Abstract
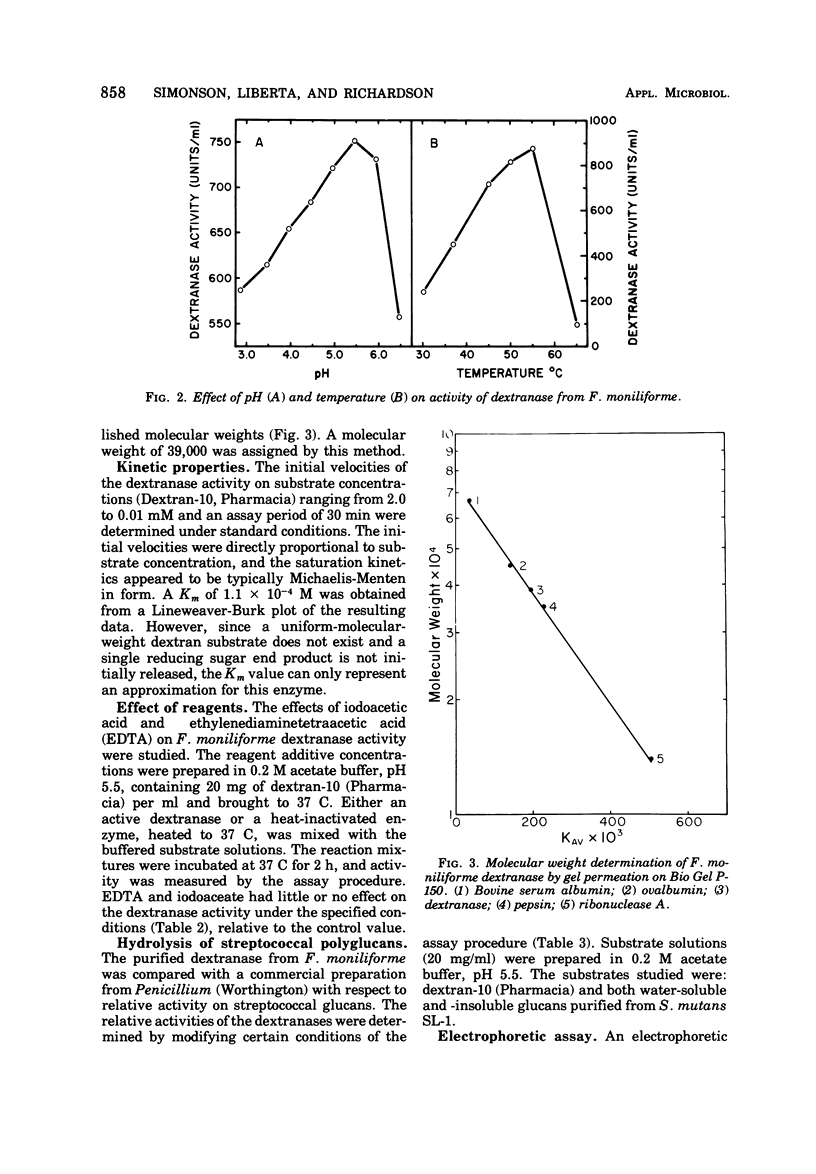
An extracellular dextranase (EC 3.2.1.11) was purified approximately 75-fold from cell-free culture filtrates of Fusarium moniliforme. The purified dextranase was of the endo type, and isomaltose was identified as the primary end product of dextran hydrolysis. The molecular weight of the dextranase was determined to be 39,000 by gel permeation chromatography. The enzyme was most active at pH 5.5, and the temperature optimum was near 55 C. Activity was not inhibited by either ethylenediaminetetraacetic acid or iodoacetate. The Km for dextran with an average molecular weight of 10,000 was estimated to be 1.1 × 10-4 M. The electrophoretic mobility of the dextranase was distinctly different from that of a Penicillium-derived commercial dextranase. The F. moniliforme dextranase was also found to differ from the commercial preparation by its greater relative activity against glucans isolated from Streptococcus mutans.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNFELD P. Enzymes of starch degradation and synthesis. Adv Enzymol Relat Subj Biochem. 1951;12:379–428. doi: 10.1002/9780470122570.ch7. [DOI] [PubMed] [Google Scholar]
- Bowen W. H. The effect of dextranase on caries activity in monkeys (Macaca irus). Br Dent J. 1971 Nov 16;131(10):445–449. doi: 10.1038/sj.bdj.4802768. [DOI] [PubMed] [Google Scholar]
- Chaiet L., Kempf A. J., Harman R., Kaczka E., Weston R., Nollstadt K., Wolf F. J. Isolation of a pure dextranase from Penicillium funiculosum. Appl Microbiol. 1970 Sep;20(3):421–426. doi: 10.1128/am.20.3.421-426.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Ebisu S., Misaki A., Kato K., Kotani S. The structure of water-insoluble glucans of cariogenic Streptococcus mutans, formed in the absence and presence of dextranase. Carbohydr Res. 1974 Dec;38:374–381. doi: 10.1016/s0008-6215(00)82375-7. [DOI] [PubMed] [Google Scholar]
- Fitzgerald R. J., Spinell D. M., Stoudt T. H. Enzymatic removal of artificial plaques. Arch Oral Biol. 1968 Jan;13(1):125–128. doi: 10.1016/0003-9969(68)90042-3. [DOI] [PubMed] [Google Scholar]
