Abstract
Proliferation of vascular smooth muscle cells (VSMCs) in response to injury plays a key role in the pathogenesis of vascular disorders. Fas ligand (FasL) induces apoptosis in Fas-bearing cells, and its expression on activated T cells contributes to the regulation of the immune response and physiological cell turnover. Here, we show that a replication-defective adenovirus encoding FasL (Ad-FasL) induced apoptosis in Fas-bearing VSMCs. When introduced locally to balloon-injured rat carotid arteries, a well characterized model of a VSMC-derived lesion, Ad-FasL functioned as a potent inhibitor of neointima formation. In rats immunized with an empty adenoviral vector, robust T cell infiltration of the vessel wall was detected after local delivery of a β-galactosidase-expressing virus (Ad-βgal), whereas T cell infiltrates were not detected after local delivery of Ad-FasL. Prior immunization prevented β-galactosidase expression from Ad-βgal, whereas the expression of the FasL transgene was unaffected. When Ad-βgal and Ad-FasL were delivered together to preimmunized animals, T cell infiltration was reduced and β-galactosidase expression was restored. These data demonstrate that Fas ligand gene transfer can effectively inhibit injury-induced vessel lesion formation and can allow adenovirus-harboring cells to evade immune destruction.
Vascular lesions are caused by inflammatory and fibroproliferative responses to injury of the endothelium and vascular smooth muscle (1). Atherosclerotic lesion formation involves macrophage and T cell infiltration of the vessel wall, inducing vascular smooth muscle cell (VSMC) migration from the media to the intima, where these cells dedifferentiate, proliferate, and synthesize extracellular matrix components. These lesions can induce thrombus, leading to occlusion of the lumen and distal tissue ischemia. VSMC hyperplasia also contributes to the restenotic occlusion that occurs in 30–50% of patients who undergo percutaneous balloon angioplasty (2, 3), and it is the principal cause of restenosis within intravascular stents (4, 5). Therefore, a number of investigators have explored molecular genetic approaches that target VSMC proliferation to minimize the incidence of restenosis following percutaneous revascularization procedures (6–9).
Fas is a type I membrane protein belonging to the tumor necrosis factor receptor family that initiates an apoptotic signal when bound to its ligand, FasL (10). The Fas–FasL system has been implicated in the regulation of physiological cell turnover, particularly in the immune system. Activated T cells express both Fas and FasL, whereas most other tissues express only Fas (11). Immune privileged tissues also express FasL, where it is thought to inhibit the immune response by inducing apoptosis in infiltrating inflammatory cells (12–16). Fas-mediated apoptosis of VSMCs may also contribute to the regulation of intimal proliferation in the vessel wall (17, 18).
Here, we examined the effects of adenovirus-mediated FasL expression on the vessel wall after balloon injury. Results demonstrate that FasL gene transfer functions as a potent inhibitor of neointima formation and alters the T cell response to adenovirus infection in immune animals.
MATERIALS AND METHODS
Adenoviral Constructs.
Ad-FasL was constructed by inserting a 943-bp cDNA containing murine FasL (a generous gift from S. Nagata) into the XbaI site of the plasmid pACCMV.pLpA, which expresses transgenes from the cytomegalovirus promoter. Ad-βgal has the β-galactosidase gene under the control of the cytomegalovirus promoter (19). Viral titer was measured by standard plaque assay using 293 cells.
Flow Cytometry.
To detect cell surface expression of FasL by VSMCs after Ad-FasL infection, primary cultured rat aortic VSMCs were infected with Ad-FasL at a multiplicity of infection (moi) of 300 for 4 hr and incubated for an additional 12 hr. Cells were detached from the culture plate with 0.5% EDTA and stained with biotinylated anti-FasL monoclonal antibody (Alexis, San Diego, CA) or mouse IgG, followed by the incubation with fluorescein isothiocyanate (FITC)-conjugated ExtrAvidin (Sigma). Immunofluorescence staining was analyzed by fluorescence-activated cell sorter (FACS, Becton Dickinson). To analyze endogenous expression of Fas on VSMCs, rat VSMCs were detached from the plates with 0.5% EDTA and incubated with an anti-Fas mouse monoclonal antibody (Transduction Laboratories, Lexington, KY) or mouse IgG (Sigma), followed by the incubation with an FITC-conjugated goat antibody to mouse IgG (BioSource International, Camarillo, CA). Immunofluorescence staining was analyzed by flow cytometry. To detect apoptosis of the Ad-FasL-infected VSMCs, flow cytometric profile of DNA content was analyzed. Rat VSMCs were infected with Ad-βgal or Ad-FasL at different multiplicities of infection (mois) (10–300) for 4 hr, after which the virus was removed. Forty-eight hours after infection, cells were harvested by trypsinization, fixed with 70% ethanol, and stained with propidium iodide, and DNA content was analyzed by flow cytometry.
