Abstract
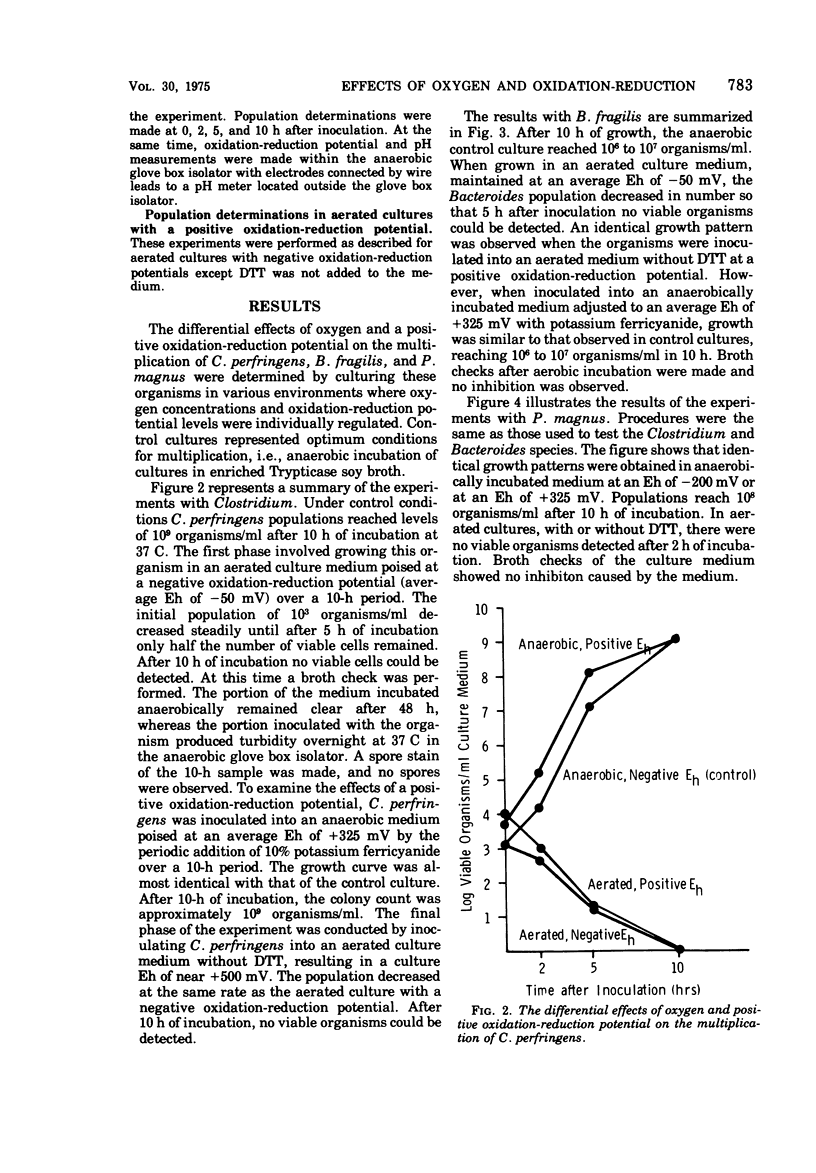
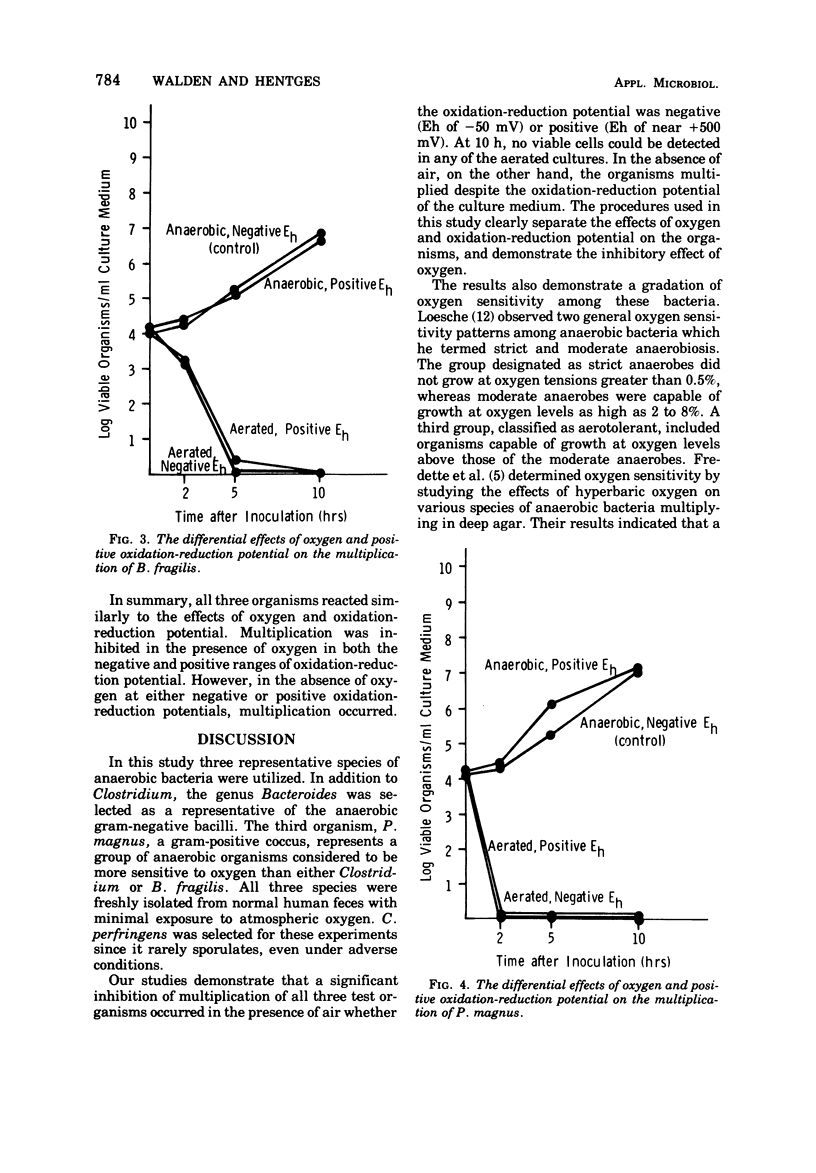
The sensitivity of three strains of anaerobic intestinal bacteria, Clostridium perfringens, Bacteroides fragilis, and Peptococcus magnus, to the differential effects of oxygen and adverse oxidation-reduction potential was measured. The multiplication of the three organisms was inhibited in the presence of oxygen whether the medium was at a negative oxidation-reduction potential (Eh of -50 mV), poised by the intermittent addition of dithiothreitol, or at a positive oxidation-reduction potential (Eh of near +500 mV). However, when these organisms were cultured in the absence of oxygen, no inhibition was observed, even when the oxidation-reduction potential was maintained at an average Eh of +325 mV by the addition of potassium ferricyanide. When the cultures were aerated, the growth patterns of the three organisms demonstrated different sensitivities to oxygen. P. magnus was found to be the most sensitive. After 2 h of aerobic incubation, no viable organisms could be detected. B. fragilis was intermediately sensitive to oxygen with no viable organisms detected after 5 h of aerobic incubation. C. perfringens was the least sensitive. Under conditions of aerobic incubation, viable organisms survived for 10 h. During the experiments with Clostridium, no spores were observed by spore staining.
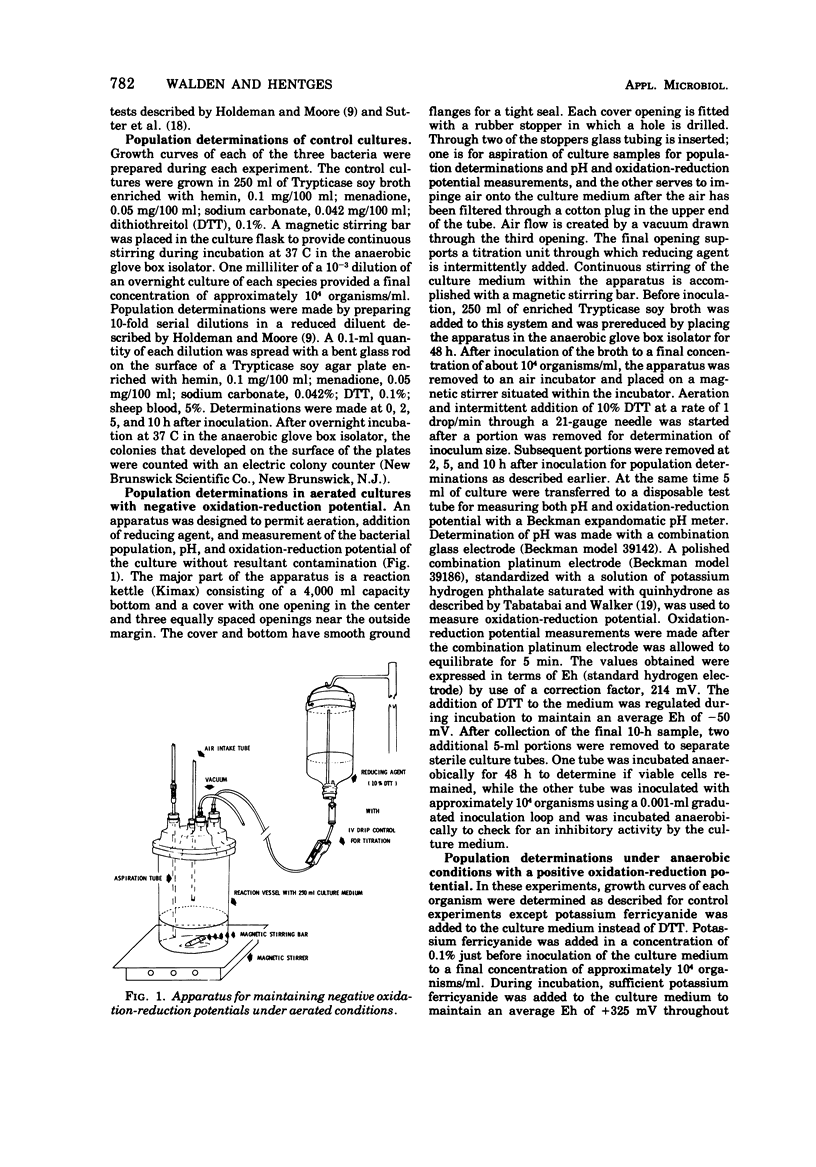
