMed] [Google Scholar]
- 6.McDonald J F, Shah A M, Schwalbe R A, Kisiel W, Dahlback B, Nelsestuen G L. Biochemistry. 1997;36:5120–5127. doi: 10.1021/bi9626160. [DOI] [PubMed] [Google Scholar]
- 7.Miletich J P, Broze G J., Jr Blood. 1987;69:1580–1586. [PubMed] [Google Scholar]
- 8.Prowse C V, Esnouf M P. Biochem Soc Trans. 1977;5:255–256. doi: 10.1042/bst0050255. [DOI] [PubMed] [Google Scholar]
- 9.Broze G J, Jr, Miletich J P. J Clin Invest. 1984;73:933–938. doi: 10.1172/JCI111317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han X, Fiehler R, Broze G J., Jr Proc Natl Acad Sci USA. 1998;95:9250–9255. doi: 10.1073/pnas.95.16.9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han X, Huang Z-F, Fiehler R, Broze G J., Jr Biochemistry. 1999;34:11073–11078. doi: 10.1021/bi990641a. [DOI] [PubMed] [Google Scholar]
- 12.Broze G J, Jr, Warren L A, Novotny W F, Higuchi D A, Girard T J, Miletich J P. Blood. 1988;71:335–343. [PubMed] [Google Scholar]
- 13.Huang Z-F, Higuchi D, Lasky N, Broze G J., Jr Blood. 1997;90:944–951. [PubMed] [Google Scholar]
- 14.Macarat M, Koffi A, Henocque G, Mathieu J-F, Guilbaud J-C. Clin Chem. 1989;35:211–214. [PubMed] [Google Scholar]
- 15.Bertina R M, Koeleman B P C, Koster T, Rosendaal F R, Dirven R J, de Ronde H, van der Velden P A, Reitsma P H. Nature (London) 1994;369:64–67. doi: 10.1038/369064a0. [DOI] [PubMed] [Google Scholar]
- 16.Sun X, Evatt B L, Griffin J H. Blood. 1994;83:3120–3125. [PubMed] [Google Scholar]
- 17.Nicolaes G A F, Tans G, Thomassen M C L G D, Hemker H C, Pabinger I, Varadi K, Schwarz H P, Rosing J. J Biol Chem. 1995;270:21158–21166. doi: 10.1074/jbc.270.36.21158. [DOI] [PubMed] [Google Scholar]
- 18.Heeb M J, Kojima Y, Greengard J S, Griffin J H. Blood. 1995;85:3405–3411. [PubMed] [Google Scholar]
- 19.Dahlback B, Hildebrand B. Proc Natl Acad Sci USA. 1994;91:1396–1400. doi: 10.1073/pnas.91.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorelli E, Kaufman R J, Dahlback B. Blood. 1999;93:2552–2558. [PubMed] [Google Scholar]
- 21.Koster T, Rosendaal F R, de Ronde F, Briet E, Vanderbroucke J P, Bertina R M. Lancet. 1993;342:1503–1506. doi: 10.1016/s0140-6736(05)80081-9. [DOI] [PubMed] [Google Scholar]
- 22.Svensson P J, Dahlback B. N Engl J Med. 1994;330:517–522. doi: 10.1056/NEJM199402243300801. [DOI] [PubMed] [Google Scholar]
- 23.Seligsohn U, Zivelin A. Thromb Haemostasis. 1997;78:297–301. [PubMed] [Google Scholar]
- 24.Jalbert L R, Rosen E D, Moons L, Chan J C Y, Carmeliet P, Collen D, Castellino F J. J Clin Invest. 1998;102:1481–1488. doi: 10.1172/JCI3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zivelin A, Rao L V, Rapaport S I. J Clin Invest. 1993;92:2131–2140. doi: 10.1172/JCI116814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eby C S. Hematol Oncol Clin North Am. 1993;7:1291–1300. [PubMed] [Google Scholar]
- 27.Gladson C L, Groncy P, Griffin J H. Arch Dermatol. 1987;123:1701a–1706a. [PubMed] [Google Scholar]
- 28.Sallah S, Abdallah J M, Gagnon G A. Haemostasis. 1998;28:25–30. doi: 10.1159/000022380. [DOI] [PubMed] [Google Scholar]
- 29.Reverdiau-Moalic P, Delahousse B, Body G, Bardos P, Leroy J, Gruel Y. Blood. 1996;88:900–906. [PubMed] [Google Scholar]