DNA Fragmentation Assay.
DNA fragmentation was measured as described previously (20). Rat VSMCs were incubated with Ad-βgal, Ad-FasL, or PBS (mock) at a moi of 100 for 4 hr, washed twice in PBS, and incubated for an additional 16 hr. Rat VSMCs or Jurkat cells were labeled with 4 μCi/ml (1 μCi = 37 kBq) [3H]thymidine for 24 hr and incubated with medium for 2 hr to minimize spontaneous release. The 3H-labeled rat VSMCs or Jurkat cells (target cells) were then applied to the infected rat VSMCs (effector cells) at a ratio of 1:2. After 7 hr of incubation, cells were harvested and the radioactivity of the retained DNA was measured in a liquid scintillation counter. Percentage of specific DNA fragmentation was calculated as 100 × [(basal count − experimental count)/basal count]. Basal count is the radioactivity of the retained DNA when only target cells were cultured.
Rat Carotid Injury Model.
The left carotid arteries of adult male Sprague–Dawley rats (400–500 g) were injured with a 2F embolectomy catheter (Baxter Edwards Healthcare, Irvine, CA) as described (17). Virus solution in 7.5% (wt/vol) Paloxamer 407 (BASF, Parsippany, NJ) was infused via a 24-gauge intravenous catheter, inserted just proximal to the carotid bifurcation into a temporarily isolated segment of the artery. The adenovirus solution was incubated for 20 min, after which the viral solution was withdrawn and the cannula was removed. Rats were sacrificed 3 or 14 days after balloon injury. The infected artery was fixed by perfusion with 4% paraformaldehyde and embedded in paraffin. Cross sections (5 μm) from four separate left carotid arterial segments of each rat were stained with hematoxylin and eosin or with Richardson’s combination elastic tissue trichrome stain. The intimal, medial, and luminal areas were measured by quantitative morphometric analysis using a computerized sketching program and expressed as mean ± SEM. The experimental protocol was approved by the Institutional Animal Care and Use Committee and complied with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals.”
Immunization Experiments.
Rats were immunized against human adenovirus by intravenous injection of an adenoviral vector lacking E1 and containing no insert [1 × 109 plaque-forming units (pfu)] via the tail vein. Two weeks after immunization, the left common carotid artery was balloon-injured, then infected with Ad-FasL (1 × 108 pfu), Ad-βgal (1 × 108 pfu), or a mixture of Ad-FasL (1 × 108 pfu) and Ad-βgal (1 × 108 pfu). Rats were sacrificed at 3 days after infection. The infected artery was removed and snap-frozen in OCT embedding compound (Miles). Cryosections (4 μm) were fixed in 2% paraformaldehyde for 10 min, rinsed in phosphate-buffered saline (PBS), and blocked with 5% goat serum and 0.01% Triton X-100 in PBS for 1 hr. A polyclonal antibody against CD3 (Sigma) was added to the sections for 1 hr. After rinsing in PBS, biotinylated goat antibody to rabbit IgG (BioGenex Laboratories, San Ramon, CA) was applied for 20 min, followed by alkaline phosphatase-conjugated streptavidin. Antibody complexes were detected by the Fast-Red system (BioGenex) followed by counterstaining with Mayer’s hematoxylin. To detect β-galactosidase expression, frozen sections (4 μm) were fixed in 2% paraformaldehyde for 10 min and stained with 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside (X-Gal; Sigma) for 16 hr at room temperature followed by counterstaining with hematoxylin. FasL expression was detected by using a monoclonal antibody against FasL (Alexis).
RESULTS AND DISCUSSION
Adenovirus-Mediated Fas Ligand Expression Induces Apoptosis in Cultured VSMCs.
