Abstract
We used Entamoeba histolytica infection in human intestinal xenografts to study the roles interleukin-1 (IL-1) and tumor necrosis factor alpha (TNF-α) in the pathogenesis of amebic colitis. We found that blockade of TNF-α reduced inflammation and intestinal damage in amebic infection, while inhibition of IL-1 reduced cytokine production but had less marked effects on inflammation and disease.
Entamoeba histolytica is a parasitic protozoan that causes amebic colitis and amebic liver abscess in humans. Studies with severe combined immunodeficient mice with human intestinal xenografts (SCID-HU-INT mice) have shown that E. histolytica infection leads to gut inflammation with intestinal production of interleukin-1β (IL-1β), IL-8, and cycloxygenase-2 (6, 9). E. histolytica-induced gut inflammation is mediated through the action of NF-κβ, and inhibition of the synthesis of the p65 subunit of NF-κβ reduces IL-1β and IL-8 levels and decreases gut inflammation and tissue damage in human intestinal xenografts infected with E. histolytica (7). Treatment with a specific inhibitor of cycloxygenase-2 also reduces inflammation and epithelial damage in amebic colitis in SCID-HU-INT mice (9). These data suggest that a significant component of the early tissue damage seen in this model of amebic colitis arises from the host inflammatory response to E. histolytica. One of the important mediators of intestinal inflammation in inflammatory bowel disease is tumor necrosis factor alpha (TNF-α) (4, 5). In a recent transcriptional analysis of human colonic xenografts infected with E. histolytica, we found increased expression of a number of genes known to be activated by TNF-α (Z. Zhang and S. L. Stanley, Jr., unpublished data). These findings led us to determine whether the blockade of either (i) TNF-α with a chimerized monoclonal antibody to TNF-α (Infliximab or Remicade; Centocor Inc., Malvern, Pa.) or (ii) IL-1 activity with a recombinant human IL-1 receptor antagonist (IL-1ra; Anakinra or Kineret; Amgen Inc., Thousand Oaks, Calif.) would alter gut inflammation and tissue damage during amebic infection.
Human intestinal xenografts were placed into the rear flanks and suprascapular regions of SCID mice (6 to 8 weeks of age) as previously described (6). Grafts were allowed to develop for at least 8 weeks before use. Human intestinal xenografts were infected with E. histolytica trophozoites with direct intraluminal inoculation of 106 HM1:IMSS trophozoites per 100 μl of TYI-S-33 medium (5). SCID-HU-INT mice were sacrificed approximately 24 h after E. histolytica infection of human intestinal xenografts, and serum and tissue were obtained for cytokine, myeloperoxidase (MPO), intestinal permeability, and histologic assays (7). A group of 10 SCID-HU-INT mice were treated by intraperitoneal injection of 100 μl of a 13.3-mg/ml solution of the IL-1ra Anakinra in sterile phosphate-buffered saline (PBS) 3 h before an amebic challenge, 8 h after an amebic challenge, and 20 h after an amebic challenge. A control group of 10 SCID-HU-INT mice were treated with PBS alone on the same schedule and challenged with an identical dose of E. histolytica HM1:IMSS trophozoites. Serum samples from five Anakinra-treated and five untreated SCID-HU-INT mice were drawn 10.5 h after the first injection and assayed for human IL-1ra activity with an enzyme-linked immunosorbent assay (ELISA; R&D Systems Inc., Minneapolis, Minn.) in accordance with the manufacturer's protocol. Serum obtained from the SCID-HU-INT mice treated with IL-1ra showed mean IL-1ra levels of 371 ± 127 ng/ml, while serum from the untreated SCID-HU-INT mice with an E. histolytica graft infection at the same time point showed a mean level of 1.9 ± 1.7 ng/ml.
A group of 14 SCID-HU-INT mice received 200 μg of Infliximab (Remicade), a chimerized anti-TNF-α monoclonal antibody, in 100 μl of sterile PBS by intraperitoneal injection 24 h prior to a challenge of the human intestinal xenografts with HM1:IMSS E. histolytica trophozoites. A control group of 11 SCID-HU-INT mice received 100 μl of sterile PBS alone 24 h prior to a challenge with E. histolytica trophozoites. One of the control mice died prior to sacrifice, and fresh tissues and serum could not be obtained for analysis. Finally, a group of 10 SCID-HU-INT mice received both Infliximab and Anakinra on the same dosage schedules described above before a challenge with E. histolytica trophozoites. A control group of 10 SCID-HU-INT mice received sterile PBS on the same dosage schedule before a challenge with E. histolytica trophozoites.
