Abstract
An important aspect of malaria vaccine development is the identification of an appropriate adjuvant which is both capable of stimulating a protective immune response and safe for use by humans. Here, we investigated the feasibility of using novel immunostimulatory molecules as adjuvants combined with a crude antigen preparation and coadsorbed to aluminum hydroxide (alum) as a vaccine against blood-stage Plasmodium chabaudi AS malaria. Prior to challenge infection, immunization of genetically susceptible A/J mice with the combination of malaria antigen plus recombinant interleukin-12 (IL-12) in alum induced a Th1 immune response with production of high levels of gamma interferon (IFN-γ) and diminished IL-4 levels by spleen cells stimulated in vitro with parasite antigen compared to mice immunized with antigen alone, antigen in alum, or antigen plus IL-12. Mice immunized with malaria antigen plus recombinant IL-12 in alum had high levels of total malaria-specific antibody and immunoglobulin G2a. Compared to unimmunized mice, immunization with antigen plus IL-12 in alum induced the highest level of protective immunity against challenge infection with P. chabaudi AS, which was evident as a significantly decreased peak parasitemia level and 100% survival. Protective immunity was dependent on CD4+ T cells, IFN-γ, and B cells and was long-lasting. Replacement of IL-12 as an adjuvant by synthetic oligodeoxynucleotides (ODN) containing CpG motifs induced a similar level of vaccine-induced protection against challenge infection with P. chabaudi AS. These results illustrate that it is possible to enhance the potency of a crude malaria antigen preparation delivered in alum by inclusion of immunostimulatory molecules, such as IL-12 or CpG-ODN.
Malaria vaccine development efforts during the past 20 years have been aimed at antigen identification, gene cloning, and expression of recombinant molecules (17). This has resulted in a number of promising blood-stage-derived recombinant antigens for inclusion in subunit vaccines, including MSP1, MSP2, MSP3, MSP4, MSP5, AMA1, PfEMP1, RESA, RAP1, and RAP2 (10, 18). Clinical trials with many of these candidates have been conducted or are ongoing (10, 13, 18). It is anticipated that the success of these trials may potentially lead to a vaccine capable of saving millions of lives each year from malaria.
An important aspect of vaccine development against infectious diseases, including malaria, is the identification of an appropriate adjuvant which is both capable of stimulating a protective immune response and safe for use by humans. Aluminum hydroxide, usually referred to as alum, which is approved as an adjuvant for use by humans, is not always the most appropriate adjuvant, given its potential to stimulate a Th2 type immune response characterized by immunoglobulin G1 (IgG1) and IgE production and the lack of induction of cytotoxic T-cell responses (5). This is particularly problematic in the development of vaccines against diseases caused by intracellular pathogens such as protozoan parasites, including intraerythrocytic Plasmodium parasites, the causative agent of malaria. Protective immunity against intracellular pathogens is generally dependent on Th1 type immune responses. However, protective immunity against blood-stage malaria is particularly complex and requires a concerted effort by a Th1 type cellular immune response and humoral immunity possibly involving a Th2 type response (24, 29). Rodent studies have revealed a role for CD4+ T cells, B cells, and antibody in mediating naturally induced immunity against primary blood-stage infection (24, 29). The importance of CD4+ T cells in immunity induced by vaccination with a defined antigen, such as MSP1, is less clear, although high titers of antigen-specific antibody correlate with protective immunity induced in mice by vaccination with the 19-kDa carboxyl-terminal fragment of MSP1 derived from Plasmodium yoelii (reviewed in reference 13).
Interleukin-12 (IL-12) plays an essential role in the differentiation of CD4+ lymphocytes by promoting Th1 while suppressing Th2 cell development, thereby favoring gamma interferon (IFN-γ) production and elevated IgG2a levels (31). Because of its potent immunoregulatory properties, this cytokine has been used successfully as a vaccine adjuvant in models of intracellular infections, such as Leishmania major (1, 21) and Listeria monocytogenes (27), which require induction of Th1 responses for protective immunity. Consistent with the ability of IL-12 to promote a Th1-dependent immune response and to dampen a Th2 response, Wynn and colleagues (46-48) demonstrated that, as an adjuvant, IL-12 not only influences the promotion of protective Th1-dependent vaccine-induced immunity against Schistosoma mansoni but also prevents Th2-dependent pathology associated with this helminth parasite. Furthermore, as a vaccine adjuvant, IL-12 enhances both cell-mediated immune responses and augments antigen-specific IgG1, IgG2a, and IgG3 antibody levels, especially when the antigen and IL-12 are simultaneously coadsorbed to alum (19, 48).
In previous studies, our laboratory demonstrated that treatment of genetically susceptible A/J mice with exogenous IL-12 during primary Plasmodium chabaudi AS infection induces protective type 1 immunity, resulting in a less severe course of infection and survival (37). Studies with IL-12 p40-deficient mice confirmed the key role of IL-12 in inducing protective Th1 responses involving IFN-γ during the acute phase of infection and demonstrated that IL-12 is also important for antibody-mediated immunity during the chronic stage and in a challenge infection (39).
In the present study, we investigated the potential of using IL-12 as an adjuvant in a vaccine against blood-stage P. chabaudi AS malaria in A/J mice. Since our focus was to define the efficacy and characterize the protective immune mechanisms induced by the combination of an immunostimulatory molecule with malaria antigen, we used murine recombinant IL-12 together with a crude preparation of whole antigen from P. chabaudi AS parasitized red blood cells (PRBC). With this combination coadsorbed to alum, we report the induction of a protective antimalarial immune response in susceptible A/J mice characterized by the production of high levels of IFN-γ and parasite-specific IgG2a and protection against challenge infection with blood-stage P. chabaudi AS. Protective immunity was found to be dependent on CD4+ T cells, B cells, and IFN-γ and to be long-lasting. Replacement of IL-12 as an adjuvant by synthetic oligodeoxynucleotides (ODN) containing CpG motifs was found to induce a similar level of vaccine-induced protection against challenge infection with P. chabaudi AS.
MATERIALS AND METHODS
Mice.
Age- and sex-matched mice, 6 to 8 weeks old, were used in all experiments. A/J mice were purchased from the Jackson Laboratory (Bar Harbor, Maine) and C57BL/6 mice were purchased from Charles River Laboratories (St. Constant, Quebec, Canada). IFN-γ knockout (GKO) mice on the C57BL/6 background were bred in the animal facility of the Montreal General Hospital Research Institute from breeding pairs of GKO mice, which were originally from Genentech, Inc. (South San Francisco, Calif.) and backcrossed onto the C57BL/6 strain for eight generations by F. P. Heinzel (Case Western Reserve University School of Medicine, Cleveland, Ohio) (16). B-cell-deficient μ-MT with targeted disruption of the membrane exon of the immunoglobulin μ-chain gene or B-cell knockout (BKO) mice were originally derived on a 129 × C57BL/6 background and backcrossed to the C57BL/10 background for 12 generations (20, 22). B-cell-deficient μ-MT and wild-type C57BL/10SgSnAi mice were obtained from Taconic Farms, Inc. (Germantown, N.Y.).
CD4+ T-cell depletion.
Monoclonal anti-CD4 antibody from the hybridoma clone GK1.5 was raised as ascites fluid in BALB/c mice as previously described (33). The ascites fluid was delipified, dialyzed, and quantitated for the concentration of rat IgG. Mice were treated with the first dose of 500 μg of anti-CD4 antibody intraperitoneally (i.p.) 3 days prior to infection. Following infection, 200 μg was administered i.p. 3 times per week until the end of the experiment. Control mice received purified rat IgG at similar dosages and timing. Treatment with GK1.5 monoclonal antibody consistently depletes >98% of CD4+ T cells based on fluorocytometric analysis (33, 37) and functional studies (33).