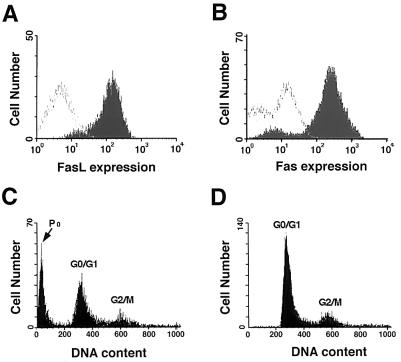
The efficacy of a replication-defective adenovirus expressing Fas ligand (Ad-FasL) was first assessed in vitro by using VSMCs derived from rat aorta. Cell surface expression of FasL by VSMCs was detected after infection with Ad-FasL (Fig. 1A). Cultures of rat VSMCs also express Fas (Fig. 1B) and thus are likely to undergo apoptotic cell death after infection. Analysis of the Ad-FasL-infected VSMC cultures revealed the presence of cells with hypodiploid DNA content indicative of apoptotic cell death (Fig. 1C), whereas infection with an adenovirus encoding β-galactosidase (Ad-βgal) did not change the DNA profile (Fig. 1D). Infection with Ad-FasL, but not Ad-βgal, also decreased cell number (not shown). The effect of Ad-FasL was dose-dependent, with increasing amounts of the apoptotic hypodiploid DNA observed with increasing mois (Table 1). Similar levels of VSMC apoptosis were observed in low serum (0.5% FBS) and in high serum (15% FBS) media (not shown), indicating that the Ad-FasL is cytotoxic to proliferating as well as quiescent VSMCs.
Figure 1.
Effect of Ad-FaL on VSMC viability in vitro. (A) FasL expression in VSMCs at 12 hr after Ad-FasL infection. Cultured rat aortic VSMCs were infected with Ad-FasL at a moi of 100 for 4 hr. After 12-hr incubation, cells were harvested and stained with biotinylated anti-FasL monoclonal antibody (filled area under curve) or with mouse IgG (open area under curve) followed by FITC-conjugated ExtrAvidin. Immunofluorescence staining was analyzed by flow cytometry. (B) Endogenous expression of Fas on VSMCs. Rat VSMCs were harvested and incubated with an anti-Fas mouse monoclonal antibody (filled area under curve) or mouse IgG (open area under curve) followed by the incubation with an FITC-conjugated goat anti-mouse IgG. (C) Flow cytometric profile of DNA content after Ad-FasL infection. Rat VSMCs were infected with Ad-FasL at a moi of 100 for 4 hr. (D) DNA profile of rat VSMCs after Ad-βgal infection at a moi of 100 for 4 hr. Forty-eight hours after infection, cells were harvested and stained with propidium iodide, and DNA content was analyzed by flow cytometry (46). For C and D the positions of the G0/G1, G2/M, and apoptotic sub-G1 (P0) DNA populations are indicated.
Table 1.
Dose-dependent production of apoptotic sub-G1 DNA population in VSMCs by Ad-FasL infection
| Treatment | moi | Sub-G1 DNA, % |
|---|---|---|
| Ad-FasL | 10 | 3.3 |
| 30 | 9.2 | |
| 100 | 35.2 | |
| 300 | 49.2 | |
| Ad-βgal | 10 | 0.2 |
| 30 | 0.2 | |
| 100 | 1.1 | |
| 300 | 0.3 | |
| Saline | — | 0.6 |
Ad-FasL-Transduced VSMCs Induce Apoptosis in Uninfected VSMCs and Jurkat Cells.
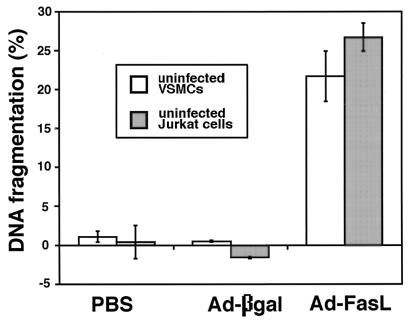
The ability of FasL-expressing VSMCs to induce apoptosis in uninfected Fas-positive target cells was assessed by incubating Ad-FasL-infected VSMCs with nontransduced VSMCs or Jurkat cells that had been preincubated with [3H]thymidine (Fig. 2). After 7 hr of co-incubation, appreciable levels of target cell DNA fragmentation were observed in both Jurkat and VSMC cultures. No DNA fragmentation was detected in target cells when incubated with either uninfected or Ad-βgal-infected VSMCs. Thus ectopic FasL expression by VSMCs can induce apoptotic cell death in neighboring nontransduced cells that express Fas.
Figure 2.
Cytotoxicity of the Ad-FasL-transduced VSMCs toward uninfected T cells and VSMCs. Rat VSMCs were briefly incubated with PBS, Ad-βgal, or Ad-FasL (effector cells), washed twice in PBS, and then cocultured with [3H]thymidine-labeled rat VSMCs or Jurkat cells (target cells). DNA fragmentation of the target cells was measured as described in the text.
