Abstract
Serum opacity factor (SOF) is a protein expressed by Streptococcus pyogenes that opacifies mammalian serum. SOF is also a virulence factor of S. pyogenes, but it has not been previously shown to elicit a protective immune response. Herein, we report that SOF evokes bactericidal antibodies against S. pyogenes in humans, rabbits, and mice. Rabbit antiserum against purified recombinant SOF2 opsonized SOF-positive M type 2, 4, and 28 S. pyogenes in human blood but had no effect on SOF-negative M type 5 S. pyogenes. Furthermore, affinity-purified human antibodies against SOF2 also opsonized SOF-positive streptococci. A combination of antisera against M2 and SOF2 proteins was dramatically more effective in killing streptococci than either antiserum alone, indicating that antibodies against SOF2 enhance the opsonic efficiency of M protein antibodies. Mice tolerated an intravenous injection of 100 μg of SOF without overt signs of toxicity, and immunization with SOF protected mice against challenge infections with M type 2 S. pyogenes. These data indicate that SOF evokes opsonic antibodies that may protect against infections by SOF-positive serotypes of group A streptococci and suggest that different serotypes of SOF have common epitopes that may be useful vaccine candidates to protect against group A streptococcal infections.
The group A streptococcus Streptococcus pyogenes causes a variety of diseases, ranging from mild and generally self-limiting infections of the pharynx and skin to more-severe and life-threatening infections, such as toxic shock syndrome and necrotizing fasciitis. The major sequelae of group A streptococcal infections are acute rheumatic fever and acute glomerulonephritis, which are thought to be due to autoimmune T- and B-cell responses induced by streptococcal products (2, 11-15, 20). Prior infections with group A streptococci may also lead to autoimmune neurological disorders (5, 34, 45).
Early efforts to develop a vaccine to prevent these diseases focused on M proteins because infections in humans were found to elicit an immune response to M proteins that was protective and long-lived (30). M proteins are the major virulence factor in group A streptococci and confer the abilities to multiply in nonimmune human blood and to attach to host cells (8, 13, 20). Structurally, M proteins are α-helical, coiled-coil proteins that radiate from the surface of the organism and that are composed of a variable N-terminal half and a highly conserved C-terminal half (20). The N-terminal 40 to 50 amino acids are hypervariable and elicit type-specific antisera. Both the conserved and variable domains of M proteins are targets of current vaccine efforts, and each approach has its own strengths and weaknesses.
The major strength of a vaccine based on the conserved domains of M proteins is that protection against both homologous and heterologous serotypes is provided (1, 4, 6, 7, 36-38). The major concern is that these conserved domains may stimulate T- and B-cell responses that target human tissues (12, 14, 15). Good and coworkers have, however, identified a peptide in the C repeats of M proteins that elicits bactericidal antibodies that do not cross-react with human tissues (1, 36, 37), but the level of bactericidal antibodies may not be adequate in some cases.
The major strengths of a vaccine based on the variable N termini of M proteins are that a strong bactericidal antibody response is evoked and that these antibodies are less likely to cross-react with human tissues (17, 23). The major problem is that protection is generally type specific, and there are more than 100 different M types produced by group A streptococci. This problem has been addressed by developing multivalent vaccines that target prevalent serotypes causing pharyngitis, invasive diseases, and rheumatic fever (23). Thus, a 26-valent vaccine targeted 84% of all group A streptococcal isolates and 74% of invasive isolates identified from 1998 to 2000 within the United States (23).
More-recent investigations have identified a number of other vaccine candidates, including the R28 protein (44), Spa (16, 32), C5a peptidase (25), the group A carbohydrate (41), Sfb1 (also termed protein F1) (22, 33, 43), FBP54 (27), SpeA (40), SpeB (26), SpeC (31), and lipoteichoic acid (LTA) (18). Some of these antigens elicit protection against only a limited number of serotypes, while other antigens, such as the group A carbohydrate, may require high concentrations of antibodies to be effective. Furthermore, the C5a peptidase, SpeA, SpeB, SpeC, SfB1, and the R28 protein have not been shown to induce antibodies that opsonize group A streptococci. FBP54 evoked opsonic antibodies against two different serotypes, but its degree of coverage and efficacy of protection have not yet been thoroughly investigated (27). LTA induced antibodies that blocked colonization (18), but almost all gram-positive bacteria produce LTA. Therefore, a vaccine utilizing LTA would not be selective in the bacteria it targets. Because of these considerations, the M protein-based vaccine is considered to be very promising. However, not all types of M proteins evoke a protective antibody response (6), and there are serotypes for which a protective antigen (an antigen that evokes a protective immune response) has not yet been identified. Moreover, the current 26-valent vaccine targets serotypes primarily found in the United States, and these serotypes may not be representative of those causing infections in other areas such as Australia and Asia. Thus, there is a need to broaden the protective coverage of vaccines and to define the protective antigen in some serotypes. Herein, we report on the potential of the serum opacity factor (SOF) of group A streptococci to meet this need.
