Abstract
Toll-like receptors mediate macrophage recognition of microbial ligands, inducing expression of microbicidal molecules and cytokines via the adapter protein MyD88. We investigated the role of MyD88 in regulating murine macrophage responses to a pathogenic yeast (Candida albicans) and mold (Aspergillus fumigatus). Macrophages derived from bone marrow of MyD88-deficient mice (MyD88−/−) demonstrated impaired phagocytosis and intracellular killing of C. albicans compared to wild-type (MyD88+/+) macrophages. In contrast, ingestion and killing of A. fumigatus conidia was MyD88 independent. Cytokine production by MyD88−/− macrophages in response to C. albicans yeasts and hyphae was substantially decreased, but responses to A. fumigatus hyphae were preserved. These results provide evidence that MyD88 signaling is involved in phagocytosis and killing of live C. albicans, but not A. fumigatus. The differential role of MyD88 may represent one mechanism by which macrophages regulate innate responses specific to different pathogenic fungi.
Fungi are a diverse group of organisms, as demonstrated by variable cell surface components, secreted products, and differential ability to cause disease in plants and mammals. Macrophages play a critical role in regulating defenses against fungi, with an ability to phagocytose and kill microbes and regulate secondary responses through production of cytokines. Central to these functions are receptors that are involved in microbe internalization (phagocytic receptors) and receptors that participate in microbe-specific inflammatory responses (Toll-like receptors [TLRs]) (2, 5, 15, 23).
Phagocytic receptors recognize microbial cell surface components like mannan and glucan or recognize opsonins that bind to specific components of the microbial surface, such as antibodies, mannose-binding lectin, complement fragments, and surfactant proteins. Most microbes can be internalized by multiple receptors, largely dependent on the microbial environment and the activation state of the macrophage (3). Signaling for phagocytosis has not been fully delineated, but there is evidence that the mechanisms that regulate expression and function of each receptor are unique (4, 35).
The extracellular domain of TLRs recognizes pathogen components, and intracellular regions transmit signals to adapter proteins that mediate downstream cellular events, such as cytokine production and activation of specific effector pathways (5). One adapter protein is myeloid differentiation factor 88 (MyD88), which mediates a signaling pathway that results in translocation of NF-κB and subsequent transcription of proinflammatory cytokine genes. Macrophages harvested from MyD88-deficient (MyD88−/−) mice fail to secrete proinflammatory cytokines in response to bacterial products, and mice exhibit an increased susceptibility to lethal infection after bacterial (Staphylococcus aureus) challenge (1, 26-29). There is evidence that TLRs may also be involved in activation of specific antimicrobial effector pathways (7, 30), although their role in regulating activity against a diverse group of pathogens is unknown.
TLRs have an important role in regulating macrophage antifungal responses. TLR2, TLR4, and TLR6 have been implicated in signaling proinflammatory cytokine production in response to components of nonpathogenic and pathogenic yeasts and molds (19, 22, 23, 25, 31, 34). Herein, we describe the role of MyD88 in regulating macrophage responses to live Candida albicans and Aspergillus fumigatus, two of the most common opportunistic pathogens in humans. Results uncover a role for MyD88 in regulating phagocytosis of C. albicans and show that murine macrophages utilize different pathways to mediate responses to different pathogenic fungi.
MATERIALS AND METHODS
Fungi and reagents.
The A. fumigatus strain used in these studies was obtained from a patient who developed pulmonary aspergillosis at the National Institutes of Health (B-5233; gift from June Kwon Chung). C. albicans strain 3153a (ATCC 28367), a well-described pathogenic isolate, was used. Fungi were maintained on potato dextrose agar (A. fumigatus at 35°C and C. albicans at 30°C) and plated fresh on RPMI 1640 agar prior to use. Suspensions of A. fumigatus conidia and C. albicans yeasts were prepared by flooding colonies with sterile RPMI 1640, and cells were enumerated with a hemacytometer. All media used for serial dilution of A. fumigatus (suspension and agar) contained 0.025% Tween to minimize conidial clumping and allow for quantification of colonies.
RPMI 1640, phosphate-buffered saline (PBS), and trypsin-EDTA were obtained from BioWhittaker (Walkersville, Md.). All experiments were performed at 37°C in humidified air (+5% CO2) in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin, and streptomycin. Media contained less than 0.03 U of endotoxin/ml, as certified by the manufacturers. FUN-1 was obtained from Molecular Probes (Eugene, Oreg.).
Mice and macrophage culture.
MyD88−/− mice (129SvJ × C57BL/6 background) were derived as previously described (1) and backcrossed for three generations with C57BL/6 mice. For experiments, MyD88−/− mice were obtained by crossing heterozygous (MyD88+/−) F3 mice. Littermate MyD88+/+ mice were used as controls. Standard PCR-based genotyping was performed with DNA from tail snip specimens to identify MyD88+/+, MyD88−/−, and MyD88+/− mice. MyD88−/− homozygosity was confirmed by quantification of lipopolysaccharide (LPS)-induced tumor necrosis factor alpha (TNF-α) production by enzyme-linked immunosorbent assay (murine TNF-α Duoset; R & D Systems, Minneapolis, Minn.). LPS-induced TNF-α production was decreased by >90% in macrophages isolated from MyD88−/− mice compared to macrophages from wild-type (MyD88+/+) mice.