Adenovirus-Mediated Fas Ligand Expression Inhibits Neointima Formation in Balloon-Injured Rat Carotid Arteries.
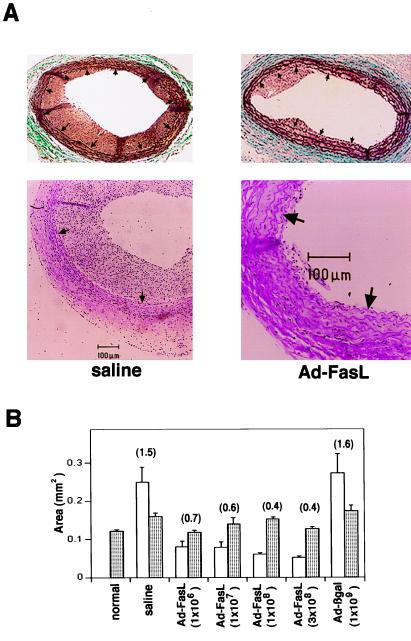
Injured rat carotid arteries develop a reproducible neointimal lesion that is dependent on VSMC migration and proliferation (21). Ad-FasL was delivered to rat carotid arteries immediately following balloon injury to determine the effect of FasL expression on lesion formation. Morphometric analyses revealed that neointimal lesion formation was significantly reduced by treatment with Ad-FasL (Fig. 3A). At 14 days after injury robust neointimal lesions were detected in vessels treated with either saline or Ad-βgal (1 × 109 pfu). In contrast, lesion size was significantly reduced in arteries treated with Ad-FasL at doses ranging from 1 × 106 to 3 × 108 pfu (Fig. 3B). The effect of Ad-FasL on neointima formation was particularly potent compared with other adenovirally delivered cytotoxic or cytostatic genes, including herpes virus thymidine kinase (8, 22), mutant or wild-type Rb (23, 24), or other growth-inhibitory genes (9, 25), where effective doses typically range from 1 to 2 × 109 pfu.
Figure 3.
Effect of Ad-FasL on neointimal formation in rat carotid arteries 2 weeks after balloon injury. (A) Representative cross sections from arteries treated with either saline or Ad-FasL (1 × 108 pfu) at the time of injury. Cross sections were stained with Richardson’s combination elastic tissue trichrome stain (Upper) or with hematoxylin and eosin (Lower). Arrows indicate the internal elastic lamina. (B) Intimal (open bar) and medial (shaded bar) areas of carotid artery cross sections at 2 weeks after injury (n = 5 arteries for each group). The intimal/medial area ratio is reported in parentheses above the bars.
At 14 days after injury, the medial layers of the Ad-FasL-treated vessels appeared normal with regard to size and cellularity (Fig. 3). Balloon injury results in the immediate loss of VSMCs because of barotrauma-induced apoptosis, followed by rapid VSMC proliferation and repopulation of the medial layer (26, 27). Analyses of histological sections revealed normal VSMC density in the media at 3 days after injury in the saline- and Ad-βgal-infected vessels. However, VSMC density was decreased by a factor of 3 at this time point in the vessels infected with Ad-FasL (Table 2). By 14 days after injury, medial cell density had returned to normal levels in the Ad-FasL-treated vessels (Table 2), and FasL expression was no longer detectable by immunohistochemistry (data not shown), presumably because the FasL-expressing VSMCs had themselves undergone apoptosis by this time.
Table 2.
Cellular density of media after balloon injury
| Time after injury, days | Cells per mm2
|
||
|---|---|---|---|
| Saline | Ad-βgal (1 × 108 pfu) | Ad-FasL (1 × 108 pfu) | |
| 3 | 3,905 ± 419 | 3,686 ± 335 | 1,365 ± 224* |
| 14 | 3,764 ± 494 | 3,699 ± 279 | 3,845 ± 263 |
Cellular density was calculated by determining the number of nuclei and the area of the media for each section (n = 4 for each group). The results are expressed as mean ± SEM.
P < 0.05 vs. normal artery (3,408 ± 124 cells per mm2).