P. chabaudi AS infection and antigen preparation.
P. chabaudi AS was maintained as previously described (33). Naive and immunized mice were infected i.p. with 106 PRBC. The course and outcome of infection were monitored by previously described procedures (33). For the determination of cytokine and antibody levels in sera, mice were sacrificed at the indicated times and blood was obtained by cardiac puncture, allowed to clot for 30 min at 4°C, and centrifuged at 3,000 × g for 3 min. Sera were collected and stored at 4°C for measurement of IL-12 p70 or at −20°C for determination of the levels of other cytokines and malaria-specific antibodies.
Antigen was prepared by modification of a freeze-thaw protocol described by Amante and Good (2). Briefly, blood from A/J mice with parasitemias of 40 to 45% was collected, pooled, and centrifuged at 300 × g for 10 min. The red blood cell pellet was subjected to 2 rounds of lysis with distilled H2O and centrifugation at 10,000 × g for 25 min. After 2 washes with phosphate-buffered saline (PBS), the parasite pellet was resuspended in PBS and subjected to 3 cycles of freeze-thaw at −70°C and 37°C. The suspension, containing both soluble and particulate antigens, was further disrupted by passage 2 to 3 times through a syringe with a 25-gauge needle.
Immunization protocol.
An amount of malaria antigen equivalent to 1 × 107 to 1.5 × 107 PRBC was mixed with 1 μg of murine recombinant IL-12 (a kind gift from Wyeth Genetics Institute, Cambridge, Mass.) to a volume of 50 μl with PBS. An equal volume of alum (Imject alum; Pierce Chemical Co., Rockford, Ill.) was added, and the suspension was mixed thoroughly. Mice were immunized subcutaneously (s.c.) with 0.1 ml on the nape of the neck. Other groups of mice were also immunized in a similar manner with the following vaccine combinations: antigen suspended in PBS, antigen admixed in alum, and antigen admixed with 1 μg of murine recombinant IL-12 in PBS. Three weeks later, the antigen-treated groups were boosted with the same amount of antigen in 0.1 ml of PBS injected i.p. Mice were challenged i.p. with 106 PRBC 2 weeks later.
CpG DNA.
ODN containing CpG motifs (CpG-ODN no. 1826) and control ODN (no. 1982) were a kind gift of Coley Pharmaceuticals Canada (Ottawa, Ontario, Canada). One hundred micrograms of CpG-ODN or control ODN was admixed with antigen and alum and used according to the standard immunization protocol described above.
Spleen cell culture and proliferation assay.
Spleens from immunized mice were removed aseptically and pressed through a sterile fine-wire mesh with 10 ml of RPMI 1640 (Gibco-Invitrogen, Burlington, Ontario, Canada) supplemented with 5% heat-inactivated fetal calf serum (HyClone Laboratories, Logan, Utah), 25 mM HEPES (Gibco-Invitrogen), 0.12% gentamicin (Schering, Montreal, Quebec, Canada), and 2 mM glutamine (Gibco-Invitrogen). Cell suspensions were centrifuged at 350 × g for 10 min. Red blood cells were lysed with 0.175 M NH4Cl, and the cells were washed twice in fresh medium. Membrane debris was removed by filtering the cell suspensions through sterile gauze. The viability of the cells was determined by trypan blue exclusion and was always >90%. Total cell counts were performed on individual samples. For proliferation assays, spleen cells were adjusted to 2.5 × 106 cells/ml and aliquots of 0.1 ml were plated in triplicate in 96-well flat-bottom plates, stimulated with 106 washed PRBC/ml (as the malaria parasite antigen) or medium (as the control), and incubated for 72 h at 37°C in a humidified CO2 incubator. During the last 16 h of culture, 1 μCi of [3H]thymidine (specific activity, 6.7 Ci/mmol) was added to each well, the cells were harvested with an automatic cell harvester, and the incorporated radioactivity was measured in a liquid scintillation counter. For determination of cytokine production, spleen cells were adjusted to 5 × 106 cells/ml and aliquots of 1 ml were plated in triplicate in 24-well tissue culture plates in the presence or absence of 106 PRBC, as described above, and incubated for 48 h at 37°C in a humidified CO2 incubator. Supernatants were collected, centrifuged at 350 × g for 5 min, and stored at 4°C or at −20°C until assayed for cytokine levels.
Cytokine ELISAs.
Cytokine levels in sera and spleen cell supernatants were measured by using two-site sandwich enzyme-linked immunosorbent assays (ELISAs) for IFN-γ and tumor necrosis factor alpha (TNF-α) as previously described (34, 37). For IL-4, the capturing and detecting antibodies were BVD4-1D11 monoclonal antibody (MAb) and biotinylated BVD6-24G2 MAb, respectively. For IL-10, JES5.2A5 MAb (American Type Culture Collection, Rockville, Md.) and biotinylated SXC-1 MAb (BD Bioscience, Mississauga, Ontario, Canada) were used as the capturing and detecting antibodies, respectively. Standard curves for each cytokine were generated by using recombinant cytokines (BD Bioscience). Reactivity was revealed by using 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) substrate (Roche, Laval, Quebec, Canada) and optical density (OD) values were read in a microplate reader at 405 nm, with a reference wavelength of 492 nm.
Malaria-specific antibody ELISA.
Serum levels of P. chabaudi AS-specific antibody isotypes were determined by ELISA. P. chabaudi AS antigen was prepared as described previously (49). Immulon II plates (Dynatech, Chantilly, Va.) were coated with parasite antigen at a concentration of approximately 4 to 5 μg/ml in PBS based on an OD at 280 nm overnight at 4°C and subsequently blocked with 1% bovine serum albumin in PBS for 1 h. Individual serum samples were serially diluted twofold, and 50 μl of each dilution was added to the plate and incubated for 2 h at room temperature. The data shown are based on values obtained at the following dilutions: total Ig, 1:20; IgG1, 1:10; IgG2a, 1:10. After extensive washing, horseradish peroxidase-conjugated goat anti-mouse isotype antibodies (SBA, Birmingham, Ala.) were added and incubated at room temperature for another 2 h. Reactivity was visualized with ABTS substrate, and OD values were read in a microplate reader at 405 nm, with a reference wavelength of 492 nm. Antibody levels in serum are expressed as relative OD.
Statistical analysis.
Data are presented as means ± standard errors of the means. The statistical significance of differences in means between experimental and control groups was analyzed by Student's t test with SAS/STAT software (SAS Institute, Cary, N.C.). A P value of <0.05 was considered significant.
RESULTS
Immunization with malaria antigen plus IL-12 in alum induces a Th1 immune response.
