Abstract
Toxoplasma gondii, an obligate intracellular parasite pathogen which initially invades the intestinal epithelium before disseminating throughout the body, may cause severe sequelae in fetuses and life-threatening neuropathy in immunocompromised patients. Immune protection is usually thought to be performed through a systemic Th1 response; considering the route of parasite entry it is important to study and characterize the local mucosal immune response to T. gondii. Despite considerable effort, Toxoplasma-targeted vaccines have proven to be elusive using conventional strategies. We report the use of mesenteric lymph node dendritic cells (MLNDCs) pulsed ex vivo with T. gondii antigens (TAg) as a novel investigation approach to vaccination against T. gondii-driven pathogenic processes. Using a murine model, we demonstrate in two genetically distinct mouse strains (C57BL/6 and CBA/J) that adoptively transferred TAg-pulsed MLNDCs elicit a mucosal Toxoplasma-specific Th2-biased immune response in vivo and confer strong protection against infection. We also observe that MLNDCs mostly traffic to the intestine where they enhance resistance by reduction in the mortality and in the number of brain cysts. Thus, ex vivo TAg-pulsed MLNDCs represent a powerful tool for the study of protective immunity to T. gondii, delivered through its natural route of entry. These findings might impact the design of vaccine strategies against other invasive microorganisms known to be delivered through digestive tract.
Most viral, bacterial, and parasitic microorganisms gain entry into the host through mucosal tissues. Mucosa harbor potent, specific protective immune defenses against those pathogens, and increasing attention is being paid to mucosal surfaces as an attractive route by which to immunize. Dendritic cells (DCs), the most potent and versatile professional antigen-presenting cells (APCs), are the initiators of T-cell responses against invasive microorganisms due to their capacity to stimulate naive T cells (30). Mucosal DCs deserve to be more characterized.
The coccidian protozoan Toxoplasma gondii is an obligate intracellular parasite of humans and other warm-blooded animals. It is a significant hazard to the fetuses of mothers who acquire the infection during pregnancy, and it has been established as a cause of life-threatening disease in immunocompromised individuals (27). After the primary infection in an immunocompetent individual, the immune response of the host usually limits the replication of tachyzoites, resulting in the formation of the dormant bradyzoite form. C57BL/6 susceptible mice do not limit the replication of tachyzoites and die, whereas CBA/J mice, which limit this replication, become chronically infected with persistent cysts and are considered to be resistant to toxoplasmosis. This effective immune reaction against parasites results in the chronic infection of an immunocompetent individual with T. gondii usually being contained (12). Immune protection is thought to be performed through a Th1 systemic response (17), but the importance of gut mucosal immunity against infection with T. gondii is less well understood.
The oral route is the natural portal of entry of T. gondii. Ingested organisms are released from cysts or oocysts within the gastrointestinal tract and initially invade the intestinal epithelium before disseminating throughout the body. After stimulation by DCs the initial activation of naive T cells probably occurs in Peyer's patches and mesenteric lymph nodes (MLNs). Because DCs are difficult to isolate in large numbers, the majority of DC studies have been performed using DC precursor populations differentiated and expanded in vitro with recombinant cytokines (15). However, it is important to take into account that DCs generated in vitro from a defined precursor population may differ in their phenotype and more importantly in their APC potential, depending on the cytokines used (26).
In this sense, we decided to fully define the MLN DCs (MLNDCs) as natural adjuvant for immune protection against Toxoplasma and to explore how the MLNDCs were involved in the immune protection of susceptible C57BL/6 and resistant CBA/J mice against toxoplasmosis.
Our results show that adoptively transferred MLNDCs pulsed ex vivo with T. gondii antigens (TAg) produce great resistance to T. gondii infection in vivo and that this immunity correlates with the development of a protective systemic and mucosal T-cell response, a shift of the cytokine expression towards a Th2-like pattern in the mucosa, a production of specific secretory immunoglobulin A (IgA), and a traffic to the spleen and to the intestine. In comparison, TAg-pulsed spleen DCs (SPDCs) produce a weaker resistance to T. gondii infection correlated with a Th1 splenic T-cell response and a traffic only to the spleen (2). These findings suggest that MLNDCs could establish long-term immunity at the systemic and mucosal level to recurrent infection with T. gondii.
Finally, these findings may well be applicable to many mucosal pathogens.
MATERIALS AND METHODS
Animals.
Female CBA/J (H-2k) and C57BL/6 (H-2b) mice 8 to 10 weeks old (Janvier) were used in all experiments.
Parasites.
Tachyzoites of the RH strain of T. gondii were harvested from the peritoneal fluid of Swiss OF1 mice intraperitoneally infected 3 to 4 days earlier. Cysts of the 76K strain of T. gondii were obtained from the brains of orally infected CBA/J mice.
Preparation of Toxoplasma sonicate.
T. gondii RH tachyzoites were washed, sonicated, and centrifuged as previously described (29). The supernatant from the last centrifugation, which was used as the source of antigen, was concentrated through dialysis tubing to achieve aliquots. The concentration was determined by a protein assay reagent kit (Bio-Rad) with bovine serum albumin as the standard. The aliquots of T. gondii tachyzoite sonicate were stored at −20°C.
Bradyzoites of the 76K strain of T. gondii were harvested from the brains of CBA/J mice orally infected 1 month earlier with 80 cysts of the 76K strain. Brains were homogenized in 5 ml of phosphate-buffered saline (PBS) with a pestle and mortar and filtered through nylon mesh. The cysts were obtained by centrifugation through a Percoll layer (100% isotonic) (45 ml of Percoll plus 5 ml of NaCl, 1.5 M). The pellet contained the cysts and red blood cells, which were lysed at −20°C overnight. After centrifugation, the cysts were counted and sonicated. The concentration was determined by a protein assay reagent kit (Bio-Rad) with bovine serum albumin as the standard. The aliquots of T. gondii bradyzoite sonicate were stored at −20°C.
Preparation of MLNDCs and SPDCs.
DCs were prepared from the MLNs and the spleens of naive CBA/J and C57BL/6 6- to 10-week-old mice. The DCs were purified by a procedure described by Iwasaki and Kelsall for Peyer's patch DCs (16). MLNs and spleen were dissected and digested with collagenase D (400 Mandl units/ml; Boehringer Mannheim) plus DNase (15 μg/ml) (DNase I; Boehringer Mannheim) in 10 ml of IMDM (Gibco/BRL) supplemented with a solution containing 10% heat-inactivated fetal calf serum, penicillin (100 U/ml), streptomycin (100 μg/ml), 50 μM 2-mercaptoethanol, and 2 mM l-glutamine for 30 min at 37°C. For isolation of CD11c+ cells using directly conjugated magnetism-activated cell sorting magnetic beads (Miltenyi Biotec), CD11c beads were added according to the manufacturer's instructions. After incubation for 20 min at 4°C, cells were washed and passed over a magnetism-activated cell sorting column. Purity was checked routinely by fluorescence-activated sell sorting and was found to be greater than 97% (not shown). DCs were cultured for 18 h with 5 ml of supplemented IMDM containing T. gondii tachyzoite and bradyzoite sonicates (TAg) (50 μg/ml). They were thoroughly washed, counted, and used for immunization and homing.
The overnight culture supernatants (5 ml) were concentrated to 1 ml through dialysis tubing and were tested for the presence of interleukin-12 (IL-12), IL-4, IL-10, gamma interferon (IFN-γ), and transforming growth factor beta (TGF-β).