Because the systemic administration of anti-Fas antibody or Ad-FasL can cause severe liver damage and morbidity (19, 28), we addressed the issue of systemic toxicity. The lowest effective dose of Ad-FasL examined here, 1 × 106 pfu, is less than 1/6000 of the lethal dose (19). At the highest Ad-FasL dose, 3 × 108 pfu, no grossly detectable lesions were observed in tissue sections from liver, heart, lung, kidney, spleen, skeletal muscle, or the contralateral carotid artery at days 3 or 14 after infection. Biochemical markers of liver function in serum at 3 days after gene transfer did not significantly differ between a relatively high dose of Ad-FasL (1 × 108 pfu) and nontreated groups. Serum levels of alkaline phosphatase were 165 ± 36 and 108 ± 21 international units/liter in control and Ad-FasL-treated rats, respectively; glutamine pyruvate transferase levels were 59 ± 2 and 58 ± 8 international units/liter in control and Ad-FasL-treated rats, respectively; and glutamine oxaloacetic transferase levels were 87 ± 8 and 137 ± 28 international units/liter (P > 0.05) in control and Ad-FasL-treated rats, respectively. Therefore, local delivery of Ad-FasL to the vessel wall can effectively inhibit injury-induced lesion formation without detectable toxicity to the major organs. In light of these observations, it may be possible to deliver a therapeutic dose of the FasL gene to the site of angioplasty and minimize systemic toxicity by expressing FasL from a conditional or smooth muscle-specific promoter.
FasL Gene Transfer Overrides the Adenovirus-Mediated T Cell Response.
A major limitation of adenovirus-mediated gene therapy is the cellular immune response to viral and transgene antigens that induce the destruction of the genetically altered cells (29). Therefore, repeated administration of the recombinant virus is associated with confounding immune responses and a low gene transfer efficiency both in animal models and in cystic fibrosis patients receiving adenovirus-mediated gene therapy (30–32). This issue is also a concern for proposed anti-restenosis therapies that employ a single delivery of an adenoviral construct, because a large portion of the adult patient population have developed immunity to several adenovirus serotypes. Moreover, it has been reported that exposure of uninjured rat and rabbit arteries to adenovirus vectors can result in pronounced neointimal lesion formation and T cell infiltration throughout the arterial wall (33, 34).
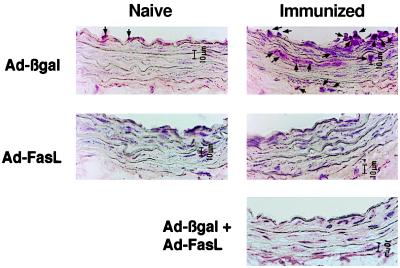
FasL expression has been identified in immune-privileged tissues (11), and it has been proposed that it functions to kill Fas-bearing inflammatory cells as they enter the tissue (12–16). Thus we tested whether ectopic FasL expression could modulate the cellular immune response to adenoviral antigen. T cell infiltration in the vessel wall was assessed in naive rats and in rats immunized with intravenous injection of an E1-deleted adenovirus that lacked a transgene. Little or no T cell infiltration was detected 3 days after transduction with Ad-βgal or Ad-FasL in naive rats (Fig. 4). In contrast, immunized rats displayed marked T cell infiltration of the vessel wall after local delivery of Ad-βgal, but not Ad-FasL. However, T cell infiltration was essentially eliminated in immunized rats when Ad-βgal and Ad-FasL were co-administered to the vessel wall. These data suggest that the differential T cell response results from the proactive effects of FasL on the vessel wall rather than from differences in the immunogenic properties of the viral transgenes.
Figure 4.
In situ detection of T cell infiltration in carotid arteries of naive and preimmunized rats 3 days after balloon injury and viral infection. Viral solutions of Ad-βgal (1 × 108 pfu), Ad-FasL (1 × 108 pfu), or mixture of Ad-βgal and Ad-FasL (1 × 108 pfu each) were introduced into the balloon-injured carotid arteries of naive or immunized rats (n = 4 arteries for each group). Rats were immunized with an adenoviral vector lacking a transgene (1 × 109 pfu) 2 weeks prior to injury/infection. T cells were detected by using an anti-CD3 polyclonal antibody (arrows).
