Abstract
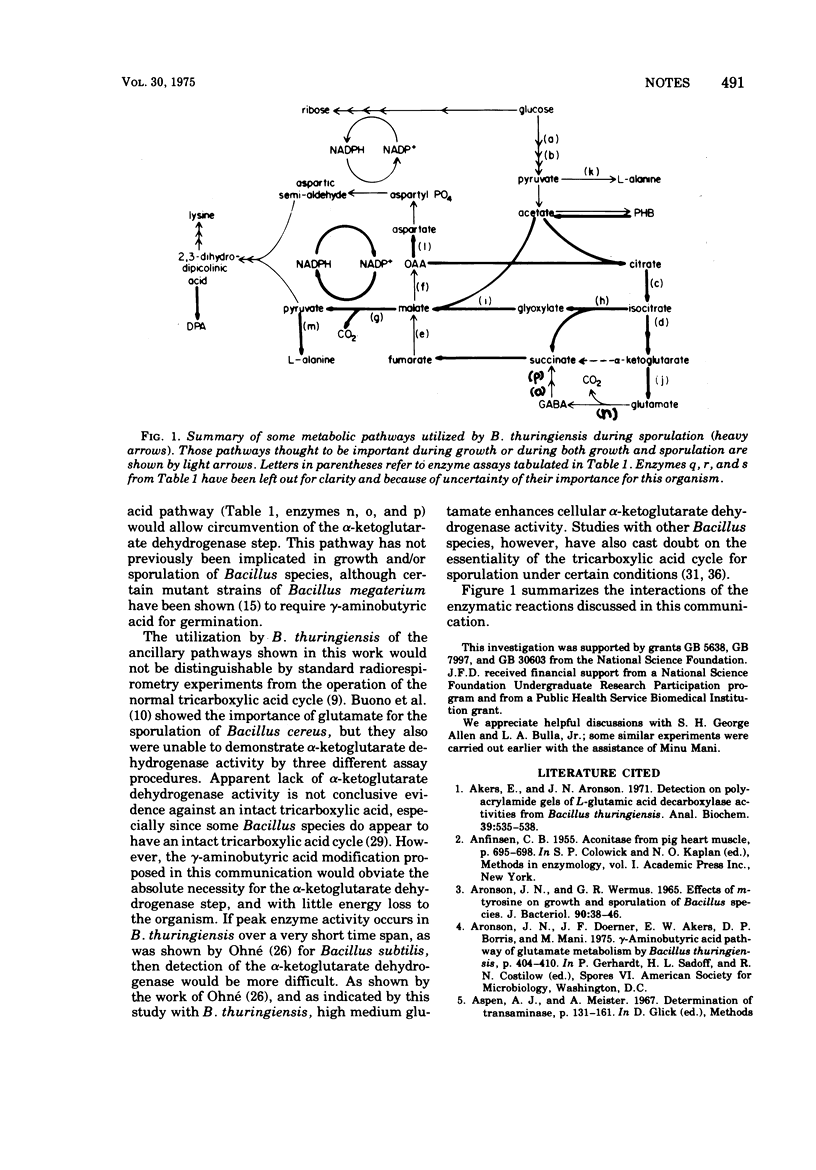
Enzymatic analyses of Bacillus thuringiensis extracts suggested that a modified Krebs tricarboxylic acid cycle (without α-ketoglutarate dehydrogenase) can operate during sporulation in conjunction with the glyoxylic acid cycle and the γ-aminobutyric acid pathway.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akers E., Aronson J. N. Detection on polyacrylamide gels of L-glutamic acid decarboxylase activities from Bacillus thuringiensis. Anal Biochem. 1971 Feb;39(2):535–538. doi: 10.1016/0003-2697(71)90446-5. [DOI] [PubMed] [Google Scholar]
- Aronson J. N., Wermus G. R. Effects of m-Tyrosine on Growth and Sporulation of Bacillus Species. J Bacteriol. 1965 Jul;90(1):38–46. doi: 10.1128/jb.90.1.38-46.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER E. L. Inhibition of complement activity by di-isopropyl fluorophosphate. Nature. 1955 Dec 3;176(4492):1073–1073. doi: 10.1038/1761073a0. [DOI] [PubMed] [Google Scholar]
- Bernlohr R. W. Changes in amino acid permeation during sporulation. J Bacteriol. 1967 Mar;93(3):1031–1044. doi: 10.1128/jb.93.3.1031-1044.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair A. H., Barker H. A. Assay and purification of (+)-citramalate hydro-lyase components from Clostridium tetanomorphum. J Biol Chem. 1966 Jan 25;241(2):400–408. [PubMed] [Google Scholar]
- Bruce P., Sims K., Pitts F. N., Jr Synthesis and purification of succinic semialdehyde. Anal Biochem. 1971 May;41(1):271–273. doi: 10.1016/0003-2697(71)90211-9. [DOI] [PubMed] [Google Scholar]
- Bulla L. A., Jr, St Julian G., Rhodes R. A. Physiology of sporeforming bacteria associated with insects. 3. Radiorespirometry of pyruvate, acetate, succinate, and glutamate oxidation. Can J Microbiol. 1971 Aug;17(8):1073–1079. doi: 10.1139/m71-170. [DOI] [PubMed] [Google Scholar]
- Buono F., Testa R., Lundgren D. G. Physiology of growth and sporulation in Bacillus cereus. I. Effect of glutamic and other amino acids. J Bacteriol. 1966 Jun;91(6):2291–2299. doi: 10.1128/jb.91.6.2291-2299.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMMARATA P. S., COHEN P. P. Spectrophotometric measurement of transamination reactions. J Biol Chem. 1951 Nov;193(1):45–52. [PubMed] [Google Scholar]
- Carls R. A., Hanson R. S. Isolation and characterization of tricarboxylic acid cycle mutants of Bacillus subtilis. J Bacteriol. 1971 Jun;106(3):848–855. doi: 10.1128/jb.106.3.848-855.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster C. W., Foerster H. F. Glutamic acid decarboxylase in spores of Bacillus megaterium and its possible involvement in spore germination. J Bacteriol. 1973 Jun;114(3):1090–1098. doi: 10.1128/jb.114.3.1090-1098.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersters K. Rapid screening assays for soluble and particulate bacterial dehydrogenases. Antonie Van Leeuwenhoek. 1967;33(1):63–72. doi: 10.1007/BF02045535. [DOI] [PubMed] [Google Scholar]