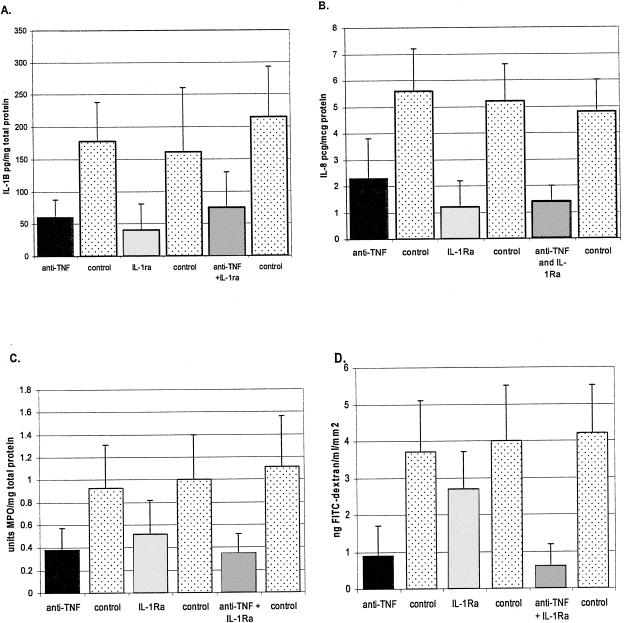
We have previously shown that increased levels of both human IL-1β and IL-8 can be detected in human intestinal xenografts infected with E. histolytica trophozoites. IL-1β levels were measured from segments of human intestinal xenografts by ELISA as previously described (7). We found that pretreatment of SCID-HU-INT mice with IL-1ra significantly inhibited the increase in IL-1β usually associated with E. histolytica infection (Fig. 1A) (P < 0.01). A statistically significant decrease in IL-1β production was also seen in the E. histolytica-infected SCID-HU-INT mice treated with the anti-TNF-α monoclonal antibody (Fig. 1A) (P < 0.01). Combination therapy of SCID-HU-INT mice with both IL-1ra and the anti-TNF-α antibody also reduced the IL-1 levels in E. histolytica-infected human colonic xenografts (P < 0.01 compared with E. histolytica-infected human colonic xenografts from untreated SCID-HU-INT mice), but no obvious additive effect of combined treatment was detectable. Similar results were obtained for IL-8 (Fig. 1B) (measured by ELISA as previously described [5]) with E. histolytica-infected human colonic xenografts from IL-1ra-treated SCID-HU-INT mice showing levels of IL-8 that were 75% lower than those seen in E. histolytica-infected human intestinal xenografts from untreated SCID-HU-INT mice (P < 0.01). Levels of IL-8 were also lower in human colonic xenografts from E. histolytica-infected SCID-HU-INT mice treated with anti-TNF-α (P < 0.02 compared to controls) and in those from E. histolytica-infected SCID-HU-INT mice treated with both anti-TNF-α and IL-1ra (P < 0.01 compared to controls).
FIG. 1.