SOF is a >100-kDa, surface-bound and released protein of S. pyogenes that opacifies mammalian serum by interacting with high-density lipoproteins (42, 46, 47). It is composed of alternating variable and conserved domains and a highly conserved C-terminal domain with an LPASG anchoring motif (9, 28, 39). The C-terminal domain contains a tandemly repeated peptide that binds fibronectin and fibrinogen (9, 10, 28, 39). The opacification of serum can be inhibited by antisera against type-specific determinants of SOF, and this inhibition is the basis for the SOF typing scheme of group A streptococci (3). Interestingly, the type-specific determinants of SOF usually covary with those of M proteins in a given strain, and thus the M type can be predicted based on the SOF type (3). Inactivation of SOF decreased the virulence of M type 2 S. pyogenes in a mouse model, indicating that it is a virulence determinant (9). Because other virulence factors have been found to elicit protective immune responses, SOF was tested for its ability to induce protective antibodies. The results indicate that SOF evokes antibodies that protect against infections by SOF-positive group A streptococci.
MATERIALS AND METHODS
Organisms and growth conditions.
The SOF-positive strains of S. pyogenes used in this study were the M type 2 strain T2MR, the M type 4 strain 52936, and the M type 28 strain 92448. The M type 5 strain Manfredo is SOF negative. The organisms were grown in Todd-Hewitt broth supplemented with 1.5% yeast extract at 37°C.
Preparation of SOF, its peptides, and antisera.
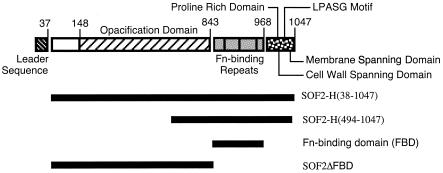
The sof2, sof4, and sof28 genes from strains T2MR, 52936, and 92448, respectively, were amplified by PCR, ligated into the pTricHis vector, introduced into Escherichia coli Top10, expressed as histidine fusion proteins, which were purified by metal affinity chromatography as previously described (9). SOF2-H(38-1047), SOF2-H(494-1047), and SOF2-H(38-843) are truncated forms of SOF2 spanning the indicated amino acid residues that were constructed and purified as previously described (9). Hereafter, SOF2-H(38-843) is referred to as SOF2ΔFBD to emphasize that the fibronectin-binding domain (FBD) has been deleted. FBD is a recombinant, histidine-tagged peptide that encompasses amino acid residues 844 to 968 of SOF2 and that contains the fibronectin-binding repeats. A schematic of SOF and the locations of recombinant peptides of SOF used in this study are illustrated in Fig. 1. Rabbit antiserum against SOF2-H(38-1047) was prepared as previously described (9). The sequences of sof2 and sof28 were previously published and have GenBank accession numbers AF01890 and AF082074, respectively, (9). The sof4 gene was ligated into pCRII and sequenced using M13 forward and reverse primers.
FIG. 1.
Model of SOF and recombinant peptides of SOF, indicating the locations of the functional domains of SOF and the recombinant peptides of SOF used in this study. The locations of the functional domains are based on the findings of Rakonjac et al. (39), Kreikemeyer et al. (28), and Courtney et al. (9). Fn, fibronectin.
Preparation of anti-sM2(1-35) serum.
The first 35 amino acids of the mature M2 protein were synthesized with a C-terminal cysteine residue used to cross-link the peptide to keyhole limpet hemocyanin as previously described (7). The conjugated peptide (500 μg/ml) was emulsified in complete Freund's adjuvant (CFA) and injected subcutaneously into New Zealand White rabbits. Booster injections of 500 μg in phosphate-buffered saline (PBS) were given at 4, 8, 10, and 15 weeks.
Enzyme-linked immunoassays (ELISA).