Bone marrow-derived macrophages were prepared by cultivation in L929 cell-conditioned medium (6). In brief, bone marrow cells were flushed from tissue-free femurs with sterile RPMI 1640 and cultured on plastic petri dishes (100 by 15 mm; Falcon, Becton Dickinson, Franklin Lakes, N.J.) for 5 to 7 days in medium consisting of a 1:1 mixture of RPMI 1640-20% FBS (supplemented with 10 mM HEPES, 0.2 mM l-glutamine, 25 U of penicillin/ml, and 25 μg of streptomycin/ml) and L929 cell-conditioned medium, prepared as the culture supernatant from confluent L929 cells grown in high-glucose (4.5 g/liter) Dulbecco's modified Eagle medium-10% FBS (supplemented with 10 mM HEPES, 0.2 mM l-glutamine, 25 U of penicillin/ml, and 25 μg of streptomycin/ml) for 3 days. Cells that were adherent after 4-day incubations were washed with PBS and used for phagocytosis and killing experiments and cytokine analyses.
Microbial phagocytosis and killing assays.
For phagocytosis experiments, C. albicans yeasts and A. fumigatus conidia were prelabeled with FUN-1 (5 μM). Labeling was performed in the dark with gentle shaking (30°C) for 30 (C. albicans) or 45 min (A. fumigatus) (20). Dye uptake and inoculum viability was confirmed by visualization of green to orange fluorescence using a Texas Red fluorescein filter. Cells were washed twice with PBS, resuspended in RPMI 1640, and stored at 4°C until use. Bone marrow-derived macrophages were incubated with C. albicans yeasts (multiplicity of infection [MOI], 1:1 or 10:1), A. fumigatus conidia (MOI, 1:1 or 10:1), fluorescein isothiocyanate (FITC)-labeled dextran (1 μg/ml, Sigma, St. Louis, Mo.), or tetramethyl rhodamine isocyanate (TRITC)-labeled zymosan (MOI, 10:1; Molecular Probes) and centrifuged briefly (500 × g) to ensure particle exposure. Macrophages were allowed to ingest particles for differing time periods, exposed to 5 mM Na-azide to arrest phagocytosis, and lifted from wells with gentle pipetting with PBS containing 1 mM EDTA (pH 7.5). FUN-1 green, FITC, or TRITC fluorescence in recovered macrophages was measured with a FACScan cytometer (Becton Dickinson, San Jose, Calif.), and analyzed with Cellquest software (Becton Dickinson). External zymosan particles were digested prior to analysis with 15-min incubations (37°C) in lyticase (100 U/ml; Sigma) (31).
To distinguish between adherent and ingested conidia and yeasts, FUN-1 fluorescence was measured in control macrophages that were pretreated with Na-azide (5 mM) for 1 h. To further distinguish between adherent and ingested fungal cells, Calcofluor white M2R (Sigma), a fungus-specific stain, was used. In brief, macrophages were allowed to ingest FUN-1-labeled microbes on sterile glass coverslips, and adherent cells were gently washed with PBS and counterstained with Calcofluor white (25 μM). Cells were incubated for 15 min at 4°C in the dark. Cells were washed, fixed, and viewed with a Deltavision wide-field fluorescent microscope (Applied Precision Inc., Issaquah, Wash.) fitted with a FITC and rhodamine filter set. The number of cells containing FUN-1 green (internal) and Calcofluor white blue (external adhered) organisms was quantified microscopically. Phagocytosis indices (the percentage of phagocytic macrophages multiplied by the mean number of organisms internalized per macrophage) were calculated.
Experiments to measure killing of C. albicans and A. fumigatus were performed by serial dilution techniques, as described and standardized elsewhere (20, 22, 33). Organisms were harvested from macrophages after 6 h by osmotic freeze-thaw lysis, and serial dilutions were plated on yeast extract-based medium (yeast extract-peptone-dextrose). This method of elaborating organisms from cells results in <10% killing of both Candida and Aspergillus (20). CFU were quantified after 2 (C. albicans) or 3 (A. fumigatus) days of growth, and killing indices (KIs) were calculated from triplicate platings in three experiments by the formula KI = 1 − (CFU 6 h/CFU 1 h).
