Abstract
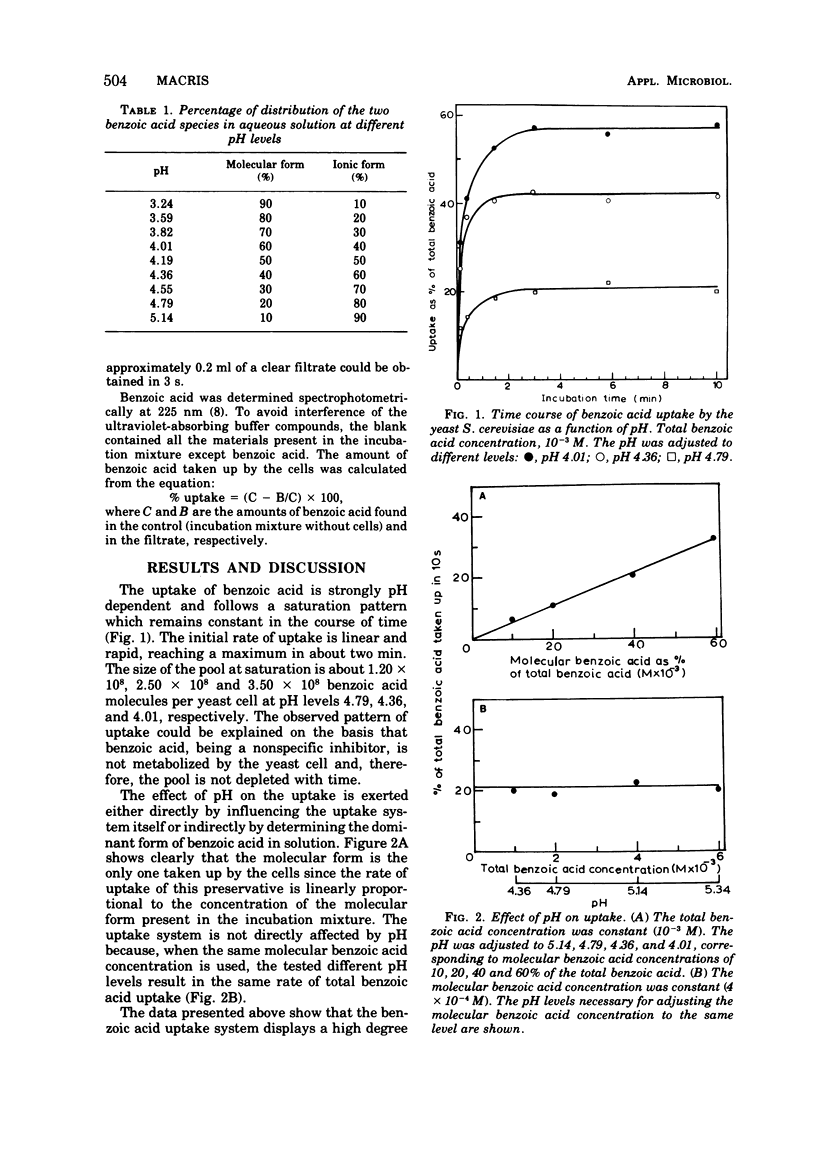
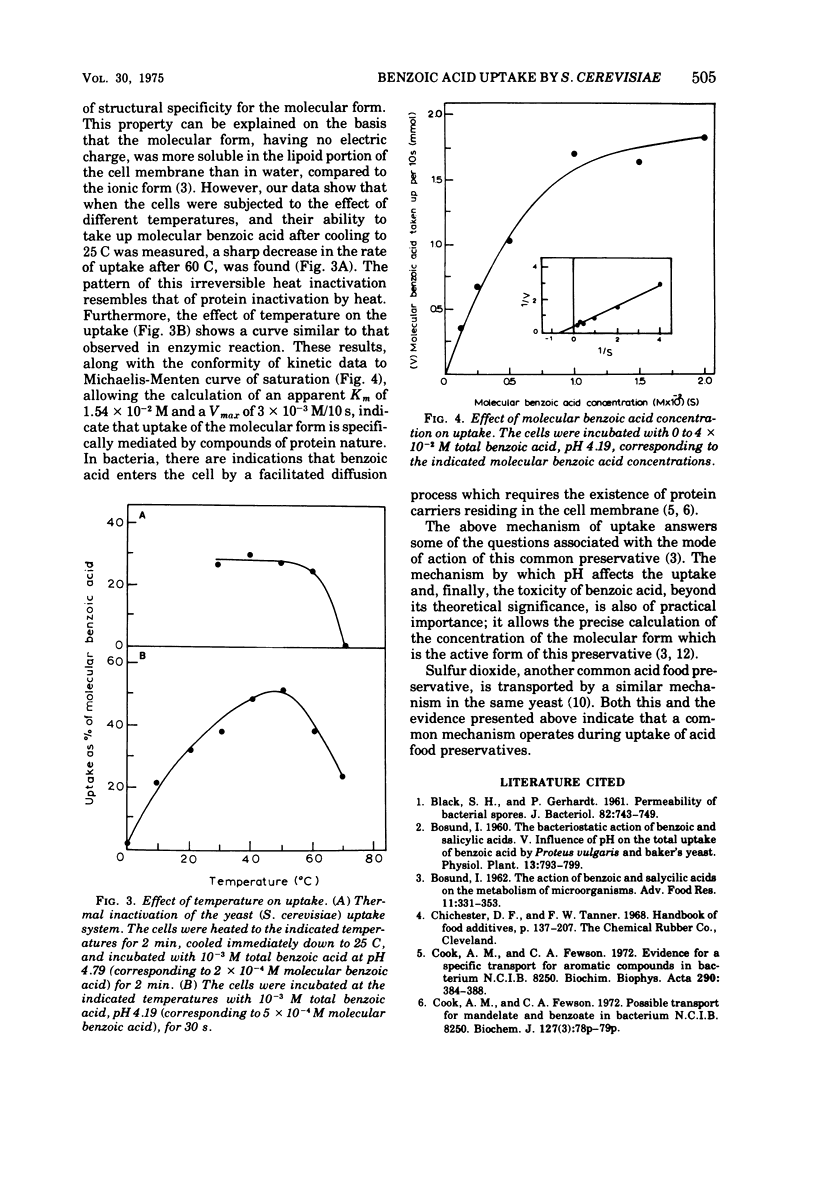
A fast uptake of the preservative benzoic acid was observed in Saccharomyces cerevisiae, reaching saturation in about two min and then remaining constant at this level. The strong dependence of benzoic acid uptake on pH was due to the relative distribution of molecular and ionic forms in solution and not to the pH itself. The molecular form was the only one taken up by the cells. The specificity of the uptake mechanism was evidenced by the pattern of irreversible heat inactivation of the uptake system resembling protein denaturation by heat. Furthermore, the effect of temperature on the uptake was similar to that observed in enzymic reactions, whereas the kinetic data of uptake conformed to the Michaelis-Menten curve of saturation with a Km of 1.54 × 10-2 M and Vmax of 3 × 10-3 M/10s. The evidence presented in this paper indicates that compounds of protein nature are involved in the uptake of this preservative.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK S. H., GERHARDT P. Permeability of bacterial spores. I. Characterization of glucose uptake. J Bacteriol. 