Since a strong Th1 immune response is associated with protective immunity to acute blood-stage P. chabaudi AS during a primary infection, the type of immune response induced by inclusion of IL-12 in a vaccine formulation was first evaluated. P. chabaudi AS susceptible A/J mice were immunized s.c. with a freeze-thaw preparation of blood-stage malaria antigen alone, antigen in alum, antigen plus IL-12, or antigen plus IL-12 in alum and boosted 3 weeks later by i.p. injection with antigen alone. Two weeks later, prior to challenge infection, immunized mice and untreated, control A/J mice were sacrificed and proliferation and cytokine production by spleen cells were analyzed in vitro. As shown in Table 1, immunization with either antigen in alum or the combination of antigen plus IL-12 in alum resulted in significantly increased antigen-specific proliferation compared to the response of control A/J mice (P = 0.02 and P = 0.037, respectively). However, the combination of antigen plus IL-12 in alum resulted in a greater than twofold increase in proliferation compared to antigen in alum, which represents a significant difference between the two groups. Furthermore, in comparison with spleen cells from mice immunized with antigen in alum, spleen cells from mice immunized with the combination of antigen plus IL-12 in alum produced significantly higher levels of the Th1 cytokines, IFN-γ and TNF-α, and significantly lower levels of IL-4. Spleen cells from mice immunized with the combination of antigen plus IL-12 in alum also produced modest levels of IL-10, which were significantly higher than the response of cells from mice immunized with antigen in alum.
TABLE 1.
Antigen-specific spleen cell proliferation and cytokine responses in immunized mice prior to P. chabaudi AS challenge infection
| Treatment groupa | Antigen proliferation (cpm ± SEM) | Level of cytokine (Mean ± SEM):
|
|||
|---|---|---|---|---|---|
| IFN-γ (ng/ml) | TNF-α (pg/ml) | IL-4 (pg/ml) | IL-10 (pg/ml) | ||
| Untreated | 526 ± 71 | 0.39 ± 0.39 | 98.41 ± 10.22 | 254.27 ± 52.80 | 0.76 ± 0.01 |
| Antigen | 486 ± 44 | 5.13 ± 0.36 | 122.03 ± 17.78 | 178.55 ± 45.13 | 1.07 ± 0.11 |
| Antigen + alum | 1,162 ± 128 | 3.83 ± 0.86 | 175.03 ± 20.93 | 234.52 ± 20.60 | 1.27 ± 0.10 |
| Antigen + IL-12 | 768 ± 130 | 11.14 ± 2.27 | 189.85 ± 28.58 | 134.19 ± 33.22 | 1.01 ± 0.09 |
| Antigen + IL-12 + alum | 2,768 ± 622b | 43.13 ± 5.20c | 341.94 ± 44.26c | 165.87 ± 42.82d | 1.44 ± 0.14e |
Groups of A/J mice (5 per group) were immunized s.c. with malaria antigen alone, antigen in alum, antigen plus 1.0 μg of IL-12, or antigen plus 1.0 μg of IL-12 in alum and boosted 3 weeks later by i.p. injection with antigen. Data from one of two replicate experiments are presented.
P < 0.05 for antigen plus alum versus antigen plus IL-12 plus alum.
P < 0.0001 for antigen plus alum versus antigen plus IL-12 plus alum.
P < 0.01 for antigen plus alum versus antigen plus IL-12 plus alum.
P < 0.008 for antigen plus alum versus antigen plus IL-12 plus alum.
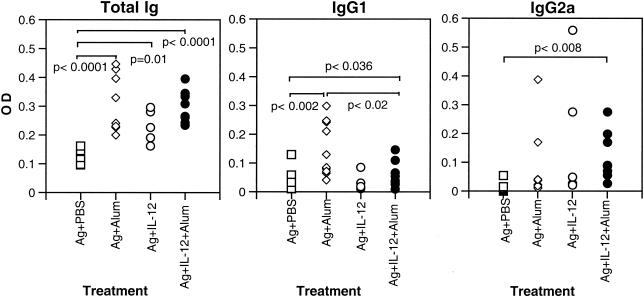
The levels of total malaria-specific antibody and IgG1 and IgG2a in the sera of immunized A/J mice were also analyzed 2 weeks after boosting prior to challenge infection. Total malaria-specific antibody was significantly and similarly increased in the three groups of immunized animals compared with the levels of total specific antibody in mice immunized with antigen alone (Fig. 1A). Malaria-specific IgG1 was significantly increased in the groups immunized with antigen in alum and the combination of antigen plus IL-12 in alum compared to IgG1 levels in mice immunized with antigen alone (Fig. 1B). However, the level of malaria-specific IgG1 was significantly higher in the group immunized with antigen in alum than in those immunized with the combination of antigen plus IL-12 in alum. The levels of specific IgG2a were significantly increased compared to controls only in mice immunized with the combination of antigen plus IL-12 in alum (Fig. 1C). These findings demonstrate that immunization with the combination of malaria antigen plus IL-12 in alum induced high levels of production of the Th1 cytokine IFN-γ and parasite-specific IgG2a. In addition, mice immunized with this combination produced significantly lower levels of antigen-specific IL-4 and IgG1 than did mice immunized with antigen in alum in the absence of IL-12.
FIG. 1.
Levels of malaria-specific antibodies in the sera of A/J mice immunized s.c. with antigen (Ag) alone, antigen in alum, antigen plus IL-12, or antigen plus IL-12 in alum and boosted 3 weeks later by i.p. injection with antigen. Two weeks later, sera were collected from immunized mice and the levels of total malaria-specific antibody, IgG1, and IgG2a were determined by ELISA. Data represent OD values for individual mice and are pooled from 2 experiments.
Immunization with malaria antigen plus IL-12 in alum induces protection against challenge infection with blood-stage P. chabaudi AS.
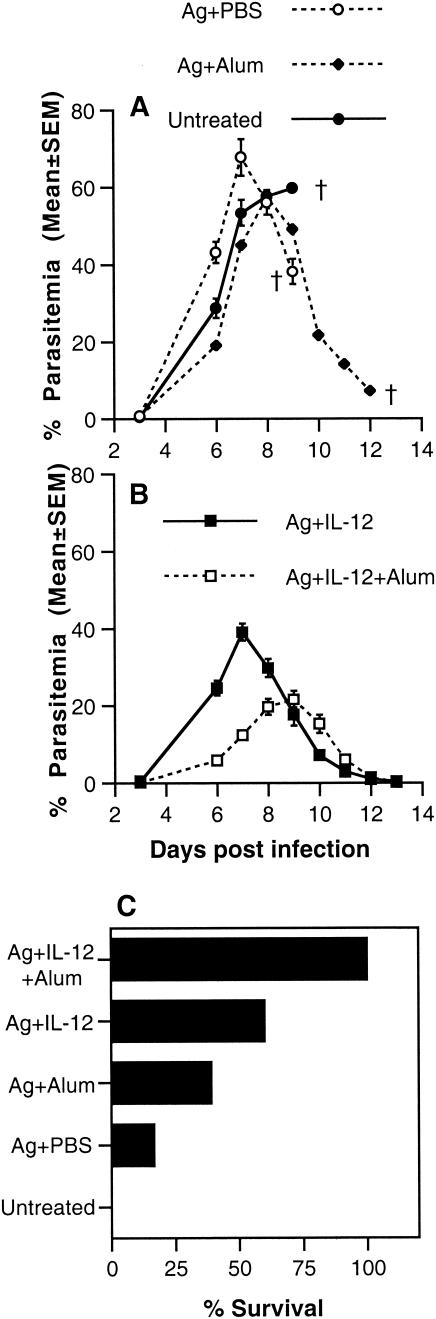
To compare the efficacy of vaccination with the various combinations in conferring protective immunity, groups of A/J mice, immunized as described above, were challenged i.p. with P. chabaudi AS 2 weeks after boosting and the course of parasitemia and the outcome of infection were monitored. Similar to control mice, mice immunized with antigen alone or antigen in alum suffered a severe course of parasitemia with high peak parasitemia levels and high mortality (Fig. 2A and C). Mice immunized with antigen plus IL-12 or antigen plus IL-12 in alum experienced less severe courses of infection with significantly lower peak parasitemia levels than control mice (P < 0.001 and P < 0.001, respectively) (Fig. 2B). In the case of mice immunized with antigen plus IL-12 in alum, there was a delay of 1 to 2 days in peak parasitemia level compared to nonimmunized mice. Although antigen plus IL-12 was effective in significantly reducing peak parasitemia compared to control mice, only 60% (9 of 15) of mice immunized with this combination survived while 100% (25 of 25) of mice immunized with the combination of antigen plus IL-12 in alum survived challenge infection with P. chabaudi AS. These results indicate that antigen plus IL-12 in alum was the best combination for conferring protection against blood-stage malaria in terms of reduced parasitemia and enhanced survival.