Because it was previously shown that serial dilution techniques yield a large amount of error, killing of A. fumigatus was confirmed by a previously developed fluorescence-based viability assay (20). Viable A. fumigatus conidia metabolize the green pigment of FUN-1 (Molecular Probes) into orange vacuoles when metabolically active (20). To measure macrophage intracellular killing of A. fumigatus conidia by flow cytometry, adherent macrophages were exposed to viable (orange) A. fumigatus conidia (prelabeled with 5 μM FUN-1), and after different time periods, intracellular conidia were harvested by freeze-thaw lysis. DNA, RNA, and protein in the suspension were digested with 10 U of DNase/ml, 500 μg of RNase/ml, and 1 μg of proteinase K/ml, and propidium iodide (PI) was added (25 μg/liter). After incubation (20 min in the dark), samples were analyzed with a FACScan cytometer (Becton Dickinson, San Jose, Calif.). Conidia were gated by forward-side scatter characteristics, and PI-positive (dead) organisms were quantified from within the nonviable (orange low) gate.
Macrophage viability and functional assays.
The baseline viability of MyD88−/− and MyD88+/+ macrophages was measured by uptake of PI. Macrophages were washed and lifted from wells by gentle pipetting with PBS (with 1 mM EDTA, pH 7.5), and uptake of PI (10 μM) was measured by flow cytometry. To measure viability and endocytic capacity after exposure to C. albicans, macrophages were allowed to ingest live C. albicans organisms for 1 h, washed extensively, and reincubated with PI (10 μM) and FITC-dextran (100 μg/ml; Molecular Probes). Uptake of FITC-dextran was quantified after 1-h incubations by flow cytometry; controls included macrophages pretreated with Na-azide (5 mM) for 1 h.
Cytokine quantification.
The proinflammatory cytokine TNF-α was quantified in macrophage culture supernatants after exposure to heat-killed fungal products and LPS (Salmonella enterica serovar Minnesota and R595 LPS from List Biological Labs). All media contained 10% FBS. To determine the stimulatory cells of C. albicans and A. fumigatus, cellular products from fungi in specific states (yeasts, conidia, or hyphal fragments) were prepared. Fungal products were killed by 30-min incubations at 80°C. To obtain hyphal fragments of A. fumigatus, a 5-day-old mycelial mat was grown in RPMI 1640 with continuous shaking (35°C), washed, and fragmented by sonication. To standardize hyphal inocula, protein was quantified by a trypan blue protein assay (Bio-Rad Laboratories). Macrophages were stimulated with FBS (10%, heat inactivated)-opsonized fungal products for 24 h, and TNF-α was measured by enzyme-linked immunosorbent assay (murine TNF-α and IL-6 Duoset; R&D Systems). Polymyxin B (10 μg/ml) was added to all fungal preparations, and all reagents (except LPS) were confirmed to be endotoxin free (<0.01 endotoxin U/ml) by the Limulus amebocyte assay (QCL-1000; BioWhittaker).
RESULTS
Intracellular killing of C. albicans, but not A. fumigatus, is MyD88 dependent.
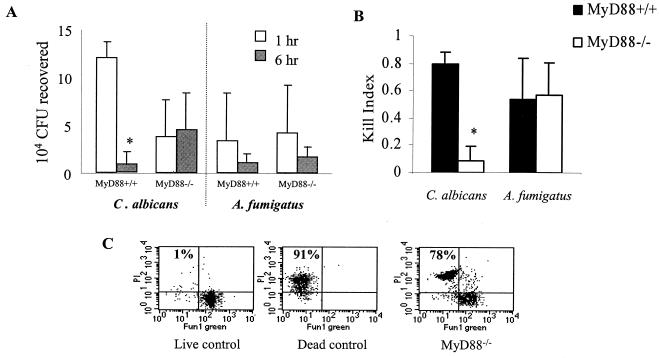
Macrophages derived from bone marrow of wild-type (MyD88+/+) and MyD88-deficient (MyD88−/−) mice were challenged with live C. albicans yeasts and A. fumigatus conidia to compare efficacy of intracellular killing. Viability of C. albicans and A. fumigatus after 1- and 6-h incubations was measured by standardized serial dilution methodologies (20). MyD88+/+ macrophages killed a significant number of C. albicans yeasts by 6 h, as demonstrated by fewer CFU recovered after 6 h than after 1 h (Fig. 1A, left panel). In contrast, there were equivalent numbers of CFU recovered from MyD88−/− macrophages after 1- and 6-h incubations (Fig. 1A, left panel). This observation is consistent with impaired intracellular killing of C. albicans yeasts in MyD88−/− macrophages. Since there were fewer C. albicans CFU recovered from MyD88−/− macrophages than from MyD88+/+ macrophages after 1 h, there was indication of impaired phagocytosis as well.
FIG. 1.