- Freedman M. L., Tanzer J. M. Dissociation of plaque formation from glucan-induced agglutination in mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):189–196. doi: 10.1128/iai.10.1.189-196.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto J., Tsuji H., Tsuru D. Studies on mold dextranases. I. Penicillium luteum dextranase: its production and some enzymatic properties. J Biochem. 1971 Jun;69(6):1113–1121. doi: 10.1093/oxfordjournals.jbchem.a129564. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Banghart S. B. Synthesis of extracellular dextran by cariogenic bacteria and its presence in human dental plaque. Arch Oral Biol. 1967 Jan;12(1):11–23. doi: 10.1016/0003-9969(67)90137-9. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J. Formation and significance of bacterial polysaccharides in caries etiology. Caries Res. 1968;2(2):164–171. doi: 10.1159/000259554. [DOI] [PubMed] [Google Scholar]
- Johnson M. C., Bozzola J. J., Shechmeister I. L. Morphological study of Streptococcus mutans and two extracellular polysaccharide mutants. J Bacteriol. 1974 Apr;118(1):304–311. doi: 10.1128/jb.118.1.304-311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes P. H., Hicks M. A., Goldman M., McCabe R. M., Fitzgerald R. J. 3. Dispersion of dextranous bacterial plaques on human teeth with dextranase. J Am Dent Assoc. 1971 Jan;82(1):136–141. doi: 10.14219/jada.archive.1971.0016. [DOI] [PubMed] [Google Scholar]
- Simonson L. G., Liberta A. E. New sources of fungal dextranase. Mycologia. 1975 Jul-Aug;67(4):845–851. [PubMed] [Google Scholar]
- Sugiura M., Ito A., Ogiso T., Kato K., Asano H. Studies on dextranase. Purification of dextranase from Penicillium funiculosum and its enzymatic properties. Biochim Biophys Acta. 1973 Jun 6;309(2):357–362. doi: 10.1016/0005-2744(73)90034-x. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- TSUCHIYA H. M., JEANES A., BRICKER H. M., WILHAM C. A. Dextran-degrading enzymes from molds. J Bacteriol. 1952 Oct;64(4):513–519. doi: 10.1128/jb.64.4.513-519.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Freedman M. L., Fitzgerald R. J., Larson R. H. Diminished virulence of glucan synthesis-defective mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):197–203. doi: 10.1128/iai.10.1.197-203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINNER D. D., JABLON J. M., ARAN A. P., SASLAW M. S. EXPERIMENTAL CARIES INDUCED IN ANIMALS BY STREPTOCOCCI OF HUMAN ORIGIN. Proc Soc Exp Biol Med. 1965 Mar;118:766–770. doi: 10.3181/00379727-118-29964. [DOI] [PubMed] [Google Scholar]