Effect of IL-1 blockade or TNF-α blockade on gut inflammation and damage in human colonic xenografts infected with E. histolytica. Values are the means ± standard deviations for E. histolytica-infected human colonic xenografts from 14 anti-TNF-α-treated SCID-HU-INT mice (anti-TNF-α), 10 IL-1ra-treated SCID-HU-INT mice (IL-1ra), 10 mice treated with both anti-TNF-α and IL-1ra (anti-TNF-α and IL-1ra), and 10 donor-matched, untreated SCID-HU-INT mice for each experimental group (control). Mean levels of IL-1β, expressed as picograms of IL-1β per milligram of total protein (A), were significantly lower (two-tailed t test) in human colonic xenografts from anti-TNF-α-treated (P < 0.01), IL-1ra-treated (P < 0.01), or anti-TNF-α- and IL-1ra-treated SCID-HU-INT mice (P < 0.01) than in the matched untreated group. Mean levels of IL-8, expressed as picograms of IL-8 per microgram of total protein (B), were significantly lower in human colonic xenografts from anti-TNF-α-treated (P < 0.02), IL-1ra-treated (P < 0.01), or anti-TNF-α- and IL-1ra-treated SCID-HU-INT mice (P < 0.01) than in the matched untreated group. Mean levels of MPO, expressed as units of MPO per milligram of total protein (C), were significantly lower in anti-TNF-α-treated SCID-HU-INT mice than in untreated SCID-HU-INT mice (P < 0.02) and in anti-TNF-α- and IL-1ra-treated SCID-HU-INT mice than in untreated mice (P < 0.01) but did not significantly differ between untreated SCID-HU-INT mice and mice receiving IL-1ra alone (P = 0.138). Mean levels of FITC in serum, expressed as nanograms of FITC per milliliter per square millimeter (D), were significantly lower in E. histolytica-infected human colonic xenografts from SCID-HU-INT mice treated with anti-TNF-α than in E. histolytica-infected colonic xenografts from untreated SCID-HU-INT mice (P ≤ 0.02) and in SCID-HU-INT mice treated with both IL-1ra and anti-TNF-α (P < 0.01 compared to untreated mice) but did not differ significantly between IL-1ra-treated SCID-HU-INT mice and untreated SCID-HU-INT mice (P = 0.45).
We can quantify one component of inflammation by measuring neutrophil influx into human intestinal xenografts with an assay of MPO activity (7). Previous experiments in our laboratory have shown that amebic infection in the SCID-HU-INT system is associated with neutrophil infiltration into the lumen and lamina propria of the human intestinal xenograft (7). We found that while treatment of SCID-HU-INT mice with human IL-1ra lowered mean MPO levels in E. histolytica-infected human intestinal xenografts (Fig. 1C), the difference did not reach statistical significance (P = 0.138). However, pretreatment of SCID-HU-INT mice with anti-TNF-α decreased neutrophil influx into E. histolytica-infected human intestinal xenografts, resulting in MPO levels that were significantly lower than those detected in E. histolytica-infected human intestinal xenografts from untreated SCID-HU-INT mice (Fig. 1C) (P < 0.02). Pretreatment with both IL-1ra and anti-TNF-α also significantly lowered MPO levels in E. histolytica-infected xenografts from SCID-HU-INT mice (P < 0.01).
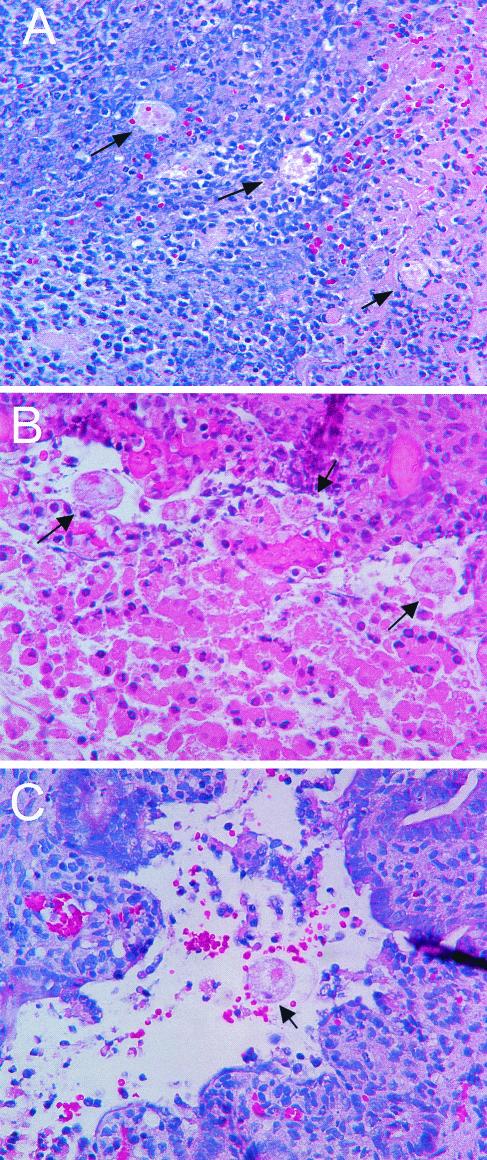