FACScan analysis.
The cell surface phenotype of purified MLNDCs was assessed by reacting cells with the fluorescein isothiocyanate-conjugated monoclonal antibody (MAb) reagents—14.4.4 (murine IgG2a anti-I-Ek,d), CD80 (1G10), CD86 (GL1), and CD8 (53-6.72) (all from BD PharMingen)—and with phycoerythrin-conjugated anti-CD11c (N418) (Serotec). Before all labeling experiments, Fc receptor blocking was performed by incubating cells with 2.4G2 (a rat anti-mouse Fc receptor MAb) for 10 min. Unrelated isotype-matched MAbs were used as control. Analysis was performed on a FACScan apparatus (Becton Dickinson & Co.). Data were evaluated both as the percentage of positive cells and as the median fluorescence intensity.
Adoptive immunizations and Toxoplasma challenge.
The potential effect of MLNDCs was tested by immunizing groups of 12 mice. For all experiments, mice were given intravenously (i.v.) 2.5 × 105 TAg-pulsed or unpulsed DCs. Control mice were untreated. Mice were orally infected with 80 cysts for CBA/J mice and 10 cysts for C57BL/6 mice of T. gondii 76K on day 5. In the study of the antigen-specific proliferative response, MLNs and spleens were harvested 28 days after passive DC transfer. 23 days after challenge, CBA/J mice were killed, and each brain was homogenized in 5 ml of PBS with a pestle and mortar. The cysts in each brain homogenate were counted microscopically (8 × 10 μl). The results are expressed as means ± standard deviations for each group. The C57BL/6 mice were observed daily for mortality. One month after challenge, survivors were killed and their brains were recovered.
Western blotting.
Serum and fresh fecal samples were collected. Fecal samples were extracted using PBS with 0.1% sodium azide (1 ml was added for every 100 mg of feces). The samples were then vortexed until all material was suspended. After centrifugation at 13,000 × g for 10 min, the supernatants were removed and immediately used for immunoblot analysis. Electrophoresis and immunoblotting using purified T. gondii tachyzoites as the source of antigen were performed as previously described (6). TAg recognized by intestinal IgA antibodies were detected using a goat anti-mouse IgA alkaline phosphatase conjugate (Sigma). T gondii antigens recognized by MAbs were detected using a goat anti-mouse IgG alkaline phosphatase conjugate (Sigma). The following anti-T. gondii MAbs were used: 3G11 (anti-p22 [SAG2]), 1E5 (anti-p30 [SAG1]), 1F12 (anti-p43 [SAG3]), 4A7 (anti-55- and 60-kDa [ROP2 to ROP4]), 1F7 (anti-gp60 [MIC1]), 2F3 (anti-p80 [MIC3]), and 4A11 (anti-p100 [MIC2]). These MAbs were generously donated by J.-F. Dubremetz (Institut National de la Santé et de la Recherche Médicale, Montpellier, France).
Measurement of antigen-specific proliferative response.
Spleens and MLNs were harvested 5 and 28 to 35 days after passive DC transfer and pressed through a stainless steel mesh. Single-cell suspensions were obtained by filtration through nylon mesh to remove tissue debris. The spleen erythrocytes were destroyed by hypotonic shock, and spleen and MLN cells were suspended in RPMI 1640 (GIBCO) supplemented with 5% fetal calf serum, HEPES (25 mM), l-glutamine (2 mM), sodium pyruvate (1 mM), β-mercaptoethanol (5 × 10−5 M), and penicillin-streptomycin (100 μg/ml) and seeded in triplicate in flat-bottomed 96-well microtiter plates (Costar) at 5 × 105 cells per well in 200 μl of culture medium, alone or containing various concentrations of TAg. The plates were incubated for 3 days in 5% CO2 at 37°C and pulsed with 1 μCi of [3H]thymidine/well for an additional 18 h. Finally, the cells were collected on glass fiber filters and the radioactivity (counts per minute) was quantified by liquid scintillation counting. Proliferation was expressed as the stimulation index (counts per minute for stimulated cells/counts per minute for unstimulated cells).
Cytokine concentration measurement.
Spleen cells and MLN cells were cultured in 24-well plates at 5 × 106 cells/well in 1 ml of culture medium alone or with TAg (10 μg/ml). Cell culture supernatants were harvested between 24 h and 4 days and assayed for IL-2, IL-4, IL-5, IL-10, IL-13, and IFN-γ activities. The total IL-12, IL-10, IFN-γ, and TGF-β in culture supernatants from unpulsed DCs and TAg-pulsed DCs were also measured. The cytokine concentrations were evaluated using a commercial enzyme-linked immunosorbent assay kit according to the manufacturer's instructions (DuoSet [Genzyme] for IL-2, IL-4, IL-5, IL-10, IL-12, TGF-β, and IFN-γ and R&D Systems for IL-13). Cytokine concentrations were determined by reference to standard curves constructed with known amounts of mouse recombinant IL-2, IL-4, IL-5, IL-10, IL-13, and IFN-γ. The sensitivity limits for the assays were 20 pg/ml for IFN-γ, 50 pg/ml for IL-10, 10 pg/ml for IL-13 and IL-2, 20 pg/ml for IL-5 and IL-4, and 60 pg/ml for TGF-β.
Short-term tissue homing.
CBA mice were used as donors and recipients for the homing experiments. MLNDCs and SPDCs were labeled with 50 μCi of 51Cr per ml for 1 h at 37°C. Unincorporated 51Cr was removed by centrifugation. The DCs were washed and 5 × 106 were injected into the tail vein of recipient mice. The mice were killed 2 h later. Blood was collected, and the intestine, lungs, kidneys, liver, spleen, brain, MLNs, and peripheral lymph nodes were removed. The peripheral lymph nodes removed were the superficial inguinal nodes, the brachial and popliteal nodes, the superficial cervical nodes, and the iliac lymph nodes. All the Peyer's patches were collected from the intestine. The intestines were flushed with 20 ml of buffer and Peyer's patches were removed. Organs were carefully homogenized in 3 ml of water-1% Triton X-100, and the radioactivity in all the organs was counted in a gamma counter. Values are expressed as percentage of radioactivity recovered in the organ and the remaining body. Two mice were used in each experiment, and each experiment was repeated at least two times.
Statistical analysis.
Parametric statistical analysis was performed using the c2 test for analyzing the observed differences in survival of mice and using Student's test for the other analyses.
RESULTS
Phenotypic analysis of isolated DCs from MLNs.
Sorted cells selected on the basis of CD11c were routinely 97 to 98% positive. DCs have been characterized on the basis of their antigen presentation potential and their phenotype (Fig. 1).
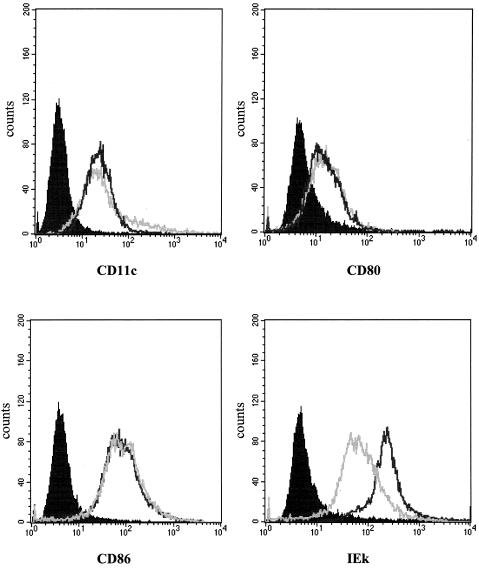
FIG. 1.