FIG. 2.
Course of parasitemia and survival in A/J mice immunized s.c. with antigen (Ag) alone, antigen in alum, antigen plus IL-12, or antigen plus IL-12 in alum and boosted 3 weeks later by i.p. injection with antigen. Two weeks later, immunized and untreated control mice were challenged i.p. with 106 P. chabaudi AS PRBC. The percentage of PRBC in peripheral blood (A and B) was determined for each group of 5 mice. Data from one of two replicate experiments are presented. Mice were examined twice daily for the duration of the experiment for survival (C). Cumulative data from 6 experiments are presented.
Immunization with malaria antigen plus IL-12 in alum induces long-lasting protection.
An important characteristic of an effective malaria vaccine is that the elicited immunity is long-lasting. To address this issue, A/J mice were immunized with the combination of antigen plus IL-12 in alum and challenged as before, that is, 2 weeks after boosting, or 12 weeks after boosting. Similar to mice challenged 2 weeks after boosting, A/J mice challenged at 12 weeks were solidly immune (Table 2). Long-lasting protection induced in these animals by malaria antigen plus IL-12 in alum was evident by a number of parameters. Importantly, there was a significant decrease in peak parasitemia compared to nonimmunized A/J mice (P < 0.001). In addition, the number of days required to clear parasites from the blood of mice challenged 12 weeks after boosting was similar to that for mice challenged 2 weeks after boosting, and there was 100% survival among all immunized mice regardless of the time of challenge infection.
TABLE 2.
Long-term protection against blood-stage malaria induced by immunization with malaria antigen plus IL-12 in alum
| Treatment groupa (n) | % Peak parasitemia (mean ± SEM) | Clearance by day: | % Survival |
|---|---|---|---|
| Untreated (10) | 41.25 ± 1.29 | 0 | |
| 2 wk postboost (5) | 15.80 ± 2.29b | 14 | 100 |
| 12 wk postboost (5) | 28.65 ± 1.29c | 15 | 100 |
Groups of A/J mice (n = 5) were immunized s.c. with antigen plus 1.0 μg of IL-12 admixed in alum and boosted 3 weeks later by i.p. injection with antigen. For each immunization group, age-matched untreated and immunized mice were infected i.p. with 106 PRBC at 2 or 12 weeks postboost. Since there were no significance differences in peak parasitemia or survival between the two untreated groups (n = 10), data have been pooled.
P < 0.001 compared to control.
P < 0.001 compared to control.
Protective immunity induced by immunization with malaria antigen plus IL-12 in alum requires CD4+ T cells and IFN-γ.
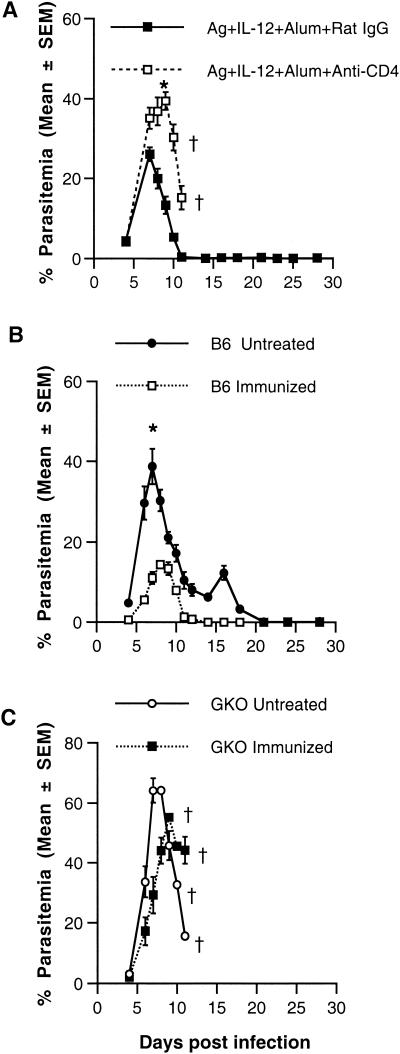
To investigate the mechanism of protective immunity induced by vaccination with the combination of antigen plus IL-12 in alum, immunized A/J mice were depleted of CD4+ T cells by treatment with GK1.5 MAb 3 days prior to and three times per week during the challenge infection with P. chabaudi AS. Parasitemia and survival were monitored for 4 weeks post-challenge infection. Consistent with the results shown above, intact immunized A/J mice suffered a mild course of infection and survived challenge infection. In contrast, CD4+-T-cell-depleted mice experienced fulminant infections with significantly higher peak parasitemia levels than rat IgG-treated mice (P = 0.008) (Fig. 3A), and the animals died by day 11 postchallenge.
FIG. 3.
Course of parasitemia and survival in immunized CD4+ T cell depleted A/J mice or in wild-type or GKO C57BL/6 mice. To deplete CD4+ in vivo, A/J mice were treated i.p. with GK1.5 monoclonal antibody or with an equivalent amount of rat IgG as a control 3 days prior to challenge infection and three times per week during infection. Two weeks after boosting, mice were challenged i.p. with 106 P. chabaudi AS PRBC and the course of parasitemia was determined (A). Female wild-type (B) and GKO (C) C57BL/6 mice were immunized with antigen (Ag) plus IL-12 in alum, and 2 weeks after boosting, mice were challenged i.p. with 106 P. chabaudi AS PRBC and the course of parasitemia was determined. Similar results were obtained in a replicate experiment using male wild-type and GKO mice. In panel A, the asterisk designates a P value of <0.001 for control versus CD4+-T-cell-depleted mice. In panel B, the asterisk designates a P value of <0.0001 for untreated versus immunized C57BL/6 mice.
To determine the role of IFN-γ in vaccine-induced protection, GKO mice on the resistant C57BL/6 background and wild-type C57BL/6 mice (36) were immunized with antigen plus IL-12 in alum. Immunized as well as untreated, control GKO and wild-type mice were challenged with P. chabaudi AS as described above. The course of parasitemia and outcome of infection were monitored for 4 weeks in control and immunized mice of both genotypes (Fig. 3B and C). As shown previously, control GKO mice developed significantly higher levels of peak parasitemia on day 7 than their wild-type counterparts (64.2 ± 3.35 versus 38.7 ± 4.43, respectively; P < 0.0001). Furthermore, immunized wild-type C57BL/6 mice had a significantly lower peak parasitemia level which occurred 1 day later than that for wild-type mice without immunization (P < 0.0001) (Fig. 3B), indicating that immunization with antigen plus IL-12 in alum induced protection in resistant C57BL/6 as well as susceptible A/J hosts. In contrast to increased protection, as defined by the level of peak parasitemia, observed in wild-type mice, there was no significant difference in peak parasitemia levels in immunized versus untreated GKO mice (55.31 ± 1.37 versus 64.2 ± 3.35, respectively; P = 0.05). The timing of the peak parasitemia was delayed from day 7 to day 9 in immunized mice compared to control GKO mice. However, 100% of GKO mice, whether immunized or not, succumbed to challenge infection by day 12 (40; data not shown). Taken together, these results demonstrate the crucial roles of CD4+ T cells and IFN-γ in the development of protective immunity against blood-stage malaria induced by immunization with P. chabaudi AS antigen plus IL-12 coadsorbed to alum.