We measured changes in the permeability of the human colonic xenografts to fluoresceinated dextrans (fluorescein isothiocyanate [FITC]-dextran) to quantify damage to the intestinal permeability barrier. Uninfected human intestinal xenografts are relatively impermeable to FITC-dextran, while E. histolytica-mediated damage permits passage of FITC-dextran from the lumen of the human intestinal xenograft to the serum of the SCID-HU-INT mouse (7). Pretreatment of SCID-HU-INT mice with IL-1ra led to mean levels of FITC-dextran in the serum of E. histolytica-infected mice that did not differ significantly (P = 0.45) from those seen in the serum of untreated SCID-HU-INT mice with E. histolytica-infected human intestinal xenografts (Fig. 1D). In contrast, pretreatment of SCID-HU-INT mice with anti-TNF-α reduced the damage to the intestinal permeability barrier in human intestinal xenografts infected with E. histolytica. As shown in Fig. 1D, FITC-dextran levels were significantly lower in the serum of SCID-HU-INT mice that had been treated with anti-TNF-α (P ≤ 0.02) than in that of uninfected controls. Pretreatment of SCID-HU-INT mice with both IL-1ra and TNF-α significantly reduced serum FITC-dextran levels as well, consistent with reduced damage to the intestinal permeability barrier in human intestinal xenografts from treated SCID-HU-INT mice (P < 0.01). Examination of histologic sections from human intestinal xenografts from each of the three groups showed evidence of E. histolytica-mediated tissue damage in almost all xenografts. Neutrophil infiltration into the mucosal and submucosal tissues accompanied E. histolytica invasion in the control (untreated) E. histolytica-infected human intestinal xenografts (Fig. 2A) However, sections from E. histolytica-infected human intestinal xenografts from SCID-HU-INT mice that had received the anti-TNF-α monoclonal antibody showed areas of mucosal invasion without marked neutrophil infiltration (Fig. 2B). Some increased cellular infiltrate was seen in the mucosal regions in sections from E. histolytica-infected SCID-HU-INT mice that had received IL-1ra (Fig. 2C), but neutrophilic infiltration appeared less prominent than that seen in E. histolytica-infected human intestinal xenografts from untreated SCID-HU-INT mice. Histologic findings on intestinal xenografts infected with E. histolytica trophozoites from SCID-HU-INT mice pretreated with both IL-1ra and anti-TNF-α did not differ from those on xenografts from mice treated with anti-TNF-α alone (data not shown).
FIG. 2.
Morphological findings on E. histolytica-infected human colonic xenografts from SCID-HU-INT mice. Photomicrographs of hematoxylin-and-eosin-stained sections of E. histolytica-infected human colonic xenografts from an untreated SCID-HU-INT mouse (A), an anti-TNF-α-treated SCID-HU-INT mouse (B), and an IL-1ra-treated mouse (C) are shown. Extensive mucosal destruction and inflammation with neutrophil infiltration and multiple E. histolytica trophozoites (arrows) that have invaded the mucosa and submucosal tissues are visible in the section from the untreated SCID-HU-INT mouse (A). In the E. histolytica-infected human colonic xenograft from the anti-TNF-α-treated mouse, extensive mucosal damage and multiple invading E. histolytica trophozoites are also visible (arrows) but no marked cellular infiltrate is present (B). In the E. histolytica-infected human colonic xenograft from the IL-1ra-treated mouse (C), extensive mucosal damage and hemorrhage are present near a single E. histolytica trophozoite (arrow) but no marked neutrophilic infiltration is apparent. Original magnification of all three panels, ×400.