Surface phenotype analysis of sorted DC populations from SP and MLN. Sorted CD11c+ DCs from SP and MLN were analyzed for expression of various surface molecules. The results are shown as histograms with fluorescence intensity on the x axis and cell number on the y axis. The light gray lines represent staining of SPDCs, and the black lines represent staining of MLNDCs. Isotype-matched control is indicated for each antibody set with a solid peak. The data depicted here represent four independent experiments producing similar results.
We found that DC markers (CD11c) as well as costimulatory molecules (CD80 and CD86) were similar between SPDCs and MLNDCs. However, MLNDCs expressed higher levels of major histocompatibility complex class II antigens compared with SPDCs.
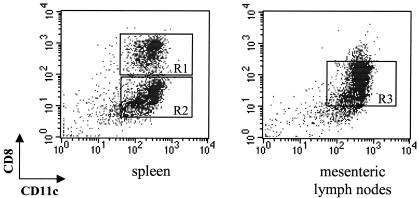
As illustrated in Fig. 2, SPDCs are subdivided in two SPDC subpopulations on the basis of CD8 expression. CD8+ and CD8− subsets represent approximately 60 and 40% of total SPDCs, respectively. MLNDCs are subdivided in three DC populations. Apart from a CD8+ lymphoid DC subpopulation and a CD8− myeloid DC subpopulation equivalent to those described in the spleen, a third DC subpopulation exists, from low to intermediate levels of CD8. CD8+, CD8int, and CD8− MLNDC subpopulations represent approximately 20, 60, and 20%, respectively, in the MLN. These results are in agreement with those obtained by Anjuere et al. (1).
FIG. 2.
Definition of DC subpopulations from spleen and MLNs. Dot plots show the CD8-versus-CD11c profile of SPDCs and MLNDCs. CD8+ and CD8− DC subpopulations can be defined (R1 and R2) in the spleen. In the MLNs an additional CD8int DC subset exists (R3). These data are representative of four experiments with similar results.
We also found no difference between the staining of CBA/J and C57BL/6 MLNDCs (data not shown). Hence, these MLNDCs had a mature phenotype compatible with antigen presentation and T-cell sensitization in vivo.
Cytokine production by isolated MLNDCs.
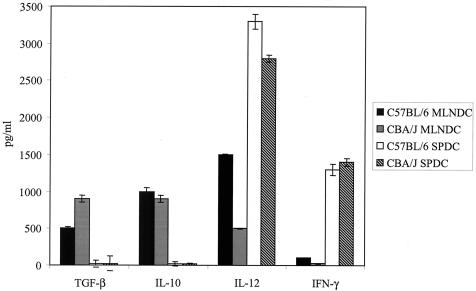
Supernatants from MLNDCs cultured overnight in the presence of TAg were assessed for the presence of IL-12, IL-10, IFN-γ, and TGF-β (Fig. 3).
FIG. 3.
Cytokine production by MLNDCs and SPDCs after TAg stimulation. Fluorescence-activated cell sorting-purified CD11c+ DCs (106 per well) from CBA/J and C57BL/6 mice were incubated overnight in the presence of TAg (50 μg/ml). The overnight culture supernatants (5 ml) were concentrated to 1 ml through dialysis tubing and were tested for the presence of IL-12, IL-10, IFN-γ, and TGF-β by enzyme-linked immunosorbent assay. Each column is representative of three separate experiments. Results are expressed as the means ± standard errors of the means.
CBA/J MLNDCs secreted large amounts of IL-10 (1,000 pg/ml) and TGF-β (950 pg/ml), low levels of IL-12 (500 ng/ml), and insignificant amounts of IFN-γ. C57BL/6 MLNDCs secreted similar levels of IL-10 (1,000 pg/ml), twofold-lower levels of TGF-β (500 pg/ml), and threefold-higher levels of IL-12 (1,500 pg/ml) and synthesized IFN-γ (150 pg/ml).
SPDCs secreted large amounts of IL-12 (3,000 ng/ml), IFN-γ (1,400 pg/ml), and no TGF-β and IL-10, regardless of the mouse strain.
No specific release of IL-4 was detected in the supernatants from MLNDCs and SPDCs (data not shown).
Protection of mice from acute infection with T. gondii by MLNDCs pulsed with T. gondii antigen.
Since susceptibility is genetically determined, two different mouse strains were assayed for these experiments. C57BL/6 (highly susceptible) and CBA (highly resistant) were infected by oral gavage with either 10 or 80 cysts, respectively. C57BL/6 mice usually succumb to infection within 8 to 10 days after the infection. CBA/J mice are highly resistant to parasite infection, and morbidity is best quantified by enumeration of brain cyst load.
The ability of pulsed MLNDCs to protect against acute parasite challenge was evaluated in susceptible C57BL/6 mice (Fig. 4).
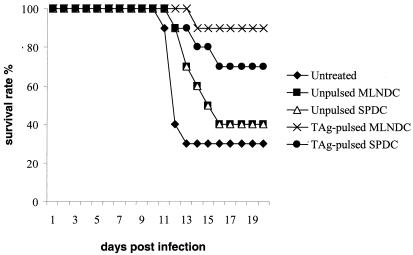
FIG. 4.
Survival of C57BL/6 mice following immunization with unpulsed MLNDCs, unpulsed SPDCs, TAg-pulsed MLNDCs and TAg-pulsed SPDCs. Mice immunized with 2.5 × 105 unpulsed DCs or TAg-pulsed MLNDCs and untreated mice were orally infected with 10 cysts of T. gondii 5 days later. Mice were observed daily for mortality. One representative experiment of three is shown.
C57BL/6 mice were injected with 2.5 × 105 TAg-pulsed or unpulsed MLNDCs and with 2.5 × 105 TAg-pulsed or unpulsed SPDCs. Control mice were untreated. The mice were then orally challenged with 10 cysts of the 76K strain of T. gondii and observed daily for mortality. TAg-pulsed MLNDCs protected the mice (90% survival) (P < 0.0017) more than TAg-pulsed SPDCs (70% survival) (P < 0.0057). Unpulsed MLNDCs and unpulsed SPDCs protect the recipient less against virulent challenge (P < 0.0105), and only 40% of mice survived within 12 days postinfection (Fig. 4). A lower survival rate of untreated mice (30%) was observed. These observations showed that pulsed MLNDCs protected C57BL/6 against an oral challenge with T. gondii and that the degree of protection is significantly superior compared with that obtained with pulsed SPDCs (P < 0.0073).
Resistance to cyst formation induced by MLNDCs pulsed with T. gondii antigen.