Wells of a microtiter plate were coated with purified recombinant SOF2, SOF4, and SOF28 (10 μg/ml in 0.01 M sodium bicarbonate, pH 9.5). Control wells were coated with bovine serum albumin (BSA). After being coated, all wells were blocked with BSA (1 mg/ml in PBS). Serial 1:2 dilutions of a 1:1,000 dilution of rabbit anti-SOF2-H(38-1047) or preimmune serum were added to the wells and incubated for 30 min at 37°C. The wells were washed, and a 1:2,000 dilution of peroxidase-labeled goat anti-rabbit immunoglobulins (Ig) was added. After 30 min, the wells were washed and the substrate tetramethylbenzidine was added. After color development, the absorbance at 650 nm was measured. The average value for wells coated with BSA served as a blank and was subtracted from all other values. All samples were tested in duplicate.
In other assays comparing the reactivities of human and rabbit serum, the microtiter wells were coated with FBD or SOF2-H(38-1047) and blocked with BSA as described above. Wells coated with BSA served as negative controls. The wells were reacted with 1:100 or 1:1,000 dilutions of human serum that neutralized SOF2 or rabbit antiserum against SOF2-H(38-1047) for 30 min. The wells were washed and reacted with a 1:2,500 dilution of peroxidase-conjugated goat anti-rabbit Ig or peroxidase-conjugated goat anti-human Ig. The wells were then washed, the substrate was added, and the absorbance was measured after color development. All samples were tested in triplicate.
Bactericidal assays.
Streptococci were grown in Todd-Hewitt broth supplemented with 1.5% yeast extract to an optical density of ∼0.08 at 530 nm and diluted 1:10,000. Twenty microliters of this dilution was added to a tube containing 200 μl of anti-SOF2 serum or preimmune serum and 400 μl of heparinized human blood from a nonimmune donor. The blood was rotated for 3 h at 37°C, and the number of CFU was determined by plating dilutions on blood agar plates. The bactericidal assays were repeated on three separate occasions. In assays testing the combined effects of anti-sM2(1-35) serum and anti-SOF2 serum, 100 μl of the serial 1:2 dilutions of anti-sM2(1-35) was added to 100 μl of anti-SOF2 or normal rabbit serum (NRS). The mixtures were added to 400 μl of heparinized human blood and treated as described above. The percentage of streptococci killed in the bactericidal assays was calculated by the following formula: percent killing = [1 − (number of CFU in anti-SOF2 serum/number of CFU in preimmune serum)] × 100.
The serum opacity reaction and its inhibition.
The ability of SOF in the culture supernatant of streptococci to opacify serum was tested by centrifugation of overnight cultures of the organisms, sterilization of the media by filtration, and addition of 100 μl of the filtrate to 1 ml of horse serum. After incubation at 37°C for 3 h, the absorbance at 405 nm was recorded. Assays for neutralization of the opacity reaction consisted of preincubating 100 μl of neutralizing serum and 100 μl of culture supernatant for 30 min at 37°C and then adding 1 ml of horse serum and recording the absorbance at 405 nm after 3 h and after an overnight incubation. In some cases, purified recombinant SOF (1 μg/ml) was used instead of culture supernatants in the inhibition experiments described above.
Purification of human antibodies against SOF.
A donor whose serum inhibited the serum opacity reaction of SOF2 was selected. The donor's serum was first chromatographed over a quaternary aminoethyl (QAE)-Sephadex column to remove other serum proteins that may bind to SOF. The QAE flowthrough containing the antibodies was then added to a column of SOF2-H(38-1047) or SOF2ΔFBD covalently linked to agarose. The columns were washed with buffer, and bound proteins were eluted with 0.05 M sodium acetate-0.1 M glycine, pH 3.0. The pH of the eluate was immediately neutralized by dialysis against PBS. The eluted antibodies retained their ability to inhibit the serum opacity reaction of SOF2.
Mouse toxicity and protection assays.
Five NIH Swiss mice received intravenous (i.v.) injections in the tail vein of 100 μg of SOF2-H(38-1047) in 0.1 ml of PBS, and five mice were injected i.v. with 100 μg of SOF2-H(494-1047) in 0.1 ml of PBS. The mice were evaluated daily for signs of toxicity, such as ruffled fur, lethargy, weight loss, abnormal movements, and death. After 10 days, all 10 mice received an intraperitoneal (i.p.) booster injection of 100 μg of SOF2-H(494-1047). At day 21, mice were challenged with ∼5 × 107 CFU of T2MR by i.p. injection, and the number of deaths was recorded daily. As a control, 15 nonimmunized mice were injected i.p. with ∼5 × 107 CFU of T2MR. These mice were of the same age, sex, and weight as the immunized mice but were not mock immunized prior to challenge.