The efficacy of anti-TNF-α in inhibiting gut inflammation in this model raised the question of whether human or murine TNF-α can be detected in human colonic xenografts or the serum of E. histolytica-infected or control SCID-HU-INT mice. Neither human nor murine TNF-α was detectable by specific ELISAs (Pharmingen OptEIA Human TNF-α Set and Pharmingen OptEIA Mouse TNF-α Mono/Mono Set; BD Biosciences, San Diego, Calif.) in either the serum or human colonic xenografts from uninfected SCID-HU-INT mice (data not shown). Murine TNF-α was detectable in the human colonic xenografts of SCID-HU-INT mice after E. histolytica infection (Table 1), and lower levels were seen in mice treated with IL-1ra and anti-TNF-α. Human TNF-α was detectable in the colonic xenografts of E. histolytica-infected SCID-HU-INT mice at low levels and was decreased in E. histolytica-infected human colonic xenografts from IL-1ra- and anti-TNF-α-treated mice, but the difference did not reach statistical significance. We were not able to detect murine or human TNF-α in the serum of E. histolytica-infected SCID-HU-INT mice (data not shown). These data indicate that both human and murine TNF-α can be detected in E. histolytica-infected human colonic xenografts, probably secondary to production from epithelial cells or fibroblasts of human origin and influxing inflammatory cells of murine origin.
TABLE 1.
Human and murine TNF-α levels in human colonic xenografts infected with E. histolytica
| Samplea source | Mean TNF-α level (pg/ml) ± SDb
|
|
|---|---|---|
| Human | Murine | |
| Untreated mice | 68 ± 50 | 194 ± 163 |
| Anti-TNF-α- + IL-1ra-treated mice | 28 ± 35 | 80 ± 66 |
E. histolytica-infected human colonic xenograft tissue samples were collected from groups of 10 untreated or TNF-α- and IL-1ra-treated SCID-HU-INT mice.
The values for human TNF-α differed between untreated and anti-TNF-α- and IL-1ra-treated SCID-HU-INT mice at P = 0.08. The values for murine TNF-α differed between untreated and anti-TNF-α- and IL-1ra-treated SCID-HU-INT mice at P ≤ 0.05.
Both interleukin-1 and TNF-α have been linked to gut inflammation in animal models of disease and in humans (1, 2, 4, 5). Blockade of IL-1 activity with IL-1ra led to decreased colitis in a rabbit model of infection, while animals with a targeted disruption of the interleukin-1-converting enzyme (ICE) gene show resistance to the development of colitis (2, 3, 8). We have previously shown that IL-1β levels are increased in human intestinal xenografts infected with E. histolytica (6) and that E. histolytica cysteine proteinases are capable of mimicking the action of ICE and can cleave and activate pIL-1β to form the active mature cytokine (11), suggesting that IL-1 could play a role in the inflammation associated with amebic colitis. We found that administration of IL-1ra inhibited the increase in IL-1β and IL-8 that normally accompanies amebic infection in the SCID-HU-INT model of disease. Given the known role of IL-1 in stimulating its own production, as well as that of IL-8, these data are consistent with the idea that IL-1 is a key mediator in stimulating cytokine production in this system. However, while there was a trend toward decreased inflammation and tissue damage in E. histolytica-infected human intestinal xenografts from IL-1ra-treated SCID-HU-INT mice, the results were not statistically significant. This suggests that blockade of IL-1 alone cannot completely inhibit inflammation in this system, and other mediators may be important components of the E. histolytica-induced inflammatory response. One obvious candidate is TNF-α, which has been strongly linked to gut inflammation in several forms of colitis (4). A chimerized monoclonal antibody to TNF-α (Infliximab) is highly effective in treating the intestinal inflammation seen in Crohn's disease (4). In addition, a recent study of inflammation arising from ischemia and perfusion injuries found that many inflammatory mediators, including IL-1β, IL-6, and IL-8, were significantly reduced in Tnfsrf1a−/− mice, suggesting that TNF-α plays a primary role in mediating inflammation (1). We found that pretreatment of SCID-HU-INT mice with the anti-TNF-α monoclonal antibody was very effective in reducing the gut inflammation seen in amebic colitis in this model. Blockade of TNF-α also reduced the damage to the intestinal permeability barrier. These data show that TNF-α plays a role in the intestinal inflammation and damage to the intestinal permeability barrier induced by E. histolytica infection. Recent studies indicate that TNF-α can directly damage the intestinal permeability barrier, offering another mechanism for the efficacy of anti-TNF-α in inhibiting damage to the intestinal permeability barrier in our model (10). Treatment of SCID-HU-INT mice with both TNF-α and IL-1ra was also effective in reducing cytokine production, inflammation, and tissue damage in human colonic xenografts in this model, but we were unable to detect an additive effect from the use of both immunosuppressants.