1961 Nov;82:743–749. doi: 10.1128/jb.82.5.743-749.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A. M., Fewson C. A. Evidence for specific transport mechanisms for aromatic compounds in bacterium N.C.I.B. 8250. Biochim Biophys Acta. 1972 Dec 1;290(1):384–388. doi: 10.1016/0005-2736(72)90081-8. [DOI] [PubMed] [Google Scholar]
- Cook A. M., Fewson C. A. Possible transport mechanisms for mandelate and benzoate in bacterium N.C.I.B. 8250. Biochem J. 1972 Apr;127(3):78P–79P. doi: 10.1042/bj1270078p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruess W. V., Richert P. H. EFFECT OF HYDROGEN ION CONCENTRATION ON THE TOXICITY OF SODIUM BENZOATE TO MICROORGANISMS. J Bacteriol. 1929 May;17(5):363–371. doi: 10.1128/jb.17.5.363-371.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macris B. J., Markakis P. Transport and toxicity of sulphur dioxide in Saccharomyces cerevisiae var ellipsoideus. J Sci Food Agric. 1974 Jan;25(1):21–29. doi: 10.1002/jsfa.2740250104. [DOI] [PubMed] [Google Scholar]
- SCHULTZ A. S., McMANUS D. K. Amino acids and inorganic sulfur as sulfur source for the growth of yeasts. Arch Biochem. 1950 Feb;25(2):401–409. [PubMed] [Google Scholar]