Cellular killing of C. albicans and A. fumigatus. The ability of macrophages to kill fungi was calculated by assaying organism viability after adherent macrophages were allowed to ingest C. albicans yeasts or A. fumigatus conidia (MOI, 10:1). Killing assays were performed in six-well plates. After 1 h, control wells (1-h) were washed, macrophages were lysed, and fungi were serially plated. The remaining wells were washed extensively to remove adherent fungi, fresh medium was added, and wells were reincubated to allow for intracellular killing of the phagocytosed organisms. (A) Viability of C. albicans or A. fumigatus was assessed by enumeration of CFU. Results shown were derived from at least four independent experiments, each with triplicate measurements. Fewer C. albicans organisms were recovered from MyD88−/− macrophages than from MyD88+/+ macrophages after 1-h incubations (P < 0.05 by Student's t test), consistent with impaired phagocytosis. *, P < 0.05 relative to MyD88+/+ control by Student's t test. (B) KIs were calculated by the formula KI = 1 − (CFU 6 h/CFU 1 h). Mean KIs (± standard deviations) were derived from at least four independent experiments, each with triplicate measurements. Killing of C. albicans yeasts was also impaired in alternative experiments using lower MOIs (1:1) (data not shown). *, P < 0.05 relative to MyD88+/+ control by Student's t test. (C) A. fumigatus conidial killing was measured by flow cytometry. Intracellular conidia were harvested by freeze-thaw lysis of MyD88−/− macrophages after 6 h, and PI uptake was quantified within the population of conidia that were not viable, as measured by metabolism of FUN-1 green to orange (see Materials and Methods and reference 20). Numbered quadrants indicate percentages of dead (heat-killed) conidia (high PI uptake) in live and dead control populations and in populations after exposure to MyD88−/− macrophages. The results shown are from one experiment, which is representative of three independent experiments.
The absolute number of CFU recovered is dependent not only on phagocytosis and intracellular killing but also on the inocula, which may vary between experiments due to slight errors in quantifying microbes. To standardize the results of multiple measurements, KIs were calculated. Because these ratios represent organism viability after 6 h of incubation relative to 1-h incubation with macrophages [KI = 1 − (CFU 6 h/CFU 1 h)], KIs provide values that reflect intracellular killing independently of phagocytosis or inoculum variation (20). The efficiency of killing of C. albicans by MyD88+/+ macrophages was greater than that by MyD88−/− macrophages (Fig. 1B). In contrast, both MyD88−/− and MyD88+/+ macrophages killed A. fumigatus conidia equally well (Fig. 1A and B).
Because a substantial amount of error is produced by serial dilution measurements of Aspergillus CFU (20), macrophage conidial killing was verified by flow cytometry (20). After 6 h of incubation with MyD88+/+ and MyD88−/− macrophages, the majority of conidia recovered were dead (Fig. 1C). Killing by MyD88−/− (Fig. 1C) and MyD88+/+ (data not shown) macrophages was equivalent.
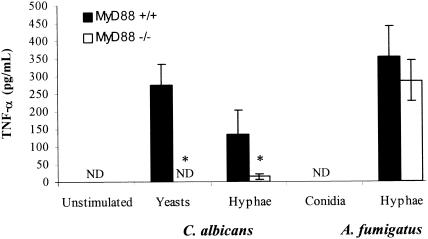
Cytokine production in response to C. albicans, but not A. fumigatus, is MyD88 dependent.
Cytokine production in response to C. albicans and A. fumigatus products was measured. To determine which specific cellular components are stimulatory, responses to 10% FBS-opsonized heat-killed A. fumigatus conidia, C. albicans yeasts, and hyphal products from each organism were measured (Fig. 2). Macrophages produced TNF-α in response to A. fumigatus hyphal products only. Incubation with Aspergillus conidia failed to induce detectable production of TNF-α from either MyD88+/+ or MyD88−/− macrophages (Fig. 2). Both C. albicans yeasts and hyphal products stimulated low but detectable amounts of TNF-α. Compared to MyD88+/+ macrophages, MyD88−/− cells produced less TNF-α in response to C. albicans yeasts and hyphae (Fig. 2). In contrast, MyD88−/− macrophages produced small but equivalent amounts of TNF-α after exposure to A. fumigatus hyphal fragments. No TNF-α was produced by unstimulated macrophages (Fig. 2) or LPS-treated MyD88−/− macrophages (data not shown).
FIG. 2.
Cytokine secretion in response to fungal stimuli. TNF-α was measured in macrophage culture supernatants after 24-h incubation with C. albicans yeasts and hyphae and with A. fumigatus conidia and hyphae, each with an MOI of 10:1, in the presence of 10% heat-inactivated FBS. Results shown are means (± standard deviations) from triplicate measurements in one experiment, which was representative of three independent experiments. ND, not detectable (<15 pg/ml); *, P < 0.05 relative to MyD88+/+ control by Student's t test.
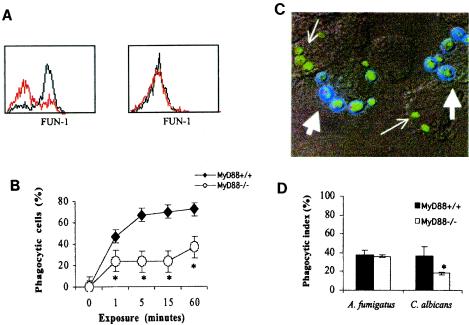
Phagocytosis of C. albicans, but not A. fumigatus, is impaired in MyD88−/− macrophages.