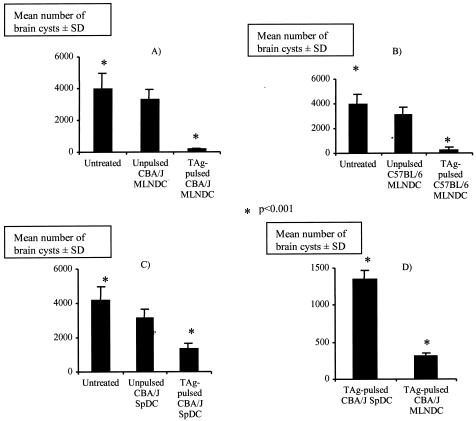
CBA/J mice infected with 80 cysts of the 76K parasite strain develop substantial numbers of intracerebral cysts containing bradyzoites. The long-term protection provided by pulsed MLNDCs and SPDCs was evaluated by counting brain cysts 1 month after an oral challenge (Fig. 5A and C). CBA/J mice immunized with 2.5 × 105 pulsed MLNDCs were highly resistant to cyst formation (93% fewer brain cysts) (Fig. 5A).
FIG. 5.
Assay for protection against oral challenge. CBA/J mice were immunized with 2.5 × 105 unpulsed MLNDCs and TAg-pulsed MLNDCs (A) or with 2.5 × 105 unpulsed SPDCs and TAg-pulsed SPDCs (C). (B) CBA/J mice were orally infected with 80 cysts. C57BL/6 mice were immunized with 2.5 × 105 unpulsed MLNDCs or TAg-pulsed MLNDCs. (D) C57BL/6 mice were orally infected with 10 cysts of T. gondii 5 days later. A comparison of protection between CBA/J mice immunized with TAg-pulsed MLNDCs and TAg-pulsed SPDCs was performed. Cyst burden was enumerated by counting brain cysts at 30 days postchallenge in the CBA/J mice and in the C57BL/6 survivors + standard deviation (error bars). Results of one of three similar experiments are shown. *, P < 0.001.
A lower decrease in the number of cysts (70%) was observed in mice immunized with TAg-pulsed SPDCs (Fig. 5C) but not in untreated mice given the same number of unpulsed MLNDCs and SPDCs, respectively (P < 0.001). TAg-pulsed MLNDCs were statistically more efficient in inducing resistance to cyst formation than TAg-pulsed SPDCs (Fig. 5D) (P < 0.001)
The decrease in the number of intracerebral cysts was not confined to CBA/J mice. C57BL/6 mice which were given pulsed MLNDCs and survived acute infection were also very much more resistant to cyst formation than unpulsed MLNDCs and untreated mice; they had 85% fewer cysts than controls (P < 0.01) (Fig. 5B). Thus, MLNDCs loaded with TAg were effective APCs inducing great resistance to cyst formation.
Toxoplasma antigens recognized by serum IgG and intestinal IgA antibodies.
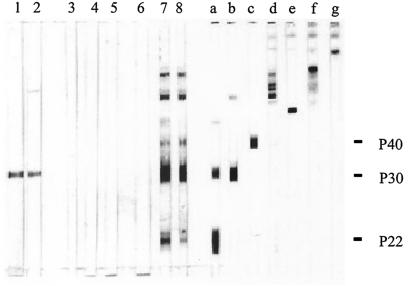
Intestinal anti-T. gondii IgA antibodies were detected by Western blotting only in fecal samples from mice injected 21 days earlier with TAg-pulsed MLNDCs (Fig. 6).
FIG. 6.
Western blot analysis of TAg recognized by intestinal IgA antibodies and MAbs. Fresh fecal samples were collected 21 days after infection of CBA/J mice with T. gondii cysts (strain 76K) (lanes 1 and 2), 21 days after injection of CBA/J mice with unpulsed SPDCs (lane 3), with TAg-pulsed SPDCs (lanes 4 and 5), with unpulsed MLNDCs (lane 6), or with TAg-pulsed MLNDCs (lanes 7 and 8). The following MAbs were used: 3G11 (anti-p22 [SAG2]) (lane a), 1E5 (anti-p30 [SAG1]) (lane b), 1F12 (anti-p43 [SAG3]) (lane c), 4A7 (anti-55- and 60-kDa [ROP2 to ROP4]) (lane d), 1F7 (anti-gp60 [MIC1]) (lane e), 2F3 (anti-p80 [MIC3]) (lane f), and 4A11 (anti-p100 [MIC2]) (lane g). The molecular masses (kilodaltons) of protein standards are given on the right.
Intestinal anti-T. gondii IgA antibodies were not detected in fecal samples from mice injected 21 days earlier with TAg-pulsed SPDCs. The intestinal IgA antibody response was directed against antigens with apparent molecular masses of 21, 30, 38, and 55 to 60 kDa. All these bands were not detected by intestinal IgA antibodies from mice injected 21 days earlier with unpulsed SPDCs or unpulsed MLNDCs. Intestinal anti-T. gondii IgA antibodies from mice infected 21 days earlier with T. gondii recognized a 30-kDa antigen as previously described (6). The 21-, 30-, 38-, and 55- to 60-kDa antigens recognized by anti-T. gondii intestinal IgA antibodies showed a migration pattern similar to that of major TAg (SAG2, SAG1, SAG3, and ROP2 to ROP4, respectively) as detected by specific MAbs.
Serum anti-T. gondii IgG antibodies were detected by Western blotting in mice injected 21 days earlier with TAg-pulsed SPDCs or with TAg-pulsed MLNDCs. A similar pattern was observed for the two groups of mice (data not shown).
Cellular proliferative response induced by MLNDCs.
TAg-induced proliferation was assessed with spleen and MLN cells from C57BL/6 and CBA/J mice immunized with unpulsed or pulsed MLNDCs 28 days after DC transfer (Fig. 7 and 8).
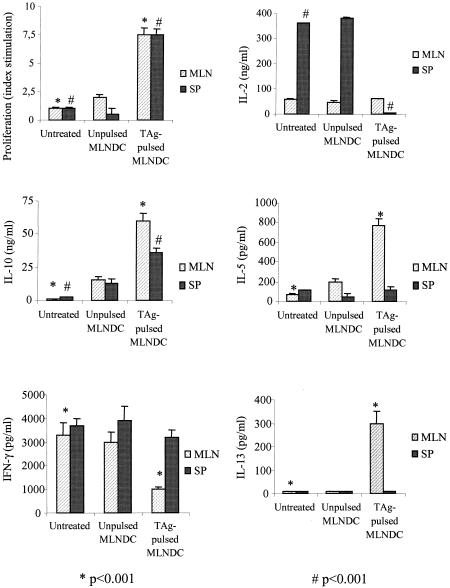
FIG. 7.
Cellular proliferative response and cytokine production following i.v. immunization with 2.5 × 105 TAg-pulsed CBA/J MLNDCs after challenge with 80 cysts of T. gondii 76K. At 28 days spleen and MLN cells were isolated and stimulated in vitro with TAg (10 μg/ml). Proliferation was assessed after 4 days. Values for IL-2 and IL-4 were measured at 24 h, for IFN-γ at 72 h and for IL-10 and IL-5 at 96 h of culture. Results are mean cytokine concentrations or counts per minute for proliferation assays on spleen and MLN cells from three mice per experimental group. Results from one of three similar experiments are shown and are expressed as the mean ± standard error of the mean. Symbols: *, P < 0.001; #, P < 0.001.