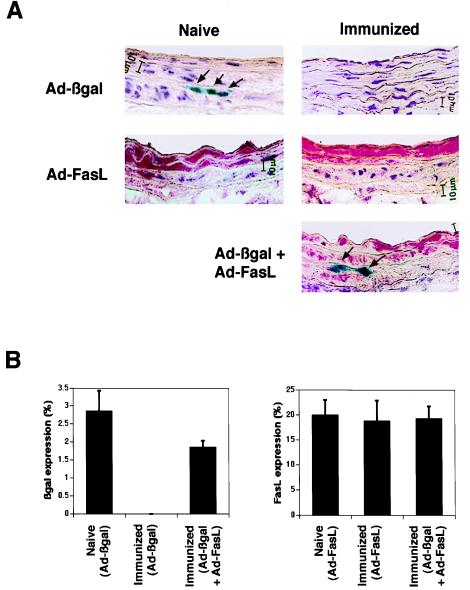
Transgene expression by viral constructs was also assessed in naive and immunized rats. The FasL transgene was expressed at similar levels at 3 days after infection in Ad-FasL-transduced vessels of both immunized and naive animals. In naive animals, β-galactosidase expression was detected in Ad-βgal-transduced vessels, but expression levels were lower relative to the FasL transgene because of the inefficiency of the histochemical detection of β-galactosidase using X-Gal (35, 36). However, in contrast to FasL, no β-galactosidase expression was detected in the vessel walls of immunized rats after infection with Ad-βgal (Fig. 5). β-Galactosidase expression in immunized rats could be restored to levels similar to the level in naive animals when injured vessels were simultaneously co-infected with Ad-βgal and Ad-FasL constructs. These results demonstrate that FasL can function in trans to create conditions that permit virus-mediated gene expression in immunized animals.
Figure 5.
Transgene expression in carotid arteries of naive and immunized rats 3 days after injury and viral infection. (A) β-Galactosidase and FasL expression in arteries infected with Ad-βgal (1 × 108 pfu), Ad-FasL (1 × 108 pfu), or a mixture of Ad-βgal (1 × 108 pfu) and Ad-FasL (1 × 108 pfu). β-Galactosidase was stained with 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside (blue), while FasL expression was revealed by using an anti-FasL monoclonal antibody (Fast-Red detection system). The Ad-βgal encodes an Escherichia coli β-galactosidase containing a nuclear localization signal under the control of cytomegalovirus promoter. Arrows indicate β-galactosidase-expressing cells. (B) Mean transduction efficiencies of Ad-βgal (Left) or Ad-FasL (Right). Transduction efficiencies were calculated by determining the percentage of cells staining positive for the presence of transgenes (n = 4 for each group). The results are expressed as mean ± SEM.
FasL expression can also produce a proinflammatory response through its ability to recruit neutrophils, limiting its potential usefulness for immunotherapy (19, 37–39). However, no neutrophil infiltration was detected in the vessel wall after transduction with Ad-FasL at any dose examined. The basis for these differences in FasL function is not known, but it is possible that the neutrophil response in the vessel wall is inhibited by the expression of transforming growth factor β, which can function to suppress neutrophil function (40–42). This cytokine is detected in human vascular lesions, and it is markedly up-regulated in rat carotid arteries after balloon injury (43–45). Alternatively, the transient nature of FasL expression in VSMCs or the extrusion of cellular debris into the lumen may minimize secondary inflammatory responses in the vessel wall.
Conclusions.
The inflammatory fibroproliferative disorders of the vessel wall provide a unique system to explore the therapeutic utility of FasL. Here, we have shown that VSMCs are susceptible to apoptotic cell death by the adenovirus-mediated delivery of FasL to VSMCs. Furthermore, Ad-FasL-transduced VSMCs could kill noninfected VSMCs and T cells in coculture assays. The local delivery of Ad-FasL to balloon-injured vessels potently suppressed neointimal lesion formation at doses that did not result in detectable systemic toxicity. The potency of Ad-FasL at the site of delivery is likely due the ability of FasL to induce cell death in a paracrine manner. Ectopic FasL expression also altered the immune status of the vessel wall. The delivery of Ad-FasL to the vessel walls of immunologically primed rats decreased T cell infiltration and restored adenoviral transgene expression. Thus, the localized expression of FasL may serve to overcome the limitations imposed by T cell responses to repeated delivery of genes with viral vectors.
Acknowledgments
This study was supported by National Institutes of Health Grants AG-15052, HL-50692, and AR-40197 to K.W. and a Juvenile Diabetes Foundation research grant to T.A.L. M.S. is a recipient of a research fellowship from the American Heart Association, Massachusetts Affiliate. D.A.M. is supported by a fellowship from the Alberta Heritage Foundation for Medical Research.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: VSMC, vascular smooth muscle cell; FasL, Fas ligand; FITC, fluorescein isothiocyanate; moi, multiplicity of infection; pfu, plaque-forming units.