Protective immunity induced by immunization with malaria antigen plus IL-12 in alum requires B cells.
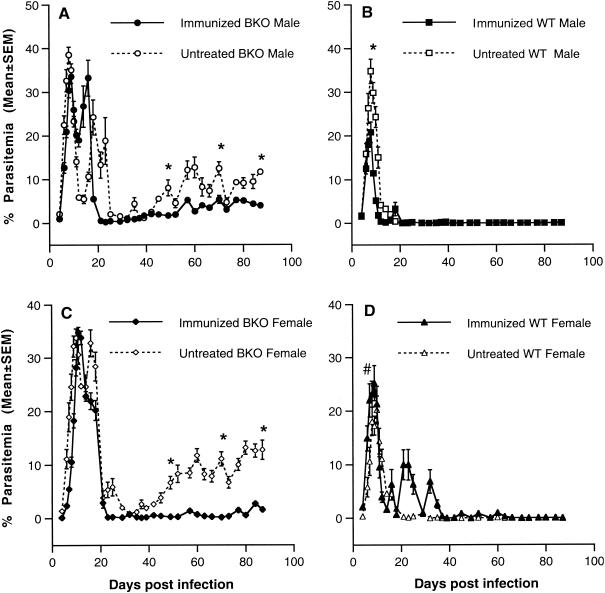
As shown above, immunization of A/J mice with malaria antigen plus IL-12 in alum induced high levels of total malaria-specific antibody, IgG2a, and IgG1, and conferred the highest level of protection against challenge infection with blood-stage P. chabaudi AS. These observations suggested to us that the B-cell response is an integral component of the mechanism of protective immunity induced by immunization with the combination of malaria antigen and IL-12 coadsorbed to alum. The role of B cells in protective immunity induced by vaccination with antigen plus IL-12 in alum was further investigated by using B-cell-deficient μ-MT mice on the resistant C57BL/10 background (36). As previously observed (41, 43, 44), nonimmunized male (Fig. 4A) and female (Fig. 4C) B-cell-deficient mice compared to intact C57BL/10 mice (Fig. 4B and D) experienced recurrent bouts of recrudescent parasitemia until the experiment was terminated 90 days after challenge infection. Following immunization, peak parasitemia levels in male and female intact C57BL/10 mice were significantly decreased (P < 0.001 for male mice and P < 0.05 for female mice). Challenge infection was cleared in both male and female immunized C57BL/10 mice, although female mice experienced several recrudescent parasitemias between 5 and 10%. Despite immunization, male and female B-cell-deficient mice experienced peak parasitemias which were not significantly reduced compared to nonimmunized, B-cell-deficient mice. Although immunized B-cell-deficient mice suffered fewer and significantly lower recrudescent parasitemias than their nonimmunized counterparts, they were unable to clear the infection completely and low levels of parasitemia (1 to 5%) persisted throughout the chronic stage of infection until the experiment was terminated on day 90.
FIG. 4.
Course of parasitemia in immunized B-cell-deficient μ-MT (BKO) and wild-type (WT) C57BL/10 mice. Groups of BKO (male, n = 6; female, n = 8) and WT (male and female, n = 10) mice were immunized s.c. with antigen plus IL-12 in alum and boosted i.p. with antigen 3 weeks later. Two weeks later, mice were challenged i.p. with 106 P. chabaudi AS PRBC and the course of parasitemia was determined in male (A and B) and female (C and D) BKO (A and C) and wild-type (B and D) mice. *, P < 0.001; #, P < 0.05 for nonimmunized versus immunized mice.
CpG-ODN can replace IL-12 as an adjuvant for immunization against blood-stage malaria.
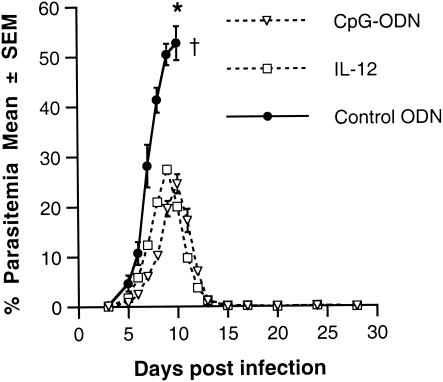
It is possible that other agents, such as CpG-ODN, with potent immunostimulatory properties could also be useful as an adjuvant in a vaccine against blood-stage malaria. CpG-ODN has been shown to induce production of IL-12, which, in turn, enhances IFN-γ production, antibody production by B cells, and cytotoxicity of NK cells and CD8+ T cells (4, 5, 7, 15, 23). To determine whether CpG-ODN can replace IL-12 as an adjuvant in the blood-stage malaria vaccine, A/J mice were immunized with malaria antigen plus 100 μg of CpG-ODN or control ODN in alum, by using the standard protocol, and challenged with P. chabaudi AS. As shown in Fig. 5, CpG-ODN was as effective as IL-12 in inducing protection against challenge infection with P. chabaudi AS. Mice immunized with malaria antigen plus CpG-ODN in alum had a course of parasitemia and 100% survival following challenge infection with P. chabaudi AS, which was similar to mice immunized with antigen plus IL-12 in alum. There was a significant decrease in the peak parasitemia levels of mice immunized with antigen plus CpG-ODN in alum compared to mice immunized with antigen plus control ODN in alum (P < 0.001), and mice in the former group cleared the parasite by 2 weeks postinfection. The combination of antigen plus control ODN in alum was not protective, and 100% of the mice in this group succumbed to challenge infection, with fulminant parasitemia levels by day 10 postinfection.
FIG. 5.
Course of parasitemia in A/J mice immunized with antigen plus IL-12 in alum or antigen plus CpG-ODN in alum. Groups of 5 A/J mice were immunized s.c. with either antigen plus IL-12 in alum (IL-12), antigen plus CpG-ODN in alum (CpG-ODN), or antigen plus ODN in alum (Control ODN) and boosted i.p. with antigen 3 weeks later. Two weeks later, mice were challenged i.p. with 106 P. chabaudi AS PRBC and the course of parasitemia was determined. *, P < 0.001 for day 9 parasitemia between antigen plus ODN in alum versus antigen plus CpG-ODN in alum and P = 0.114 for antigen plus CpG-ODN in alum versus antigen plus IL-12 in alum.
DISCUSSION
Previous studies demonstrate that coadsorption of antigen and IL-12 to alum promotes both type 1 cytokine and antibody responses (19, 21). Since both cellular and humoral responses have been implicated in protective immunity to malaria, we reasoned that immunization with the combination of malaria antigen and IL-12 coadsorbed to alum may enhance protective immunity to blood-stage malaria. To investigate this possibility, we examined the feasibility of using crude malaria antigen coadsorbed with IL-12 to alum as a vaccine against blood-stage malaria in the mouse model of P. chabaudi AS. Cellular and humoral immune responses were compared in A/J mice immunized with antigen plus IL-12 in alum as well as antigen alone, antigen in alum, or antigen plus IL-12 and boosted 3 weeks later with antigen alone prior to challenge infection.