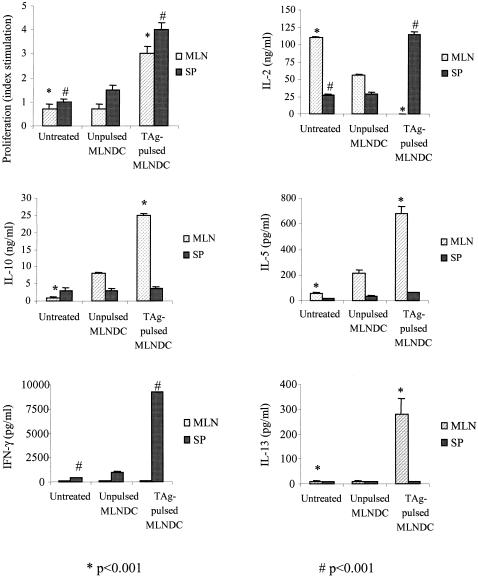
FIG. 8.
Cellular proliferative response and cytokine production following i.v. immunization with 2.5 × 105 TAg-pulsed C57BL/6 MLNDCs after challenge with 10 cysts of T. gondii 76K. At 28 days spleen and MLN cells were isolated and stimulated in vitro with TAg (10 μg/ml). Proliferation was assessed after 4 days. Values for IL-2 and IL-4 were measured at 24 h, those for IFN-γ were measured at 72 h, and those for IL-10 and IL-5 were measured at 96 h of culture. Results are mean cytokine concentrations or counts per minute for proliferation assays on spleen and MLN cells from three mice per experimental group. Results from one of three similar experiments are shown and are expressed as the mean ± standard error of the mean. Symbols: *, P < 0.001; #, P < 0,001.
An absence of lymphoproliferation to Toxoplasma antigen was observed in the splenic and MLN immune compartment from untreated or unpulsed CBA/J MLNDCs (Fig. 7) or C57BL/6 (Fig. 8) mice. MLN and spleen cells from CBA/J (Fig. 7) and C57BL/6 (Fig. 8) mice given TAg-pulsed MLNDCs showed a strong proliferative response to TAg restimulation. These results demonstrate that i.v. administration of TAg-pulsed MLNDCs triggered a systemic and local cell-mediated immunity to Toxoplasma and abrogated the immunosuppression of the Toxoplasma infection.
Cellular proliferative response induced by SPDCs.
TAg-induced proliferation was assessed with spleen and MLN cells from CBA/J mice immunized with unpulsed or pulsed SPDCs 28 days after DC transfer (Fig. 9).
FIG. 9.
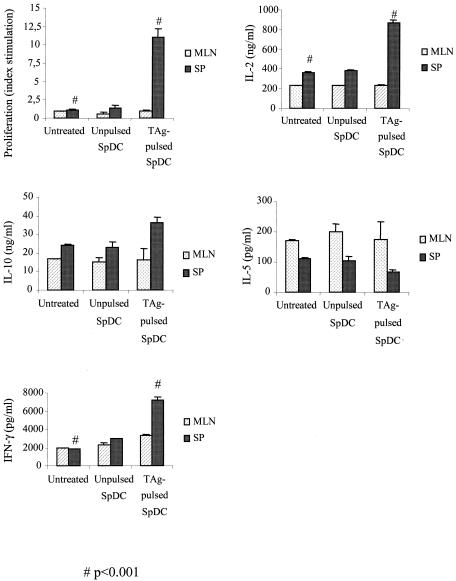
Cellular proliferative response and cytokine production following i.v. immunization with 2.5 × 105 TAg-pulsed CBA/J SPDCs after challenge with 80 cysts of T. gondii 76K. At 28 days spleen and MLN cells were isolated and stimulated in vitro with TAg (10 μg/ml). Proliferation was assessed after 4 days. Values for IL-2 and IL-4 were measured at 24 h, for IFN-γ at 72 h and for IL-10 and IL-5 at 96 h of culture. Results are mean cytokine concentrations or counts per minute for proliferation assays on spleen and MLN cells from three mice per experimental group. Results from one of three similar experiments are shown and are expressed as the mean ± standard error of the mean. Symbols: *, P < 0.001; #, P < 0.001.
An absence of lymphoproliferation to Toxoplasma antigen was observed in the MLN immune compartment from untreated, unpulsed SPDCs and TAg-pulsed SPDCs (Fig. 9). An absence of lymphoproliferation to Toxoplasma antigen was also observed in the spleen from untreated and unpulsed SPDCs (Fig. 9). Spleen cells from CBA/J mice given TAg-pulsed SPDCs showed a strong proliferative response to TAg restimulation. These results demonstrate that i.v. administration of TAg-pulsed SPDCs triggered a systemic cell-mediated immunity to Toxoplasma but was not able to abrogate the immunosuppression of the Toxoplasma infection at the mucosal site.
Cytokine production by lymph node cells from mice immunized with TAg-pulsed or unpulsed MLNDCs.
The supernatants of cultured immune MLN and spleen cells from unpulsed and pulsed MLNDC-injected C57BL/6 and CBA/J mice were evaluated for the production of IFN-γ, IL-2, IL-4, IL-5, and IL-10 in response to TAg antigen 23 days after oral challenge (Fig. 7 and 8).
MLN cells from CBA/J mice immunized with TAg-pulsed MLNDCs responded to TAg stimulation by greater production of IL-10 (60 ng/ml), IL-5 (775 pg/ml), and IL-13 (300 pg/ml) than did the lymph node cells from untreated CBA/J mice (1 ng/ml, 70 pg/ml, and 50 pg/ml) or those given unpulsed MLNDCs (15 ng/ml, 200 pg/ml, and 50 pg/ml), respectively, and by lower production of IFN-γ (1,000 pg/ml) than the lymph node cells from untreated CBA/J mice (3,300 pg/ml) or those given unpulsed MLNDCs (3,000 pg/ml). No specific release of IL-2 (Fig. 7) and IL-4 was demonstrated (data not shown).
Spleen cells from CBA/J mice immunized with TAg-pulsed MLNDCs responded to TAg stimulation by greater production of IL-10 (36 ng/ml) than did lymph node cells from untreated CBA/J mice (2.4 ng/ml) or mice given unpulsed MLNDCs (13 ng/ml). The production of IFN-γ (3,200 ng/ml) compared to untreated CBA/J mice (3,700 ng/ml) and unpulsed MLNDC CBA/J mice (3,900 ng/ml) and IL-5 (110 pg/ml) compared to untreated CBA/J mice (50 pg/ml) or unpulsed MLNDC mice (110 pg/ml) was identical (Fig. 7). The production of IL-2 (<20 ng/ml and 360 to 380 ng/ml) was lower (Fig. 7). No specific release of IL-4 was detected (data not shown).
MLN cells from C57BL/6 mice immunized with TAg-pulsed MLNDCs responded to TAg stimulation by greater production of IL-10 (25 ng/ml), IL-5 (680 pg/ml) and IL-13 (280 pg/ml) than the lymph node cells from untreated C57BL/6 mice (<0.02, 55, and <10 pg/ml) or from mice given unpulsed MLNDCs (8 ng/ml, 210 pg/ml, and <10 pg/ml), respectively. The production of IL-2 (<20, 110, and 56 ng/ml) was lower. No specific release of IFN-γ (Fig. 8) and IL-4 (data not shown) was demonstrated.
Spleen cells from C57BL/6 mice immunized with TAg-pulsed MLNDCs responded to TAg stimulation by greater production of IFN-γ (9,200 ng/ml) and IL-2 (115 ng/ml) than did lymph node cells from untreated C57BL/6 mice (460 and ng/ml) or from mice given unpulsed MLNDCs (1,000 and 27 ng/ml) (Fig. 8). No specific releases of IL-10, IL-13, and IL-5 (Fig. 8) and IL-4 were detected.