Enumeration of C. albicans CFU recovered from macrophages after 1 h (Fig. 1) suggested a phagocytic defect in MyD88−/− macrophages. To further examine phagocytosis, uptake of FUN-1-labeled C. albicans yeasts and A. fumigatus conidia was measured by flow cytometry (Fig. 3A). A. fumigatus conidia were phagocytosed efficiently by both MyD88+/+ and MyD88−/− macrophages (Fig. 3A, right panel). In contrast, C. albicans yeasts were not ingested as efficiently by MyD88−/− as by MyD88+/+ macrophages (Fig. 3A, left panel). The number of macrophages that had either bound or internalized FUN-1-labeled C. albicans yeasts was quantified by flow cytometry (Fig. 3B). Within 1 min after exposure, there was a difference in MyD88+/+ and MyD88−/− binding and/or internalization of C. albicans yeasts.
FIG. 3.
Phagocytosis of C. albicans and A. fumigatus. (A) MyD88+/+ (black histograms) and MyD88−/− (red histograms) macrophages were lifted off of wells after 15-min incubations with live FUN-1-labeled C. albicans yeasts (left panel) (MOI, 10:1) and A. fumigatus conidia (right panel) (MOI, 10:1). Macrophages were gated by light-scatter characteristics, and intracellular FUN-1 fluorescence was measured by flow cytometry. Results shown are representative of at least four independent experiments. Similar results were obtained with an MOI of 1:1 for both C. albicans and A. fumigatus (data not shown). All y axes are linear (0 to 100 events). (B) Phagocytosis of C. albicans yeasts was quantified after macrophages were exposed for the indicated periods of time (x axis). The percentage of cells expressing FUN-1 fluorescence (y axis) is shown for MyD88+/+ and MyD88−/− macrophages. Results shown are derived from triplicate measurements in one experiment, which was representative of three independent experiments. (C) MyD88+/+ macrophages exposed to live C. albicans. Intracellular (small arrows) and external, adhered C. albicans cells (large arrows) were distinguished based on fluorescence after costaining with Calcofluor white, which does not enter or stain macrophages. Calcofluor white stains the cell wall of external fungal cells blue, such that green cells have an external rim of blue fluorescence. Note that yeasts that are internalized by the macrophages appear small, while the yeasts on the exterior are bigger, budding cellular forms. The viability of internal and external yeasts is also reflected by microbial metabolism of FUN-1 green fluorescence into small vacuoles that fluoresce orange-red (20). (D) Phagocytic indices of MyD88+/+ and MyD88−/− macrophages were calculated after incubation with C. albicans yeasts and A. fumigatus conidia, as described in Materials and Methods. Results shown are means of triplicate measures in three independent experiments (± standard deviations). *, P < 0.05 relative to MyD88+/+ control by Student's t test.
To determine if the difference represented bound versus internalized yeasts, ingestion of FUN-1 fluorescent fungal cells was measured microscopically after external microbes were counterstained with the fungus-specific stain Calcofluor white. By this method, internalized microbial cells fluoresced green, but cells that remained external (adhered) were costained, yielding a rim of blue fluorescence (Fig. 3C). A phagocytic index (percentage of phagocytic macrophages multiplied by the mean number of organisms ingested per cell) was calculated after microscopic review. Results confirmed that MyD88−/− macrophages phagocytosed C. albicans less than MyD88+/+ macrophages (Fig. 3D). In contrast, MyD88−/− and MyD88+/+ macrophages ingested FUN-1-labeled A. fumigatus conidia equally well.
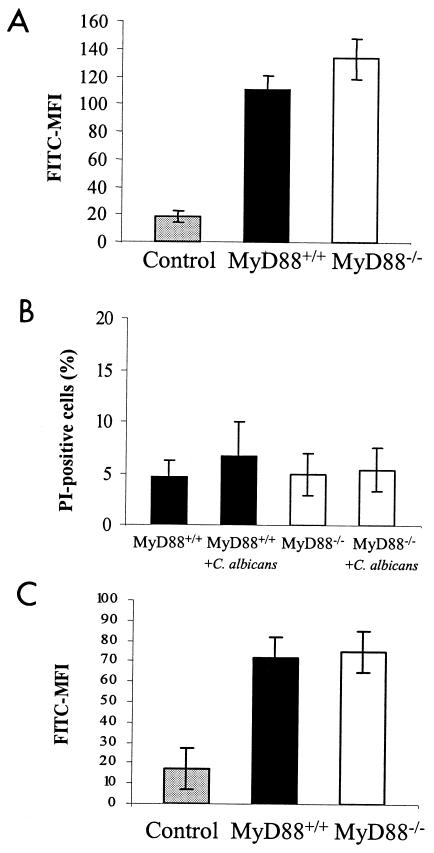
MyD88 deficiency could impact phagocytosis by decreasing macrophage viability or function; however, equivalent phagocytosis of A. fumigatus conidia makes this unlikely. We investigated this hypothesis by comparing baseline viability and function of MyD88+/+ and MyD88−/− macrophages by measuring uptake of PI and endocytosis of FITC-labeled dextran. There was no difference in endocytic capacity (Fig. 4A) or viability (Fig. 4B) before exposure to C. albicans. MyD88−/− and MyD88+/+ macrophages also ingested TRITC-labeled zymosan equally well (data not shown).