While the host inflammatory response mediated by TNF-α is clearly linked to tissue damage in this model, blockade of TNF-α does not completely suppress damage to the intestinal permeability barrier in ameba-infected human colonic xenografts. FITC-dextran levels were significantly higher in anti-TNF-α-treated, E. histolytica-infected human colonic xenografts than those we detected in uninfected xenografts. Thus, damage to the colon and its permeability barrier also arises from tissue injury caused directly by invading E. histolytica trophozoites, which could be seen in submucosal tissues in all three experimental groups. Our data are consistent with the concept that tissue damage in amebic colitis arises from both the direct effects of E. histolytica on colonic tissue and the resultant gut inflammatory response, which may be primarily mediated by TNF-α.
Acknowledgments
This work was supported by National Institutes of Health grants AI30084 to S.L.S., DK 25274 to the Washington University School of Medicine Digestive Diseases Research Core Center, and HD 00836 to the Birth Defects Research Laboratory at the University of Washington. S.L.S. is a Burroughs Wellcome Scholar in Molecular Parasitology.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Chen, L. W., L. Egan, Z. Li, F. R. Greten, M. F. Kagnoff, and M. Karin. 2003. The two faces of IKK and NF-κB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat. Med. 5:575-581. [DOI] [PubMed] [Google Scholar]
- 2.Cominelli, F., C. C. Nast, B. D. Clark, R. Schindler, R. Lierena, V. E. Eysselein, R. C. Thompson, and C. A. Dinarello. 1990. Interleukin 1 (IL-1) gene expression, synthesis, and effect of specific IL-1 receptor blockade in rabbit immune complex colitis. J. Clin. Investig. 86:972-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cominelli, F., C. C. Nast, A. Duchini, and M. Lee. 1992. Recombinant interleukin-1 receptor antagonist blocks the proinflammatory activity of endogenous interleukin-1 in rabbit immune colitis. Gastroenterology 103:65-71. [DOI] [PubMed] [Google Scholar]
- 4.Podolsky, D. K. 2002. Inflammatory bowel disease. N. Engl. J. Med. 347:417-429. [DOI] [PubMed] [Google Scholar]
- 5.Rogler, G., and T. Andus. 1998. Cytokines in inflammatory bowel disease. World J. Surg. 22:382-389. [DOI] [PubMed] [Google Scholar]
- 6.Seydel, K. B., E. Li, P. E. Swanson, and S. L. Stanley, Jr. 1997. Human intestinal epithelial cells produce proinflammatory cytokines in response to infection in a SCID mouse-human intestinal xenograft model of amebiasis. Infect. Immun. 65:1631-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seydel, K. B., E. Li, Z. Zhang, and S. L. Stanley, Jr. 1998. Epithelial cell-initiated inflammation plays a crucial role in early tissue damage in amebic infection of human intestine. Gastroenterology 115:1446-1453. [DOI] [PubMed] [Google Scholar]
- 8.Siegmund, B., H. A. Lehr, G. Fantuzzi, and C. A. Dinarello. 2001. IL-1β-converting enzyme (caspase-1) in intestinal inflammation. Proc. Natl. Acad. Sci. USA 98:13249-13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stenson, W. F., Z. Zhang, T. Riehl, and S. L. Stanley, Jr. 2001. Amebic infection in the human colon induces cyclooxygenase-2. Infect. Immun. 69:3382-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suenaert, P., V. Bulteel, L. Lemmens, M. Noman, B. Geypens, G. Van Assche, K. Geboes, J. L. Ceuppens, and P. Rutgeerts. 2002. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn's disease. Am. J. Gastroenterol. 97:2000-2004. [DOI] [PubMed] [Google Scholar]
- 11.Zhang, Z., L. Wang, K. B. Seydel, E. Li, S. Ankri, D. Mirelman, and S. L. Stanley, Jr. 2000. Entamoeba histolytica cysteine proteinases with ICE activity cause intestinal inflammation and tissue damage in amoebiasis. Mol. Microbiol. 37:542-548. [DOI] [PubMed] [Google Scholar]