Cytokine production by lymph node cells from mice immunized with TAg-pulsed or unpulsed SPDCs.
We can observe that there is no difference of cytokine synthesis (IL-2, IL-10, IL-5, and IFN-γ) by MLN cells from CBA/J mice untreated or immunized with unpulsed SPDCs or immunized with TAg-pulsed SPDCs in response to TAg stimulation (Fig. 9).
Spleen cells from CBA/J mice immunized with TAg-pulsed SPDCs responded to TAg stimulation by greater production of IL-2 (864 ng/ml) and IFN-γ (7,775 pg/ml) than did lymph node cells from untreated CBA/J mice (IL-2, 374 ng/ml; IFN-γ, 1,879 pg/ml) or mice given unpulsed SPDCs (IL-2, 387 ng/ml; IFN-γ, 2,134 pg/ml). The production of IL-10 (36 ng/ml), compared to untreated CBA/J mice (24 ng/ml) and unpulsed MLNDC CBA/J mice (23 ng/ml) was slightly superior (Fig. 9). The production of IL-5 (65 ng/ml and 105 to 111 ng/ml) was lower (Fig. 9). No specific release of IL-4 was detected (data not shown).
MLNDC traffic to the intestine and to the spleen following adoptive transfer.
To explore the importance of DC origin in relation to homing, a radioisotope trafficking study was done with MLNDCs and SPDCs to determine whether DCs home to host organs (Fig. 10).
FIG. 10.
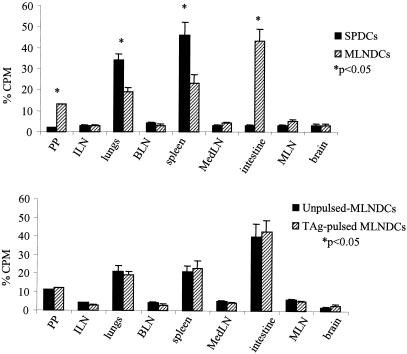
Unpulsed or TAg-pulsed SPDCs and unpulsed or TAg-pulsed MLNDCs were isolated from the same CBA/J mice. DCs were incubated with 51Cr. DCs (5 × 105) were injected and 2 h after the transfer, radioactivity was measured in each organ. Homing to different organs was compared between SPDCs and MLNDCs. Results are expressed as the percentage of radioactivity recovered from the specific organ compared with the total radioactivity in the mouse. Results from one of the three similar experiments are shown and are expressed as the mean ± standard error of the mean. *, P < 0.05. Abbreviations: PP, Peyer's patches; ILN, iliac lymph nodes; BLN, brachial lymph nodes; MedLN, mediastinal lymph nodes.
Two hours after i.v injection of 51Cr-labeled MLNDCs (5 × 105), 43% of the total radioactivity was detected in the small intestine of the recipient CBA/J mice. There was also radioisotope activity concentrated in the spleen (22%), in the lungs (19%), and in the Peyer's patches (12%). Radioactivity in other immune compartments was 5% or less. The injection of 51Cr-labeled SPDCs resulted in 48% of the total radioactivity being in the spleen and 32% in the lungs of the recipient CBA/J mice. Radioactivity in other immune compartments was 5% or less. Radioactivity was not detected in the small intestine (Fig. 10).
To explore the importance of antigen priming for DC homing, unpulsed and TAg-pulsed MLNDCs, labeled with chromium, were injected into naive host. There was no significant difference in the radioactive counts in the organs of mice given antigen-pulsed and unpulsed MLNDCs (Fig. 10).
DISCUSSION
We have shown that adoptively transferred MLNDCs pulsed ex vivo with TAg elicit a mucosal Toxoplasma specific Th2 immune response in vivo and conferred protection against Toxoplasma infection. These mucosal DCs have been shown to preferentially traffic to mucosa. These studies are the first to describe the development of a highly efficacious vaccine against Toxoplasma correlated with a mucosal and splenic homing capacity of mucosal DCs and a mucosal Th2 response.
The role of MLNDCs has been investigated in the initiation of the immune response against T. gondii. We demonstrated that genetically resistant CBA/J mice exhibited a profound reduction in cerebral parasite load (93%) following parasite challenge against Toxoplasma infection in comparison with CBA/J SPDCs, which induced a weaker protection (70%) in the same model of adoptive transfer. We also showed that susceptible C57BL/6 mice were highly protected from mortality (90%). MLNDCs conferred protection against challenge by reducing susceptibility and decreasing parasite burden. These results indicated that MLNDCs play an important role in short- and long-term host immunity against toxoplasmosis. This protection was independent of the mouse strain and was closely related to the production of a mucosal Th2 cytokine profile via TGF-β and IL-10 synthesis by MLNDCs and a production of specific secretory IgA. MLNDCs were able to induce a T. gondii antigen-specific proliferation of MLN lymphocytes, which produced IL-10, IL-13, and IL-5 and down regulated IFN-γ and IL-2. At the spleen level, CBA/J MLNDCs induced a Th1/Th2 cytokine profile (production of IL-10 and IFN-γ) and C57BL/6 MLNDCs induced a Th1 cytokine profile (production of IFN-γ and IL-2 and down regulation of IL-5 and IL-13). In contrast, the lower protection observed after TAg-pulsed SPDCs was correlated to the production of Th1 cytokine profile via an IL-12 synthesis by SPDCs. SPDCs were not able to induce a T. gondii antigen-specific proliferation of MLN lymphocytes, and no production of secretory IgA and cytokines was observed at the mesenteric level. One of the unique features of mucosal lymphoid tissues such as MLNs is their capacity to produce Th cells producing type 2 (IL-10, IL-5, and IL-4) cytokines (8, 10). IL-4 is generally considered to efficiently direct Th2 response, but IL-4 production was always undetectable in our cultures. Similar results were obtained recently by Chang et al. (5), who described a phenotypically and functionally novel monocyte-derived DC subset, designated mDC2, that lacked IL-12 synthesis, produced high levels of IL-10, and directed differentiation of Th2 cells which synthesized IL-5, IL-10, and IL-13 but produced no IL-4 (5). The mechanism that determines the ability of mucosal DCs to generate Th2 responses yet allow for the differentiation of Th1 responses after infection with pathogenic organisms is not known. The nature of the resident DCs is an important factor. It has been suggested that these cells differ in their capacity to drive T-cell differentiation (31). Everson et al. (11) produced evidence to suggest that cytokine secretion by mucosal DCs may influence T-cell activation. He showed that activation of T cells by mucosal DCs led to a Th2 pattern of cytokine secretion, whereas SPDCs induced Th1 cytokines by IL-12 synthesis. The mucosal microenvironment also appears to be particularly geared toward the induction of T-helper cells producing type 2 cytokines. The data presented show that MLNDCs stimulate the differentiation of CD4+ into a Th2 pathway in the mucosa.