FIG. 4.
Macrophage viability and function. (A) MyD88+/+ and MyD88−/− macrophage endocytosis was measured after a 1-h incubation with FITC-labeled dextran. Results are FITC mean fluorescence intensity (MFI) of lifted macrophages from triplicate measures in two independent experiments (mean ± standard deviation). Control represents FITC-dextran fluorescence of Na-azide-treated macrophages. (B) Macrophage viability was assessed at baseline and after a 1-h incubation with live C. albicans by flow-cytometric quantification of PI uptake. Results shown represent mean percentages of dead (PI-positive) macrophages in three independent experiments. More than 95% of the control (heat-killed) macrophages were PI positive (data not shown). (C) MyD88+/+ and MyD88−/− macrophages were allowed to phagocytose C. albicans yeasts for 1 h and then washed extensively and incubated in FITC-dextran for measurement of endocytosis. Results are FITC mean fluorescence intensity (MFI) of lifted macrophages from triplicate measures in two independent experiments (mean ± standard deviation). Control represents FITC-dextran fluorescence of Na-azide-treated macrophages.
An alternative hypothesis for the defect in C. albicans phagocytosis is that MyD88−/− macrophages may have increased susceptibility to Candida-derived factors that induce cellular damage or dysfunction. We investigated this hypothesis by comparing macrophage uptake of PI and endocytosis of FITC-labeled dextran before and after 1-h incubations with live C. albicans (Fig. 4B and C). After 1-h incubations with live C. albicans, there was minimal death of either MyD88+/+ or MyD88−/− macrophages, as measured by PI positivity (Fig. 4B). Also, there was no difference between FITC-dextran uptake by MyD88+/+ and MyD88−/− cells after exposure to C. albicans (Fig. 4C). These findings indicate that MyD88 does not mediate baseline viability or function (ability to ingest dextran) or alter macrophage susceptibility to cytotoxic microbial products. The defect in phagocytosis appears to be specific to C. albicans and not merely a reflection of impaired cellular viability and/or metabolic activity associated with the MyD88−/− phenotype.
DISCUSSION
Using macrophages harvested from mice with targeted genetic disruption of MyD88, we have shown that MyD88 deficiency is associated with a phagocytic defect that is specific to C. albicans. This defect is coupled with impaired intracellular killing of these yeasts and decreased cytokine secretion in response to candidal products. In contrast, intracellular killing of A. fumigatus conidia and cytokine production in response to hyphal products can occur via a MyD88-independent mechanism.
Prior studies have shown that phagocytosis mediated by specific receptors (mannose receptor and FcR) is a proinflammatory process associated with the release of multiple cytokines, including TNF-α and IL-1, occurring upon receptor ligation (3). Particle internalization and TLR recruitment are also likely to be coupled, as several TLR family members (TLR1, TLR2, TLR6) have been shown to localize to the phagosome, where they recognize molecules specific to pathogens and mediate inflammatory signaling (23, 31). MyD88 serves as an adapter protein for TLRs but has yet to be implicated in the function of phagocytic receptors. The MyD88-dependent defect in phagocytosis of C. albicans may be related to a previously unrecognized, direct role in mediating the function of specific phagocytic receptors or to indirect effects associated with impaired inflammatory signaling.
Multiple candidate phagocytic receptors for yeasts exist. Both opsonic and nonopsonic receptors (mannose and glucan receptors) have been implicated in macrophage internalization of yeasts or cell-associated components (32). Further studies will be necessary to define the mechanism by which MyD88 deficiency impacts internalization of C. albicans.
The results of this study emphasize the unique characters of different pathogenic fungi. While some cell wall components, such as chitin, mannan, and glucan, may be conserved in fungi, components of different organisms vary. For instance, the acid labile fraction of the C. albicans cell wall contains unique polysaccharide moieties presenting β-1,2-oligomannosides, and the cell surface of A. fumigatus is characterized by galactomannan (8, 10, 11, 16, 18, 24, 32). Also, fungal cell wall components may be rearranged during morphological transitions into hyphae, the invasive state of most fungi. While both yeasts and hyphae of C. albicans stimulated murine macrophages, only hyphal products of A. fumigatus triggered cytokine release. Unique cell components may account for the different responses reported for conidia and hyphae.