- LOWE I. P., ROBINS E., EYERMAN G. S. The fluorometric measurement of glutamic decarboxylase and its distribution in brain. J Neurochem. 1958 Oct;3(1):8–18. doi: 10.1111/j.1471-4159.1958.tb12604.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Megraw R. E., Beers R. J. Glyoxylate metabolism in growth and sporulation of Bacillus cereus. J Bacteriol. 1964 May;87(5):1087–1093. doi: 10.1128/jb.87.5.1087-1093.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson K. W., De Pinto J., Bulla L. A., Jr Sporulation of Bacillus thuringiensis without concurrent derepression of the tricarboxylic acid cycle. J Bacteriol. 1974 Jan;117(1):321–323. doi: 10.1128/jb.117.1.321-323.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohné M. Regulation of the dicarboxylic acid part of the citric acid cycle in Bacillus subtilis. J Bacteriol. 1975 Apr;122(1):224–234. doi: 10.1128/jb.122.1.224-234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Rosen N. L., Bishop L., Burnett J. B., Bishop M., Colman R. F. Methionyl residue critical for activity and regulation of bovine liver glutamate dehydrogenase. J Biol Chem. 1973 Nov 10;248(21):7359–7369. [PubMed] [Google Scholar]
- Rutberg B., Hoch J. A. Citric acid cycle: gene-enzyme relationships in Bacillus subtilis. J Bacteriol. 1970 Nov;104(2):826–833. doi: 10.1128/jb.104.2.826-833.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. M. Role of tricarboxylic acid cycle in bacterial sporulation. Biochem Biophys Res Commun. 1970 May 22;39(4):651–654. doi: 10.1016/0006-291x(70)90254-8. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- YOUNG I. E., FITZ-JAMES P. C. Chemical and morphological studies of bacterial spore formation. II. Spore and parasporal protein formation in Bacillus cereus var. alesti. J Biophys Biochem Cytol. 1959 Dec;6:483–498. doi: 10.1083/jcb.6.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousten A. A., Hanson R. S. Sporulation of tricarboxylic acid cycle mutants of Bacillus subtilis. J Bacteriol. 1972 Feb;109(2):886–894. doi: 10.1128/jb.109.2.886-894.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousten A. A., Rogoff M. H. Metabolism of Bacillus thuringiensis in relation to spore and crystal formation. J Bacteriol. 1969 Dec;100(3):1229–1236. doi: 10.1128/jb.100.3.1229-1236.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