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas F., Hambleton R., Rigby G. J. An investigation of the oxidation-reduction potential and of the effect of oxygen on the germination and outgrowth of Clostridium butyricum spores, using platinum electrodes. J Appl Bacteriol. 1973 Dec;36(4):625–633. doi: 10.1111/j.1365-2672.1973.tb04148.x. [DOI] [PubMed] [Google Scholar]
- Fredette V., Planté C., Roy A. Numerical data concerning the sensitivity of anaerobic bacteria to oxygen. J Bacteriol. 1967 Dec;94(6):2012–2017. doi: 10.1128/jb.94.6.2012-2017.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. F., Stutman M., Loesche W. J. Improved isolation of anaerobic bacteria from the gingival crevice area of man. Appl Microbiol. 1971 Jun;21(6):1046–1050. doi: 10.1128/am.21.6.1046-1050.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges D. J., Maier B. R. Theoretical basis for anaerobic methodology. Am J Clin Nutr. 1972 Dec;25(12):1299–1305. doi: 10.1093/ajcn/25.12.1299. [DOI] [PubMed] [Google Scholar]
- Knaysi G., Dutky S. R. The Growth of a Butanol Clostridium in Relation to the Oxidation-Reduction Potential and Oxygen Content of the Medium. J Bacteriol. 1936 Feb;31(2):137–149. doi: 10.1128/jb.31.2.137-149.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight B. C., Fildes P. Oxidation-reduction studies in relation to bacterial growth: The positive limit of oxidation-reduction potential required for the germination of B. tetani spores in vitro. Biochem J. 1930;24(5):1496–1502. doi: 10.1042/bj0241496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J. Oxygen sensitivity of various anaerobic bacteria. Appl Microbiol. 