A second mouse protection study was undertaken to determine the effectiveness of SOF2ΔFBD immunizations in protecting mice against challenge infections. Ten NIH Swiss mice were injected subcutaneously with 25 μg of SOF2ΔFBD in CFA. Nine control mice received a subcutaneous injection of CFA. After 2 weeks, the mice were boosted with an intramuscular injection of 25 μg of SOF2ΔFBD in PBS. Control mice received PBS injections. Two weeks after the booster injections, all mice were challenged by an i.p. injection of ∼1 × 107 CFU of T2MR. The number of surviving mice was recorded daily. Moribund mice were sacrificed and recorded as a dead.
Nucleotide sequence accession number.
The sof4 gene has been assigned GenBank accession no. AY162273.
RESULTS
Antiserum against SOF2 cross-reacts with other types of SOF.
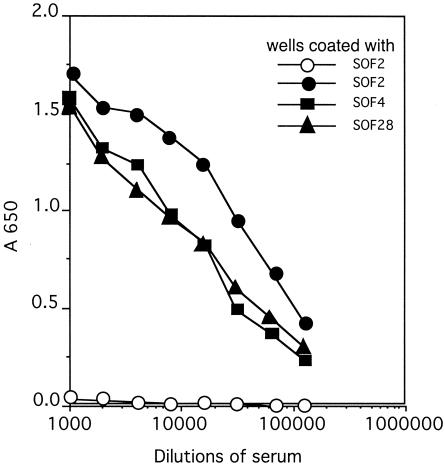
Rabbit antiserum against SOF2-H(38-1047) did not inhibit the serum opacity reaction of SOF2 but did react strongly with SOF2 in ELISA as exhibited by a positive signal at a 1:128,000 dilution (Fig. 2). The anti-SOF2 serum also strongly cross-reacted with SOF4 and SOF28. It was anticipated that the anti-SOF2 serum would cross-react with both SOF4 and SOF28, because there is ∼60% homology between SOF2 and SOF28 and ∼53% homology between SOF2 and SOF4. The degree of cross-reactivity suggests that a significant proportion of the antibodies are directed against common epitopes.
FIG. 2.
Cross-reaction of anti-SOF2 serum with SOF4 and SOF28. Microtiter wells were coated with SOF2, SOF4, or SOF28. The coated wells were reacted with dilutions of rabbit preimmune serum (open circles) or rabbit anti-SOF2 serum (filled symbols). The reaction of preimmune serum with wells coated with SOF4 and SOF28 is not shown but was similar to that shown for SOF2.
Bactericidal activity of antiserum against SOF2.
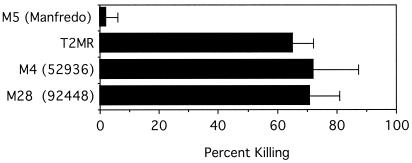
The ability of the rabbit antisera against SOF2 to opsonize M type 2, 4, and 28 S. pyogenes in nonimmune human blood was tested (Fig. 3). Rabbit antisera against SOF2-H(38-1047) not only opsonized and killed M type 2 S. pyogenes (65% killing) but also opsonized M type 4 and 28 S. pyogenes (72 and 71% killing, respectively). Two separate control experiments were performed to ensure that the antiserum did not aggregate the streptococci. In one experiment, an identical inoculum was added to preimmune serum and to anti-SOF2 serum, the mixtures were shaken, and the numbers of CFU were determined by plating. There was no difference in the numbers of CFU in the inocula, indicating that no aggregation occurred due to anti-SOF serum. In a second experiment, streptococci were added to freshly prepared human plasma containing either preimmune serum or anti-SOF2 serum. After 3 h of rotation, the numbers of CFU were determined. Again, no significant difference in the numbers of CFU between preimmune and immune serum was found, indicating that anti-SOF2 serum did not aggregate the streptococci. The results of the second experiment also demonstrate that neutrophils are needed to kill the streptococci and that antibodies and complement alone are not sufficient.
FIG. 3.