Protective immunity to T. gondii infection is generally considered to be cell-mediated. In addition to CD8+ T cells and IFN-γ, which are known to be critical for resistance (14), CD4+ cells contribute significantly to protection against chronic T. gondii infections via their role as helper cells for production of isotype-switched antibodies and cytokines (18). CD4+ T cells are important for early IFN-γ production during T. gondii infection and are essential for priming of CD8+-T-cell effector immunity against T. gondii (13). A lack of such cells leads to increased parasite multiplication in the tissues (4). IL-12 is also known to play a major role in immunity to intracellular pathogens by governing the development of IFN-γ-dependent host resistance (34). However, other immune mechanisms of protection could act otherwise or in concert with such a cellularly mediated immunity. Humoral effector mechanisms, particularly secretory IgA, may be involved in the protective response against Toxoplasma following natural infections. In vitro our preliminary results indicate that IgA inhibits T. gondii replication in enterocytes during transcytosis. The induction of efficacious mucosal immune responses against T. gondii at gut level could inhibit the parasite replication and the severity of the infection. Moreover, two recent publications indicate that specific antibodies play an important role in resistance to T. gondii infection in vivo by blocking infection of host cells by tachyzoites (19, 28). Many studies have established the role of various immune compartments in response to infection, but they do not address infection of the host by acquisition per os, the natural route of infection. Tachyzoites are given intraperitoneally in most studies. The activation of different immune compartments such as Peyer's patches and MLNs after infection has been explored by different laboratories. Chardès et al. (7) investigated the cytokine secretion of antigen-stimulated mucosal T lymphocytes and spleen T cells from T. gondii orally infected mice to describe the lymphocyte proliferation induced by T. gondii. In the CBA/J mouse model, the blastogenesis of mesenteric TAg-induced T lymphocyte is associated with significant IFN-γ, IL-4, IL-5, and IL-6 production and little IL-2 secretion, whereas TAg-specific spleen T cells produce only IFN-γ, IL-6, and IL-2. Chardès et al. (7) concluded that TAg induce an early mucosal T-cell response mainly dominated by a mesenteric Th2-type cytokine response and a later spleen response correlated with a major Th1-type response. Liesenfeld et al. (23) showed that IFN-γ mediates necrosis in the ilea of susceptible C57BL/6 mice after infection, suggesting that this IFN-γ-mediated disorder of the small intestine predisposes to death in these mice. Buzoni-Gatel et al. (3) recently demonstrated a potentially important role for gut-derived intraepithelial lymphocytes (IEL) in host immunity to T. gondii. The ability of the IEL to protect appeared dependent upon the production by these cells of TGF-β which protected mice from developing hyperinflammation and bowel necrosis. These IEL can drastically reduce the expression of the Th1 proinflammatory cytokine, IFN-γ. All these results showed that a Th2 dominant mucosal immune response is correlated to the control of T. gondii infection. More generally, studies on mucosal surfaces such as lung and gut clearly indicate that the baseline response to pathogens at these sites displays a Th2 bias (20). This protective capacity may be associated with DC trafficking. The transport of antigen from the periphery to organized lymphoid tissues by APCs is crucial for initiating an immune response. DCs are probably the main APCs contributing to this antigen transport, as they migrate and home to the T-cell areas of lymphoid tissues, after antigen uptake in the periphery (22). To determine their contribution to immunogenicity, we monitored the migration pathway of these cells and correlated it with their ability to induce immune responses. Adoptively transferred MLNDCs mostly traffic to the intestine and partly to the Peyer's patches but also to the spleen and to the lungs whereas SPDCs home to the spleen and to the lung but not to the intestine and to the Peyer's patches. This is in line with a strong primary activation in the MLNs and spleen, following i.v. injection. Therefore, the settlement of the MLNDCs in the intestine and the spleen may be a prerequisite for the final triggering of the immunological response leading to protection. The mechanism by which MLNDCs home to the appropriate organ is not known. Chemokines could be involved in the homing since recently Cook et al. have demonstrated that CCR6 is a mucosa-specific regulator of mucosal immunity and mediates DC localization and immune responses in mucosal tissue (9). The ideal vaccination protocol for inducing protective immune responses is not established. It has been shown that antigen-pulsed DCs administered i.v. are more effective than those administered subcutaneously (s.c.) in protecting against tumor or infectious challenge (33, 32). Kupiec-Weglinski (21) observed that i.v.-injected purified SPDCs were immediately sequestered in the lung, but then actively migrated into the liver and the spleen which is the principal site of DCs. DCs were also unable to enter MLNs and other peripheral lymph nodes as we have also observed. In contrast Morikawa et al. (24) found that the majority of freshly isolated splenic cells injected s.c. remained at the site of injection and did not migrate to the draining lymph node. The possibility that the route of administration affects the homing properties of DCs needs to be addressed further, particularly since there is evidence that the localization of antigen-treated DCs in the spleen induces a Th1 response, whereas localization in lymph nodes induces a Th2 response (25). Thus, it is becoming increasingly clear that the nature of the immune response mounted within the mucosa-associated lymphoid tissue is a critical event in the development of protective immunity against T. gondii. It is also clear that the presence of DCs at the site of antigenic exposure is crucial for the development of effective local and systemic immune responses. In the case of Toxoplasma infection, we have shown that MLNDCs control the development of an immunologically mediated protective response to the parasite. The findings reported in this work might impact the design of vaccine strategies to many mucosal pathogens. However it would be impractical at present to suggest the use of autologous ex vivo antigen-pulsed DCs as immunotherapies for the prevention of Toxoplasma infection of humans. Nevertheless, the results described herein clearly demonstrate the feasibility of producing a highly efficacious vaccine against Toxoplasma infection using nonviable organisms. This is an important finding, since previous immunization studies have shown that optimum protective immunity against Toxoplasma infection could only be produced using viable organisms, although lower levels of protection against mortality or brain cysts have been described after immunization of mice with Toxoplasma antigens. The work reported here demonstrates that it should be possible to develop an efficacious Toxoplasma vaccine using conventional approaches. To reach this goal it will be important to understand the immunizing property(ies) of Toxoplasma-pulsed MLNDCs that confer Th2-mediated immunity mucosally, and to identify Toxoplasma protective antigens that can be used for immunological targeting and vaccine development. The DC system described here represents a powerful means to identify Toxoplasma protective antigens through reconstitution experiments. For example, DCs pulsed ex vivo with acellular or recombinant antigens or transfected with DNA encoding antigen(s) can be used to efficiently evoke anti-Toxoplasma immunity at the intestinal mucosa.