A/J mice are susceptible to primary P. chabaudi AS infection and experience fulminant and lethal parasitemia by 10 to 13 days postinfection (36). During the first week of infection, spleen cells from these mice produce high levels of IL-4 and low levels of IFN-γ in vitro in response to the parasite antigen (38). Determination of proliferation and cytokine production in vitro by spleen cells from A/J mice immunized with the various vaccine combinations revealed that spleen cells from mice immunized with malaria antigen plus IL-12 in alum had the highest levels of proliferation as well as of IFN-γ production in response to the specific antigen. Spleen cells from these mice also produced lower levels of the Th2 cytokine IL-4 and the Th1 cytokine TNF-α and low levels of IL-10.
The finding of high levels of production of IFN-γ is consistent with observations in previous studies with IL-12 as an adjuvant together with antigen for vaccination against L. major (1), S. mansoni (46-48), or Listeria (27), which demonstrate that inclusion of this cytokine in the vaccine formulation induces a strongly polarized type 1 cytokine response with production of high levels of IFN-γ. In these studies, the combination of IL-12 and antigen was administered as a vaccine in the absence of alum or another adjuvant. However, Jankovic et al. (19) observed that administration of IL-12 and human immunodeficiency virus type 1 gp120 induces a shift from a type 2 to a type 1 cytokine profile only when coadsorbed to alum. In the case of vaccination against L. major (1) or S. mansoni (46-48) with the combination of parasite antigen and IL-12, Th2 cytokine production, including IL-4 production, is markedly diminished. Taken together, these findings in various infection models with mice are in accordance with the ability of IL-12 to promote the differentiation of CD4+ Th1 cells (12).
Our observations that the combination of malaria antigen plus IL-12 in alum induced high levels of total malaria-specific antibody and IgG2a antibody as well as moderate levels of malaria-specific IgG1 are consistent with previous studies utilizing IL-12 in a vaccine formulation. The present study did not address whether the increased protection seen in the group of mice immunized with the combination of malaria antigen plus IL-12 in alum is due to a particular subclass or isotype of antibody or due to a generalized increase in antibody titers. Wynn et al. (48) demonstrated that mice vaccinated with a combination of IL-12 and irradiated S. mansoni cercariae have significant increases in parasite-specific IgG2a, IgG2b, and IgG1. Studies by Jankovic et al. (19) demonstrated that mice vaccinated with IL-12 and recombinant gp120 envelope protein from human immunodeficiency virus type 1 coadsorbed to alum have high levels of specific IgG2a, IgG2b, and IgG3 isotypes as well as significantly increased gp120-specific IgG1 isotype levels compared to mice immunized with antigen in alum. Interestingly, in these two studies, enhanced IgG1 production occurred in the face of suppressed IL-4 production when IL-12 was included in the vaccine formulation. Similarly, our results indicate that vaccination with the combination of malaria antigen plus IL-12 coadsorbed to alum induced a Th1 immune response in vaccinated mice. The induction of a Th1 immune response by administration of malaria antigen plus IL-12 coadsorbed to alum is relevant given the important role of type 1 cell-mediated and humoral immune responses in mediating naturally induced immunity against malaria in mice infected with blood-stage P. chabaudi AS and possibly in humans (24, 29, 39, 40).
Importantly, immunization with the combination of malaria antigen plus IL-12 in alum induced strong protective immunity against challenge infection with blood-stage P. chabaudi AS in both susceptible A/J and resistant C57BL/6 mice. In contrast to control A/J mice, which experience a severe course of parasitemia and 100% mortality (36), immunization with either antigen plus IL-12 or antigen plus IL-12 coadsorbed to alum resulted in less severe courses of infection and significant decreases in peak parasitemia levels. However, only mice immunized with antigen plus IL-12 in alum experienced 100% survival. Moreover, the protection induced by this formulation was long-lasting since mice challenged 3 months after boosting were still completely protected against P. chabaudi AS. This group of animals had significant decreases in peak parasitemia levels and time to parasite clearance comparable to mice challenged 2 weeks after boosting. In both instances, there was 100% survival of vaccinated mice.
Although CD4+ T cells are known to play an important role in immunity to primary blood-stage P. chabaudi AS (24, 29), little is known about the role of these cells in vaccine-induced immunity to blood-stage malaria. Earlier studies by Langhorne and colleagues (25) demonstrated that depletion of CD4+ T cells from immune C57BL/6 mice results in a low, transient parasitemia following challenge with P. chabaudi AS, which is eventually cleared. In contrast, our results in CD4+-T-cell-depleted, immunized mice indicate that CD4+ T cells play a critical role in immunity induced by vaccination with malaria antigen and IL-12 in alum. We observed that immunized CD4+-T-cell-depleted mice experienced severe and lethal infections when challenged with P. chabaudi AS.
It is likely that CD4+ T cells participate in immunity induced by immunization with malaria antigen and IL-12 coadsorbed to alum by producing IFN-γ, although the studies performed here did not address the cellular source of this cytokine in immunized mice. Both CD4+ T cells and NK cells were found to produce IFN-γ in mice vaccinated with L. major antigen and IL-12 (1). NK cells may also be a source of IFN-γ in mice immunized with malaria antigen and IL-12 in alum. NK cells have been found to produce IFN-γ early in infection with various species of mouse malaria parasites, including P. chabaudi AS (8, 28). Recent studies with humans demonstrated that Plasmodium falciparum-infected red blood cells induce IFN-γ production by NK cells from individuals infected with P. falciparum and nonexposed donors (3). IFN-γ is considered to be a major component of innate and acquired immunity to primary blood-stage P. chabaudi infections (11, 24, 40, 42). The inability to protect GKO mice, compared to wild-type C57BL/6 mice, against challenge infection as shown here indicates that IFN-γ is also a critical cytokine in vaccine-induced immunity following immunization with malaria antigen and IL-12 coadsorbed to alum. In humans, IFN-γ production has been found to correlate with resistance to reinfection with P. falciparum as well as with protection from clinical attacks of malaria (6, 9, 26). Based on these observations, it has been concluded that IFN-γ production should be considered an important hallmark of effector T-cell function for the development of an effective malaria vaccine (14, 32). Our results in the present report support this contention.
During primary P. chabaudi AS infection, mice rendered B-cell deficient by treatment from birth with anti-IgM antibodies or μ-MT mice with targeted disruption of the membrane exon of the immunoglobulin μ-chain gene can control acute parasitemias in a manner similar to that of intact mice (41, 44). However, B-cell-deficient mice maintain a chronic low level of parasitemia, indicating that effective parasite clearance at the later, chronic stage of infection requires the presence of B cells (41, 44). In addition to their ability to produce antibody, B cells may also play a role via production of IL-10 (41) in the switch from Th1 cells producing IFN-γ, which mediates control of acute parasitemia, to Th2 cells which provide help for antibody production leading to clearance of primary blood-stage P. chabaudi AS infection. Studies in μ-MT mice also showed that B-cell-deficient animals are unable to control a challenge infection and develop parasitemia levels similar in magnitude to a primary infection (44). These findings suggest that B-cell-dependent mechanisms may be important for an effective memory response to P. chabaudi AS infection (44). In the present study, we observed that immunization of B-cell-deficient μ-MT mice with malaria antigen and IL-12 coadsorbed to alum is ineffective in providing enhanced protection against challenge infection with P. chabaudi AS, suggesting a role for a B-cell-dependent mechanism(s) in vaccine-induced immunity.