Bactericidal activity of anti-SOF2 serum. An inoculum of the indicated serotypes of S. pyogenes was mixed with rabbit anti-SOF2 serum or with preimmune serum, added to heparinized human blood, and rotated for 3 h at 37°C, and the numbers of CFU were determined as described in Materials and Methods. The means from three separate experiments ± standard deviations are shown. M type 5 strain Manfredo is a SOF-negative strain and served as a negative control.
Next, it was of interest to determine if humans also produce opsonic antibodies against SOF2. A donor whose serum inhibited the serum opacity reaction of SOF2 was selected. The antibodies against SOF2 were purified from this serum by affinity chromatography utilizing either SOF2-H(38-1047) or SOF2ΔFBD as the matrix and tested in bactericidal assays using strain T2MR. In two separate experiments, antibodies eluted from SOF2ΔFBD killed 40 and 43% of streptococci in a bactericidal assay of whole human blood (Table 1). Antibodies eluted from SOF2-H(38-1047) killed 73% of the streptococci. These results indicate that SOF stimulates the production of bactericidal antibodies in humans.
TABLE 1.
Opsonization of M type 2 S. pyogenes by affinity-purified human antibodies against SOF2a
| Expt | Affinity matrix | Inoculum (CFU) | CFU in:
|
% Killing | |
|---|---|---|---|---|---|
| Control buffer | Purified antibodies | ||||
| 1 | SOF2ΔFBD | 72 | 51,600 | 31,200 | 40 |
| 2 | SOF2ΔFBD | 40 | 44,800 | 25,600 | 43 |
| 3 | SOF2-H(38-1047) | 85 | 54,720 | 14,760 | 73 |
Human antibodies against SOF2 were purified by SOF affinity chromatography, mixed with the indicated number of CFU of S. pyogenes strain T2MR, and added to human blood as described in Materials and Methods. The number of CFU after 3 h of rotation was determined by plating dilutions of the mixtures. Controls consisted of adding human IgG equivalent to the amount of affinity-purified SOF antibodies except for experiment 1, where Tris-saline buffer was used.
The higher level of opsonization achieved with antibodies eluted from SOF2-H(38-1047) raised the possibility that antibodies against the FBD of SOF may also contribute to the opsonization of the bacteria. To evaluate this possibility, serum from the human donor was tested for antibodies that react with the FBD of SOF. Serum from the human donor did not react with the FBD of SOF (Table 2). Thus, it is unlikely that antibodies against the FBD contributed to the opsonization of T2MR by the affinity-purified antibodies. Gillen et al. (21) also found that human sera have little or no antibodies that react with the FBD of SOF. However, these investigators reported that rabbit antisera against full-length SOF did not react with the FBD of SOF. In contrast, we found that immunization of a rabbit with full-length SOF elicited antibodies that reacted with the FBD of SOF (Table 2).
TABLE 2.
Comparison of antibodies against SOF2 and the FBD of SOF2 in rabbit and human immune seruma
| Serum | Dilution | Substrate | A650 ± SD |
|---|---|---|---|
| Rabbit anti-SOF2- | 1:100 | SOF2-H(38-1047) | >2.5 |
| H(38-1047) | 1:1,000 | SOF2-H(38-1047) | 1.767 ± 0.197 |
| 1:100 | FBD | 1.634 ± 0.122 | |
| 1:1,000 | FBD | 0.349 ± 0.074 | |
| Human donor | 1:100 | SOF2-H(38-1047) | 0.444 ± 0.056 |
| (opsonizing) | 1:100 | FBD | −0.009 ± 0.005 |
Rabbit anti-SOF2 serum and human serum (from the same donor that provided antibodies listed in Table 1) were diluted as indicated and reacted with microtiter wells coated with SOF2-H(38-1047) or FBD. The wells were washed and reacted with the appropriate peroxidase-conjugated second antiserum as indicated in Materials and Methods. Wells were done in triplicate. Wells coated with BSA and treated as described above served as blanks, and the value for these was subtracted from test values. The A650s of wells coated with FBD were slightly lower than that for wells coated with BSA, resulting in a negative value for FBD.
Bactericidal effect of combining anti-M2 and anti-SOF2 sera.