1969 Nov;18(5):723–727. doi: 10.1128/am.18.5.723-727.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATELES R. I., ZUBER B. L. THE EFFECT OF EXOGENOUS CATALASE ON THE AEROBIC GROWTH OF CLOSTRIDIA. Antonie Van Leeuwenhoek. 1963;29:249–255. doi: 10.1007/BF02046065. [DOI] [PubMed] [Google Scholar]
- O'Brien R. W., Morris J. G. Oxygen and the growth and metabolism of Clostridium acetobutylicum. J Gen Microbiol. 1971 Nov;68(3):307–318. doi: 10.1099/00221287-68-3-307. [DOI] [PubMed] [Google Scholar]
- SOCRANSKY S. S., LOESCHE W. J., HUBERSAK C., MACDONALD J. B. DEPENDENCY OF TREPONEMA MICRODENTIUM ON OTHER ORAL ORGANISMS FOR ISOBUTYRATE, POLYAMINES, AND A CONTROLLED OXIDATION-REDUCTION POTENTIAL. J Bacteriol. 1964 Jul;88:200–209. doi: 10.1128/jb.88.1.200-209.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabai L. B., Walker H. W. Oxidation-reduction potential and growth of Clostridium perfringens and Pseudomonas fluorescens. Appl Microbiol. 1970 Sep;20(3):441–446. doi: 10.1128/am.20.3.441-446.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennesland B., Hanke M. E. The Oxidation-Reduction Potential Requirements of a Non-Spore-Forming, Obligate Anaerobe. J Bacteriol. 1940 Feb;39(2):139–169. doi: 10.1128/jb.39.2.139-169.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries W., Wijck-Kapteijn W. M., Stouthamer A. H. Influence of oxygen on growth, cytochrome synthesis and fermentation pattern in propionic acid bacteria. J Gen Microbiol. 1972 Aug;71(3):515–524. doi: 10.1099/00221287-71-3-515. [DOI] [PubMed] [Google Scholar]