TLRs mediate cytokine production in response to both C. albicans and A. fumigatus products. TLR2 mediates production of cytokines in response to C. albicans, and both TLR2 and TLR4 have been implicated in regulating TNF-α release from murine and human macrophages in response to A. fumigatus (19, 22). In contrast to our findings, Mambula and colleagues recently reported that TNF-α production in response to live A. fumigatus is MyD88 dependent (19). TNF-α production in response to endotoxin contamination was not a likely explanation for our observations, as polymyxin B was added to all inocula. More likely, differences in experimental conditions, including duration and quantity of antigen exposure, and the source of murine macrophages may explain the discrepant results. It is possible that cytokine production in response to Aspergillus inocula is mediated by multiple receptors that recognize different components of conidial and hyphal cells; complete elucidation of this complicated process awaits further study.
The observation that killing of C. albicans, but not A. fumigatus, is dependent on MyD88 is consistent with the growing amount of evidence that macrophage mechanisms of recognition and intracellular microbicidal mechanisms for these fungi may differ. Recently, Garlanda and colleagues showed that a soluble pattern recognition receptor, pentraxin (PTX3), binds to A. fumigatus conidia, facilitating macrophage internalization, killing, and chemokine secretion, but PTX3 did not bind to C. albicans (12). Intracellular killing of Candida occurs via both oxygen-independent and -dependent mechanisms, with apparent involvement of both reactive oxygen and nitrogen intermediates (32). In contrast, alveolar macrophage killing of A. fumigatus conidia does not involve nitric oxide (21), which is one microbicidal mechanism reported to be induced by TLR activation (7, 30) and mediated by MyD88 (13). Whether macrophage killing of A. fumigatus conidia involves TLR-dependent, MyD88-independent, gamma interferon-inducible genes (5, 17) or signaling mediated by the recently identified non-MyD88 Toll interleukin 1 receptor-binding adapter molecule(s) (9, 14) awaits further study.
In conclusion, we have described a unique role for MyD88 in mediating macrophage phagocytosis and killing of C. albicans. Macrophage responses to A. fumigatus differ, as conidia were internalized and killed by a mechanism that appears to be independent of MyD88 signaling. It is likely that differential dependence on MyD88 represents a mechanism by which macrophages produce antifungal responses that are specific to different types of pathogenic fungi.
Acknowledgments
Financial support was provided by NIH grants K08-AI01571 and R01-AI51468 (K.A.M.), K08-AI49374 (T.R.H.), R01-AI25032 (A.A.), R01-AI32972 (A.A.), and R01-HL62995 (W.C.L.). T.R.H. was supported by a postdoctoral fellowship from the Howard Hughes Medical Institute.
Editor: T. R. Kozel
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. [DOI] [PubMed] [Google Scholar]
- 2.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 3.Aderem, A., and D. M. Underhill. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593-623. [DOI] [PubMed] [Google Scholar]
- 4.Aderem, A. A., S. D. Wright, S. C. Silverstein, and Z. A. Cohn. 1985. Ligated complement receptors do not activate the arachidonic acid cascade in resident peritoneal macrophages. J. Exp Med. 161:617-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 6.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 7.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 8.Chinen, T., M. H. Qureshi, Y. Koguchi, and K. Kawakami. 1999. Candida albicans suppresses nitric oxide (NO) production by interferon-gamma (IFN-gamma) and lipopolysaccharide (LPS)-stimulated murine peritoneal macrophages. Clin. Exp. Immunol. 115:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzgerald, K. A., E. M. Palsson-McDermott, A. G. Bowie, C. A. Jefferies, A. S. Mansell, G. Brady, E. Brint, A. Dunne, P. Gray, M. T. Harte, D. McMurray, D. E. Smith, J. E. Sims, T. A. Bird, and L. A. O'Neill. 2001. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 413:78-83. [DOI] [PubMed] [Google Scholar]
- 10.Fradin, C., T. Jouault, A. Mallet, J. M. Mallet, D. Camus, P. Sinay, and D. Poulain. 1996. Beta-1, 2-linked oligomannosides inhibit Candida albicans binding to murine macrophage. J. Leukoc. Biol. 60:81-87. [DOI] [PubMed] [Google Scholar]
- 11.Fradin, C., D. Poulain, and T. Jouault. 2000. β-1,2-linked oligomannosides from Candida albicans bind to a 32-kilodalton macrophage membrane protein homologous to the mammalian lectin galectin-3. Infect Immun. 68:4391-4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garlanda, C., E. Hirsch, S. Bozza, A. Salustri, M. De Acetis, R. Nota, A. Maccagno, F. Riva, B. Bottazzi, G. Peri, A. Doni, L. Vago, M. Botto, R. De Santis, P. Carminati, G. Siracusa, F. Altruda, A. Vecchi, L. Romani, and A. Mantovani. 2002. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature 420:182-186. [DOI] [PubMed] [Google Scholar]