We also examined the possibility of replacing IL-12 with immunostimulatory CpG-ODN. Because of its ability to induce a type 1 pattern of cytokine production dominated by IL-12 and IFN-γ with little secretion of type 2 cytokines, CpG-ODN have been found to be useful as adjuvants for vaccines, including peptide vaccines, against a variety of pathogens (4, 5, 7, 15, 23, 30, 35, 45). Near and colleagues (30) recently demonstrated that vaccination with the combination of CpG-ODN and P. yoelii MSP119 in alum resulted in a dramatic elevation of IFN-γ production as well as elevated production of IL-10 by MSP119-stimulated splenocytes, suggesting induction of a mixed Th1 and Th2 response. In mice vaccinated with this formulation, IgG1 was found to be the predominant antibody isotype in sera, although increased levels of MSP119-specific IgG2a, IgG2b, and IgG3 isotype antibodies were also observed. Furthermore, increased antibody levels were found to correlate with protection against challenge infection with a high dose of P. yoelii PRBC. Our experimental results demonstrate that inclusion of immunostimulatory CpG-ODN instead of IL-12 in the vaccine formulation provided strong protection against blood-stage P. chabaudi AS infection in A/J mice. Studies in progress in our laboratory demonstrate that immunization with CpG-ODN and crude malaria antigen in alum induces high levels of malaria-specific IgG2a in A/J mice before challenge infection in comparison to immunization with control ODN and antigen in alum (unpublished data).
In conclusion, the results of this study illustrate that it is possible to enhance the potency of a crude malaria antigen in alum vaccine formulation by inclusion of agents with immunostimulatory properties, such as IL-12 or CpG-ODN. Although alum is the most commonly used adjuvant and is approved for use by humans by the U.S. Food and Drug Administration, our data indicate that this adjuvant is weak in promoting vaccine-induced protective immunity against blood-stage malaria. Furthermore, immunity induced by immunization with malaria antigen and IL-12 coadsorbed to alum induced a long-lasting, Th1 immune response required for protection against challenge infection with P. chabaudi AS infection.
Acknowledgments
We thank Sarah M. Stevenson for help with data management and statistical analyses.
This study was supported by the Canadian Institutes of Health Research (MOP14663).
Editor: J. M. Mansfield
REFERENCES
- 1.Alfonso, L. C. C., T. M. Scharton, L. Q. Vieira, M. Wysocka, G. Trinchieri, and P. Scott. 1994. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science 263:235-237. [DOI] [PubMed] [Google Scholar]
- 2.Amante, F. H., and M. F. Good. 1997. Prolonged Th1-like response generated by a Plasmodium yoelii-specific T cell clone allows complete clearance of infection in reconstituted mice. Parasite Immunol. 19:111-126. [DOI] [PubMed] [Google Scholar]
- 3.Artavanis-Tsakonas, K., and E. M. Riley. 2002. Innate immune response to malaria: rapid induction of IFN-γ from human NK cells by live Plasmodium falciparum-infected erythrocytes. J. Immunol. 169:2956-2963. [DOI] [PubMed] [Google Scholar]
- 4.Braziolot-Millan, C. L., R. Weeratna, A. M. Krieg, C. A. Siegrist, and H. L. Davis. 1998. CpG DNA can induce strong Th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc. Natl. Acad. Sci. USA 95:1555-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis, H. L., R. Weeratna, T. J. Waldschmidt, L. Tygrett, J. Schorr, and A. M. Krieg. 1998. CpG is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J. Immunol. 160:870-876. [PubMed] [Google Scholar]
- 6.Deloran, P., C. Chougnet, J.-P. Lepers, S. Tallet, and P. Coulanges. 1991. Protective value of elevated levels of γ interferon in serum against exoerythrocytic stages of P. falciparum. J. Clin. Microbiol. 29:1757-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demi, L., R. Schirmbeck, J. Reimann, H. Wolf, and R. Wagner. 1999. Immunostimulatory CpG motifs trigger a T helper-1 immune response to human immunodeficiency virus type-1 (HIV) gp160 envelope proteins. Clin. Chem. Lab. Med. 37:199-204. [DOI] [PubMed] [Google Scholar]
- 8.DeSouza, J. B., K. H. Williamson, T. Otani, and J. H. Playfair. 1997. Early gamma interferon responses in lethal and nonlethal murine blood-stage malaria. Infect. Immun. 65:1593-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodoo, D. F. Omer, J. Todd, B. Akanmori, K. Koram, and E. Riley. 2002. Absolute levels and ratios of pro-inflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to P. falciparum malaria. J. Infect. Dis. 185:971-979. [DOI] [PubMed] [Google Scholar]
- 10.Facer, C. A., and M. Tanner. 1997. Clinical trials of malaria vaccines: progress and prospects. Adv. Parasitol. 39:1-68. [DOI] [PubMed] [Google Scholar]
- 11.Favre, N., B. Ryffel, G. Bordmann, and W. Rudin. 1997. The course of Plasmodium chabaudi chabaudi infections in interferon-gamma receptor deficient mice. Parasite Immunol. 19:375-383. [DOI] [PubMed] [Google Scholar]
- 12.Gately, M. K., L. M. Renzetti, J. Magram, A. S. Stern, L. Adorini, U. Gubler, and D. H. Presky. 1998. The interleukin-12/interleukin-12 receptor system: role in normal and pathogenic immune responses. Annu. Rev. Immmunol. 16:495-521. [DOI] [PubMed] [Google Scholar]
- 13.Good, M. F. 2001. Towards a blood-stage vaccine for malaria: are we following all the leads? Nat. Rev. Immunol. 1:117-125. [DOI] [PubMed] [Google Scholar]
- 14.Good, M. F., and D. L. Doolan. 1999. Immune effector mechanisms in malaria. Curr. Opin. Immunol. 11:412-419. [DOI] [PubMed] [Google Scholar]
- 15.Gramzinski, R. A., D. L. Doolan, M. Sedegah, H. L. Davis, S. M. Krieg, and S. L. Hoffman. 2001. Interleukin-12- and gamma interferon-dependent protection against malaria conferred by CpG oligodeoxynucleotide in mice. Infect. Immun. 69:1643-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinzel, F. P., R. M. Rerko, F. Ahmed, and A. M. Hujer. 1996. IFN-γ independent production of IL-12 during murine endotoxemia. J. Immunol. 157:4521-4528. [PubMed] [Google Scholar]
- 17.Holder, A. A. 1999. Malaria vaccines. Proc. Natl. Acad. Sci. USA 96:1167-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James, S., and L. Miller. 2000. Malaria vaccine development: status report, p. 9-13. In Nature medicine special focus: malaria.