References
- 1.Ross R. Nature (London) 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz R S, Holmes D R, Topol E J. J Am Coll Cardiol. 1992;20:1284–1293. doi: 10.1016/0735-1097(92)90389-5. [DOI] [PubMed] [Google Scholar]
- 3.Nobuyoshi M, Kimura T, Ohishi H, Horiuchi H, Nosaka H, Hamasaki N, Yokoi H, Kim K. J Am Coll Cardiol. 1991;17:433–439. doi: 10.1016/s0735-1097(10)80111-1. [DOI] [PubMed] [Google Scholar]
- 4.Kearney M, Pieczek A, Haley L, Losordo D W, Andrés V, Schainfeld R, Rosenfield K, Isner J M. Circulation. 1997;95:1998–2002. doi: 10.1161/01.cir.95.8.1998. [DOI] [PubMed] [Google Scholar]
- 5.Dussaillant G R, Mintz G S, Pichard A D, Kent K M, Salter L F, Popma J J, Wong S C, Leon M B. J Am Coll Cardiol. 1995;26:720–724. doi: 10.1016/0735-1097(95)00249-4. [DOI] [PubMed] [Google Scholar]
- 6.Simons M, Edelman E R, DeKeyser J-L, Langer R, Rosenberg R D. Nature (London) 1992;359:67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- 7.Morishita R, Gibbons G H, Ellison K E, Nakajima M, Zhang L, Kaneda Y, Ogihara T, Dzau V J. Proc Natl Acad Sci USA. 1993;90:8474–8478. doi: 10.1073/pnas.90.18.8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzman R J, Hirschowitz E A, Brody S L, Crystal R G, Epstein S E, Finkel T. Proc Natl Acad Sci USA. 1994;91:10732–10736. doi: 10.1073/pnas.91.22.10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith R C, Branellec D, Gorski D H, Guo K, Perlman H, Dedieu J-F, Pastore C, Mahfoudi A, Denèfle P, Isner J M, Walsh K. Genes Dev. 1997;11:1674–1689. doi: 10.1101/gad.11.13.1674. [DOI] [PubMed] [Google Scholar]
- 10.Nagata S, Golstein P. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 11.French L E, Hahne M, Viard I, Radlgruber G, Zanone R, Becker K, Müller C, Tschopp J. J Cell Biol. 1996;133:335–343. doi: 10.1083/jcb.133.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffith T S, Brunner T, Fletcher S M, Green D R, Ferguson T A. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 13.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke R C. Nature (London) 1995;377:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 14.Hahne M, Rimoldi D, Schröter M, Romero P, Schreier M, French L E, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J-C, Tschopp J. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 15.Strand S, Hofman W J, Hug H, Müller M, Otto G, Strand D, Mariani S M, Stremmel W, Krammer P H, Galle P R. Nat Med. 1996;2:1361–1366. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 16.Lau H T, Yu M, Fontana A, Stoeckert C J. Science. 1996;273:109–112. doi: 10.1126/science.273.5271.109. [DOI] [PubMed] [Google Scholar]
- 17.Fukuo K, Hata S, Suhara T, Nakahashi T, Shinto Y, Tsujimoto Y, Morimoto S, Ogihara T. Hypertension. 1996;27:823–826. doi: 10.1161/01.hyp.27.3.823. [DOI] [PubMed] [Google Scholar]
- 18.Dong C, Wilson J E, Winters G L, McManus B M. Lab Invest. 1996;74:921–931. [PubMed] [Google Scholar]
- 19.Muruve D A, Nicolson A G, Manfro R C, Strom T B, Sukhatme V P, Libermann T A. Human Gene Ther. 1997;8:955–963. doi: 10.1089/hum.1997.8.8-955. [DOI] [PubMed] [Google Scholar]
- 20.Saas P, Walker P R, Hahne M, Quiquerez A-L, Schnuringer V, Perrin G, French L, Van Meir E G, Tribolet N, Tschopp J, Dietrich P-Y. J Clin Invest. 1997;99:1173–1178. doi: 10.1172/JCI119273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clowes A W, Reidy M A, Clowes M M. Lab Invest. 1983;49:327–333. [PubMed] [Google Scholar]
- 22.Ohno T, Gordon D, San H, Pompili V J, Imperiale M J, Nabel G J, Nabel E G. Science. 1994;265:781–784. doi: 10.1126/science.8047883. [DOI] [PubMed] [Google Scholar]