- 13.Henneke, P., O. Takeuchi, R. Malley, E. Lien, R. R. Ingalls, M. W. Freeman, T. Mayadas, V. Nizet, S. Akira, D. L. Kasper, and D. T. Golenbock. 2002. Cellular activation, phagocytosis, and bactericidal activity against group B streptococcus involve parallel myeloid differentiation factor 88-dependent and independent signaling pathways. J. Immunol. 169:3970-3977. [DOI] [PubMed] [Google Scholar]
- 14.Horng, T., G. M. Barton, and R. Medzhitov. 2001. TIRAP: an adapter molecule in the Toll signaling pathway. Nat. Immunol. 2:835-841. [DOI] [PubMed] [Google Scholar]
- 15.Janeway, C. A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 16.Jouault, T., C. Fradin, P. A. Trinel, and D. Poulain. 2000. Candida albicans-derived β-1,2-linked mannooligosaccharides induce desensitization of macrophages. Infect. Immun. 68:965-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawai, T., O. Takeuchi, T. Fujita, J. Inoue, P. F. Muhlradt, S. Sato, K. Hoshino, and S. Akira. 2001. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 167:5887-5894. [DOI] [PubMed] [Google Scholar]
- 18.Latge, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mambula, S. S., K. Sau, P. Henneke, D. T. Golenbock, and S. M. Levitz. 2002. Toll-like receptor (TLR) signaling in response to Aspergillus fumigatus. J. Biol. Chem. 277:39320-39326. [DOI] [PubMed] [Google Scholar]
- 20.Marr, K., M. Khododoust, M. Black, and S. Balajee. 2001. Early events in macrophage killing of Aspergillus fumigatus conidia: development of a new flow cytometric viability assay. Clin. Diagn. Lab. Immunol. 8:1240-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michaliszyn, E., S. Senechal, P. Martel, and L. deRepentigny. 1995. Lack of involvement of nitric oxide in killing of Aspergillus fumigatus conidia by pulmonary alveolar macrophages. Infect. Immun. 63:2075-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Netea, M. G., C. A. Van Der Graaf, A. G. Vonk, I. Verschueren, J. W. Van Der Meer, and B. J. Kullberg. 2002. The role of toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J. Infect. Dis. 185:1483-1489. [DOI] [PubMed] [Google Scholar]
- 23.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. USA 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroppel, K., M. Kryk, M. Herrmann, E. Leberer, M. Rollinghoff, and C. Bogdan. 2001. Suppression of type 2 NO-synthase activity in macrophages by Candida albicans. Int. J. Med. Microbiol. 290:659-668. [DOI] [PubMed] [Google Scholar]
- 25.Shoham, S., C. Huang, J. M. Chen, D. T. Golenbock, and S. M. Levitz. 2001. Toll-like receptor 4 mediates intracellular signaling without TNF-α release in response to Cryptococcus neoformans polysaccharide capsule. J. Immunol. 166:4620-4626. [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392-5396. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi, O., A. Kaufmann, K. Grote, T. Kawai, K. Hoshino, M. Morr, P. F. Muhlradt, and S. Akira. 2000. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 164:554-557. [DOI] [PubMed] [Google Scholar]
- 29.Takeuchi, O., K. Takeda, K. Hoshino, O. Adachi, T. Ogawa, and S. Akira. 2000. Cellular responses to bacterial cell wall components are mediated through MyD88-dependent signaling cascades. Int. Immunol. 12:113-117. [DOI] [PubMed] [Google Scholar]
- 30.Thoma-Uszynski, S., S. Stenger, O. Takeuchi, M. T. Ochoa, M. Engele, P. A. Sieling, P. F. Barnes, M. Rollinghoff, P. L. Bolcskei, M. Wagner, S. Akira, M. V. Norgard, J. T. Belisle, P. J. Godowski, B. R. Bloom, and R. L. Modlin. 2001. Induction of direct antimicrobial activity through mammalian Toll-like receptors. Science 291:1544-1547. [DOI] [PubMed] [Google Scholar]
- 31.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 32.VazquezTorres, A., and E. Balish. 1997. Macrophages in resistance to candidiasis. Microbiol. Mol. Biol. Rev. 61:170-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vonk, A. G., C. W. Wieland, M. G. Netea, and B. J. Kullberg. 2002. Phagocytosis and intracellular killing of Candida albicans blastoconidia by neutrophils and macrophages: a comparison of different microbiological test systems. J. Microbiol. Methods 49:55-62. [DOI] [PubMed] [Google Scholar]
- 34.Wang, J. E., A. Warris, E. A. Ellingsen, P. F. Jorgensen, T. H. Flo, T. Espevik, R. Solberg, P. E. Verweij, and A. O. Aasen. 2001. Involvement of CD14 and toll-like receptors in activation of human monocytes by Aspergillus fumigatus hyphae. Infect. Immun. 69:2402-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright, S. D., and S. C. Silverstein. 1983. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J. Exp. Med. 158:2016-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]