Acknowledgments
We thank A. Hosmalin, G. Milon, C. Chougnet, and I. Bourguin for critically reading the manuscript, and we thank D. Tabareau and R. Magné for technical help.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Anjuere, F., P. Martin, I. Ferrero, M. L. Fraga, G. M. del Hoyo, N. Wright, and C. Ardavin. 1999. Definition of dendritic cell subpopulations present in the spleen, Peyer's patches, lymph nodes, and skin of the mouse. Blood 93:590-598. [PubMed] [Google Scholar]
- 2.Bourguin, I., M. Moser, D. Buzoni-Gatel, F. Tielemans, D. Bout, J. Urbain, and O. Leo. 1998. Murine dendritic cells pulsed in vitro with Toxoplasma gondii antigens induce protective immunity in vivo. Infect. Immun. 66:4867-4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buzoni-Gatel, D., A. C. Lepage, I. H. Dimier-Poisson, D. T. Bout, and L. H. Kasper. 1997. Adoptive transfer of gut intraepithelial lymphocytes protects against murine infection with Toxoplasma gondii. J. Immunol. 158:5883-5889. [PubMed] [Google Scholar]
- 4.Casciotti, L. K., H. Ely, M. E. Williams, and I. A. Khan. 2002. CD8+-T-cell immunity against Toxoplasma gondii can be induced but not maintained in mice lacking conventional CD4+ T cells. Infect. Immun. 70:434-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, C.-C., A. Wright, and J. Punnonen. 2000. Monocyte-derived CD1a+ and CD1a- dendritic cell subsets differ in their cytokine production profiles, susceptibilities to transfection, and capacities to direct Th cell differentiation. J. Immunol. 165:3584-3591. [DOI] [PubMed] [Google Scholar]
- 6.Chardes, T., I. Bourguin, M. N. Mevelec, J. F. Dubremetz, and D. Bout. 1990. Antibody responses to Toxoplasma gondii in sera, intestinal secretions, and milk from orally infected mice and characterization of target antigens. Infect. Immun. 58:1240-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chardes, T., F. Velge-Roussel, P. Mevelec, M. N. Mevelec, D. Buzoni-Gatel, and D. Bout. 1993. Mucosal and systemic cellular immune responses induced by Toxoplasma gondii antigens in cyst orally infected mice. Immunology 78:421-429. [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, Y., J. Inobe, R. Marks, P. Gonnella, V. K. Kuchroo, and H. L. Weiner. 1995. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature 376:177-180. [DOI] [PubMed] [Google Scholar]
- 9.Cook, D. N., D. M. Prosser, R. Forster, J. Zhang, N. A. Kuklin, S. J. Abbondanzo, X. D. Niu, S. C. Chen, D. J. Manfra, M. T. Wiekowski, L. M. Sullivan, S. R. Smith, H. B. Greenberg, S. K. Narula, M. Lipp, and S. A. Lira. 2000. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity 12:495-503. [DOI] [PubMed] [Google Scholar]
- 10.Daynes, R. A., B. A. Araneo, T. A. Dowell, K. Huang, and D. Dudley. 1990. Regulation of murine lymphokine production in vivo. III. The lymphoid tissue microenvironment exerts regulatory influences over T helper cell function. J. Exp. Med. 171:979-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everson, M. P., D. S. McDuffie, D. G. Lemak, W. J. Koopman, J. R. McGhee, and K. W. Beagley. 1996. Dendritic cells from different tissues induce production of different T cell cytokine profiles. J. Leukoc. Biol. 59:494-498. [DOI] [PubMed] [Google Scholar]
- 12.Frenkel, J. 1988. Pathophysiology of toxoplasmosis. Parasitol. Today 4:273-278. [DOI] [PubMed] [Google Scholar]
- 13.Gazzinelli, R. T., F. T. Hakim, S. Hieny, A. Cheever, and A. Sher. 1991. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J. Immunol. 146:286-292. [PubMed] [Google Scholar]
- 14.Gazzinelli, R. T., E. Y. Denkers, and A. Sher. 1993. Host resistance to Toxoplasma gondii: model for studying the selective induction of cell-mediated immunity by intracellular parasites. Infect. Agents Dis. 2:139-149. [PubMed] [Google Scholar]
- 15.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R. M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwasaki, A., and B. L. Kelsall. 1999. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 190:229-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, L. L., and P. C. Sayles. 1997. Interleukin-12, dendritic cells, and the initiation of host-protective mechanisms against Toxoplasma gondii. J. Exp. Med. 186:1799-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, L. L., and P. C. Sayles. 2002. Deficient humoral responses underlie susceptibility to Toxoplasma gondii in CD4-deficient mice. Infect. Immun. 70:185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang, H. J. S. Remington, and Y. Suzuki. 2000. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-gamma, TNF-alpha, and inducible nitric oxide synthase. J. Immunol. 164:2629-2634. [DOI] [PubMed] [Google Scholar]
- 20.Kjerrulf, M., D. Grdic, M. Kopf, and N. Lycke. 1998. Induction of gut mucosal immune responses: importance of genetic background and Th1/Th2 cross-regulation. Scand. J. Immunol. 47:401-407. [DOI] [PubMed] [Google Scholar]
- 21.Kupiec-Weglinski, J. W., J. M. Austyn, and P. J. Morris. 1988. Migration patterns of dendritic cells in the mouse. Traffic from the blood, and T cell-dependent and -independent entry to lymphoid tissues. J. Exp. Med. 167:632-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lafferty, K. J., A. Bootes, G. Dart, and D. W. Talmage. 1976. Effect of organ culture on the survival of thyroid allografts in mice. Transplantation 22:138-149. [DOI] [PubMed] [Google Scholar]
- 23.Liesenfeld, O., J. Kosek, J. S. Remington, and Y. Suzuki. 1996. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J. Exp. Med. 184:597-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morikawa, Y., M. Furotani, K. Kuribayashi, N. Matsuura, and K. Kakudo. 1992. The role of antigen-presenting cells in the regulation of delayed-type hypersensitivity. I. Spleen dendritic cells. Immunology 77:81-87. [PMC free article] [PubMed] [Google Scholar]
- 25.Morikawa, Y., K. Tohya, H. Ishida, N. Matsuura, and K. Kakudo. 1995. Different migration patterns of antigen-presenting cells correlate with Th1/Th2-type responses in mice. Immunology 85:575-581. [PMC free article] [PubMed] [Google Scholar]
- 26.Pugh, C. W., G. G. MacPherson, and H. W. Steer. 1983. Characterization of nonlymphoid cells derived from rat peripheral lymph. J. Exp. Med. 157:1758-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remington, J. S., R. McLeod, and G. Desmonts. 1995. Toxoplasmosis, p. 140. In J. S. Remington and J. O. Klein (ed.), Infectious diseases of the fetus and newborn infant, 4th ed. The W. B. Saunders Co., Philadelphia, Pa.
- 28.Sayles, P. C., G. W. Gibson, and L. L. Johnson. 2000. B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect. Immun. 68:1026-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma, S. D., J. Mullenax, F. G. Araujo, H. A. Erlich, and J. S. Remington. 1983. Western blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J. Immunol. 131:977-983. [PubMed] [Google Scholar]
- 30.Steinman, R. M., and M. C. Nussenzweig. 1980. Dendritic cells: features and functions. Immunol. Rev. 53:127-147. [DOI] [PubMed] [Google Scholar]
- 31.Stumbles, P. A., J. A. Thomas, C. L. Pimm, P. T. Lee, T. J. Venaille, S. Proksch, and P. G. Holt. 1998. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. J. Exp. Med. 188:2019-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi, H., Y. Nakagawa, K. Yokomuro, and J. A. Berzofsky. 1993. Induction of CD8+ cytotoxic T lymphocytes by immunization with syngeneic irradiated HIV-1 envelope derived peptide-pulsed dendritic cells. Int. Immunol. 5:849-857. [DOI] [PubMed] [Google Scholar]
- 33.Toes, R. E., E. I. van der Voort, S. P. Schoenberger, J. W. Drijfhout, L. van Bloois, G. Storm, W. M. Kast, R. Offringa, and C. J. Melief. 2000. Enhancement of tumor outgrowth through CTL tolerization after peptide vaccination is avoided by peptide presentation on dendritic cells. J. Immunol. 160:4449-4456. [PubMed] [Google Scholar]
- 34.Yap, G., M. Pesin, and A. Sher. 2000. Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J. Immunol. 165:628-631. [DOI] [PubMed] [Google Scholar]