- 23.Chang M W, Barr E, Seltzer J, Jiang Y, Nabel G J, Nabel E G, Parmacek M S, Leiden J M. Science. 1995;267:518–522. doi: 10.1126/science.7824950. [DOI] [PubMed] [Google Scholar]
- 24.Smith R C, Wills K N, Antelman D, Perlman H, Truong L N, Krasinski K, Walsh K. Circulation. 1997;96:1899–1905. doi: 10.1161/01.cir.96.6.1899. [DOI] [PubMed] [Google Scholar]
- 25.Chang M W, Barr E, Lu M M, Barton K, Leiden J M. J Clin Invest. 1995;96:2260–2268. doi: 10.1172/JCI118281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perlman H, Maillard L, Krasinski K, Walsh K. Circulation. 1997;95:981–987. doi: 10.1161/01.cir.95.4.981. [DOI] [PubMed] [Google Scholar]
- 27.Wei G L, Krasinski K, Kearney M, Isner J M, Walsh K, Andrés V. Circ Res. 1997;80:418–426. [PubMed] [Google Scholar]
- 28.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Nature (London) 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, Nunes F A, Berencsi K, Furth E E, Gönczöl E, Wilson J M. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knowles M R, Hohneker K W, Zhou Z, Olsen J C, Noah T L, Hu P C, Leigh M W, Engelhardt J F, Edwards L J, Jones K R. N Engl J Med. 1995;333:823–831. doi: 10.1056/NEJM199509283331302. [DOI] [PubMed] [Google Scholar]
- 31.Crystal R G, McElvaney N G, Rosenfeld M A, Chu C-S, Mastrangeli A, Hay J G, Brody S L, Jaffe H A, Eissa N T, Danel C. Nat Genet. 1994;8:42–51. doi: 10.1038/ng0994-42. [DOI] [PubMed] [Google Scholar]
- 32.Zabner J, L A, C, Gregory R J, Graham S M, Smith A E, Welsh M J. Cell. 1993;75:207–216. doi: 10.1016/0092-8674(93)80063-k. [DOI] [PubMed] [Google Scholar]
- 33.Newman K D, Dunn P F, Owens J W, Schulick A H, Virmani R, Sukhova G, Libby P, Dichek D A. J Clin Invest. 1995;96:2955–2965. doi: 10.1172/JCI118367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulick A H, Vassalli G, Dunn P F, Dong G, Rade J J, Zamarron C, Dichek D A. J Clin Invest. 1997;97:209–219. doi: 10.1172/JCI119149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clesham G J, Browne H, Efstathiou S, Weissberg P L. Circ Res. 1996;79:1188–1195. doi: 10.1161/01.res.79.6.1188. [DOI] [PubMed] [Google Scholar]
- 36.Couffinhal T, Kearney M, Sullivan A, Silver M, Tsurumi Y, Isner J M. Human Gene Ther. 1997;8:929–934. doi: 10.1089/hum.1997.8.8-929. [DOI] [PubMed] [Google Scholar]
- 37.Yagita H, Seino K, Kayagaki N, Okumura K. Nature (London) 1996;379:682. doi: 10.1038/379682a0. [DOI] [PubMed] [Google Scholar]
- 38.Seino K, Kayagaki N, Okumura K, Yagita H. Nat Med. 1997;3:165–170. doi: 10.1038/nm0297-165. [DOI] [PubMed] [Google Scholar]
- 39.Kang S-M, Schneider D B, Lin Z, Hanahan D, Dichek D A, Stock P G, Steinunn B. Nat Med. 1997;3:738–743. doi: 10.1038/nm0797-738. [DOI] [PubMed] [Google Scholar]
- 40.Wilbanks G A, Mammolenti M, Streilein J W. Eur J Immunol. 1992;22:165–173. doi: 10.1002/eji.1830220125. [DOI] [PubMed] [Google Scholar]
- 41.Gresham H D, Ray C J, O’Sullivan F X. J Immunol. 1991;146:3911–3921. [PubMed] [Google Scholar]
- 42.Lowrance J H, O’Sullivan F X, Caver T E, Waegell W, Gresham H D. J Exp Med. 1994;180:1693–1703. doi: 10.1084/jem.180.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikol S, Isner J M, Pickering J G, Kearney M, Leclerc G, Weir L. J Clin Invest. 1992;90:1582–1592. doi: 10.1172/JCI116027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madri J A, Reidy M A, Kocher O, Bell L. Lab Invest. 1989;60:755–765. [PubMed] [Google Scholar]
- 45.Majesky M W, Lindner V, Twardzik D R, Schwartz S M, Reidy M A. J Clin Invest. 1991;88:904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Guo K, Walsh K. Cancer Res. 1997;57:351–354. [PubMed] [Google Scholar]