- 19.Jankovic, D., P. Caspar, M. Zweig, M. Garcia-Moll, S. D. Showalter, F. R. Vogel, and A. Sher. 1997. Adsorption to aluminum hydroxide promotes the activity of IL-12 as an adjuvant for antibody as well as type 1 cytokine responses to HIV-1 gp120. J. Immunol. 159:2409-2417. [PubMed] [Google Scholar]
- 20.Jankovic, D., T. A. Wynn, M. C. Kullberg, S. Hieny, P. Caspar, S. James, A. W. Cheever, and A. Sher. 1999. Optimal vaccination against Schistosoma mansoni requires the induction of both B cell- and IFN-γ-dependent effector mechanisms. J. Immunol. 162:345-351. [PubMed] [Google Scholar]
- 21.Kenney, R. T., D. L. Sacks, J. P. Sypek, L. Vilela, A. A. Gam, and K. Evans-Davis. 1999. Protective immunity using recombinant human IL-12 and alum as adjuvants in a primate model of cutaneous leishmaniasis. J. Immunol. 163:4481-4488. [PubMed] [Google Scholar]
- 22.Kitamura, D., J. Roes, R. Kühn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon on the immunoglobulin mu chain gene. Nature 350:423-426. [DOI] [PubMed] [Google Scholar]
- 23.Klinman, D., A.-K. Yi, S. L. Beaucage, J. Conover, and A. M. Krieg. 1996. CpG motifs present in bacterial DNA rapidly induce lymphocytes to secrete IL-6, IL-12 and IFN-γ. Proc. Natl. Acad. Sci. USA 93:2879-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langhorne, J., S. J. Quin, and L. A. Sanni. 2002. Mouse models of blood-stage malaria infections: immune responses and cytokines involved in protection and pathology, p. 204-228. In P. Perlmann and M. Troye-Blomberg (ed.), Malaria immunology. Karger, Basel, Switzerland. [DOI] [PubMed]
- 25.Langhorne, J., B. Simon-Haarhaus, and S. J. Meding. 1990. The role of CD4+ T cells in the protective immune response to Plasmodium chabaudi in vivo. Immunol. Lett. 25:101-108. [DOI] [PubMed] [Google Scholar]
- 26.Luty, A. J. F., B. Lell, R. Schmidt-Ott, L. G. Lehman, D. Luckner, B. Greve, P. Matousek, K. Herbich, D. Schmidt, F. Migot-Nabias, P. Deloran, R. S. Nussenzweig, and P. G. Kremnser. 1999. Interferon-γ responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J. Infect. Dis. 179:980-988. [DOI] [PubMed] [Google Scholar]
- 27.Miller, M. A., M. J. Skeen, and H. K. Ziegler. 1997. A synthetic peptide administered with IL-12 elicits immunity to Listeria monocytogenes. J. Immunol. 159:3675-3679. [PubMed] [Google Scholar]
- 28.Mohan, K., P. Moulin, and M. M. Stevenson. 1997. Natural killer cell cytokine production, not cytotoxicity, contributes to resistance against blood-stage Plasmodium chabaudi AS infection. J. Immunol. 159:4990-5004. [PubMed] [Google Scholar]
- 29.Mohan, K., and M. M. Stevenson. 1998. Acquired immunity to asexual blood stages, p. 467-493. In I. W. Sherman (ed.), Malaria: parasite biology, pathogenesis, and protection. American Society Microbiology, Washington, D.C.
- 30.Near, K. A., A. W. Stowers, D. Jankovic, and D. C. Kaslow. 2002. Improved immunogenecity and efficacy of the recombinant 19-kilodalton merozoite surface protein 1 by addition of oligodeoxynucleotide and aluminum hydroxide gel in a murine malaria vaccine model. Infect. Immun. 70:692-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Garra, A., and K. Murphy. 1994. Role of cytokines in determining T-lymphocyte function. Curr. Opin. Immunol. 6:458-466. [DOI] [PubMed] [Google Scholar]
- 32.Plebanski, M., and A. Hill. 2000. The immunology of malaria infection. Curr. Opin. Immunol. 12:437-441. [DOI] [PubMed] [Google Scholar]
- 33.Podoba, J. E., and M. M. Stevenson. 1991. CD4+ and CD8+ T lymphocytes both contribute to acquired immunity to blood-stage Plasmodium chabaudi AS. Infect. Immun. 59:51-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sam, H., and M. M. Stevenson. 1999. In vivo IL-12 production and IL-12 receptors β1 and β2 mRNA expression in the spleen are differentially upregulated in resistant B6 and susceptible A/J mice during early blood-stage Plasmodium chabaudi AS malaria. J. Immunol. 162:1582-1589. [PubMed] [Google Scholar]
- 35.Stacey, K. J., and J. M. Blackwell. 1999. Immunostimulatory DNA as an adjuvant in vaccination against Leishmania major. Infect. Immun. 67:3719-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevenson, M. M., J. J. Lyanga, and E. Skamene. 1982. Murine malaria: genetic control of resistance to Plasmodium chabaudi. Infect. Immun. 38:80-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevenson, M. M., M. F. Tam, S. F. Wolf, and A. Sher. 1995. IL-12 induced protection against blood-stage Plasmodium chabaudi AS requires IFN-γ and TNF-α and occurs via an NO-dependent mechanism. J. Immunol. 155:2545-2556. [PubMed] [Google Scholar]
- 38.Stevenson, M. M., and M. F. Tam. 1993. Differential induction of helper T cell subsets during blood-stage Plasmodium chabaudi AS infection in resistant and susceptible mice. Clin. Exp. Immunol. 92:77-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su, Z., and M. M. Stevenson. 2002. IL-12 is required for antibody-mediated protective immunity against blood-stage Plasmodium chabaudi AS malaria infection in mice. J. Immunol. 168:1348-1355. [DOI] [PubMed] [Google Scholar]
- 40.Su, Z., and M. M. Stevenson. 2000. Central role of endogenous gamma interferon in protective immunity against blood-stage Plasmodium chabaudi AS infection. Infect. Immun. 68:4399-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor-Robinson, A. W., and R. S. Philips. 1994. B cells are required for the switch from TH1- to TH2-regulated immune response to Plasmodium chabaudi chabaudi infection. Infect. Immun. 62:2490-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Heyde, H. C., B. Pepper, J. Batchelder, F. Cigel, and W. P. Weidanz. 1997. The time course of selected malarial infections in cytokine-deficient mice. Exp. Parasitol. 88:206-213. [DOI] [PubMed] [Google Scholar]
- 43.von der Weid, T., and J. Langhorne. 1993. Altered response of CD4+ T cell subsets to Plasmodium chabaudi chabaudi in B cell-deficient mice. Int. Immunol. 5:1343-1348. [DOI] [PubMed] [Google Scholar]
- 44.von der Weid, T., N. Honarvar, and J. Langhorne. 1996. Gene-targeted mice lacking B cells are unable to eliminate a blood stage malaria infection. J. Immunol. 156:2510-2516. [PubMed] [Google Scholar]
- 45.Weeratna, R. D., M. J. McCluskie, Y. Xu, and H. S. Davis. 2000. CpG DNA induces stronger immune responses with less toxicity than other adjuvants. Vaccine 18:1755-1762. [DOI] [PubMed] [Google Scholar]
- 46.Wynn, T. A., A. W. Cheever, D. Jankovic, R. W. Poindexter, P. Caspar, F. A. Lewis, and A. Sher. 1995. An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature 376:594-596. [DOI] [PubMed] [Google Scholar]
- 47.Wynn, T. A., D. Jankovic, S. Hieny, A. W. Cheever, and A. Sher. 1995. IL-12 enhances vaccine-induced immunity to Schistosoma mansoni in mice and decreases T helper 2 cytokine expression, IgE production, and tissue eosinophilia. J. Immunol. 154:4701-4709. [PubMed] [Google Scholar]
- 48.Wynn, T. A., A. Reynolds, S. James, A. W. Cheever, P. Caspar, S. Hieny, D. Jankovic, M. Strand, and A. Sher. 1996. IL-12 enhances vaccine-induced immunity to schistosomes by augmenting both humoral and cell-mediated immune responses against the parasite. J. Immunol. 157:4068-4078. [PubMed] [Google Scholar]
- 49.Yap, G. S., and M. M. Stevenson. 1994. Differential requirements for an intact spleen in induction and expression of B-cell-dependent immunity to Plasmodium chabaudi AS. Infect. Immun. 62:4219-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]