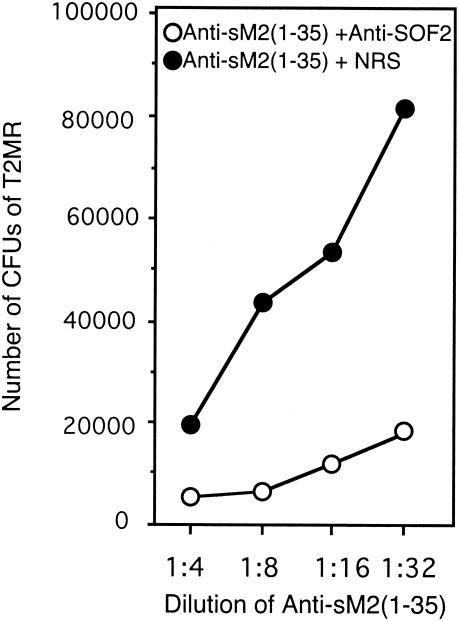
To further evaluate the potential of SOF as a vaccine candidate, the ability of anti-SOF2 serum to enhance the opsonic effect of antiserum against the M protein was assessed. Serial 1:2 dilutions of rabbit antisera against a synthetic peptide copying the first 35 amino acids from the N terminus of the M type 2 protein from S. pyogenes, anti-sM2(1-35), were added to NRS or to rabbit anti-SOF2 serum. S. pyogenes strain T2MR and nonimmune human blood were added, and the mixtures were treated as described for the bactericidal assays. Antiserum against SOF dramatically enhanced the ability of antisera against the M2 protein to opsonize and kill group A streptococci (Fig. 4).
FIG. 4.
Combined effects of anti-SOF2 serum and anti-M2 serum on opsonization of M type 2 S. pyogenes in human blood. Serial twofold dilutions of rabbit anti-sM2(1-35) serum were added to an equal volume of NRS or anti-SOF2. An inoculum of ∼175 CFU and nonimmune human blood were added. The mixtures were rotated for 3 h, and the numbers of CFU were determined as described in Materials and Methods. When used alone without anti-sM2(1-35) serum, anti-SOF2 serum killed 33% of the streptococci. Note that the concentration of anti-SOF2 serum used in this experiment is one-half of that used in the experiments shown in Fig. 3.
Mouse toxicity and protection experiment.
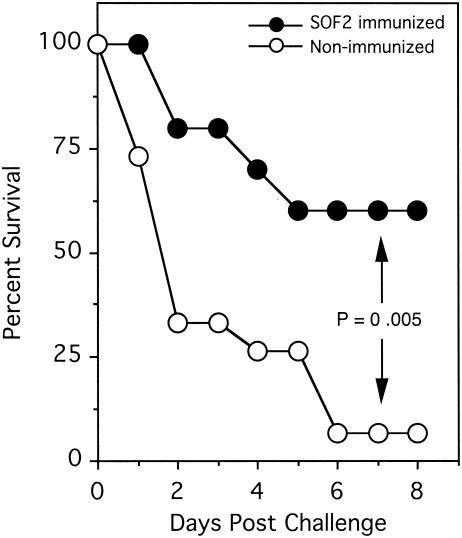
The mouse toxicity and protection experiment was initially designed to determine if SOF was toxic to mice. Five mice were injected i.v. with 100 μg of SOF2-H(38-1047) and five mice were injected i.v. with 100 μg of SOF2-H(494-1047). SOF2-H(38-1047) encompasses the mature SOF2 protein and opacifies serum. SOF2-H(494-1047) does not opacify serum and served as a negative control. None of the mice exhibited any visible signs of illness, indicating that SOF2 is not overtly toxic to mice under these conditions. The mice were then used to determine if vaccination against SOF2 would protect against group A streptococcal infections. The mice were boosted by an i.p. injection of SOF2-H(494-1047) and challenged i.p. with ∼5 × 107 CFU of M type 2 strain T2MR 11 days later. As a negative control, 15 nonimmunized mice were also challenged i.p. with T2MR. There was no difference in survival rate between mice immunized with SOF2-H(494-1047) and mice immunized with SOF2-H(38-1047); therefore, the two groups were combined. Thus, only 4 of the 10 mice immunized with SOF2 died, whereas, 14 of the 15 mice that were not immunized died (Fig. 5). These results suggest that immunization with SOF2 protects mice against infections by SOF-positive group A streptococci.
FIG. 5.
Survival plots demonstrating that immunization of mice with SOF2 protects against challenge infections with SOF-positive group A streptococci. Groups of five mice were immunized by i.v. injections of SOF2(38-1047) or SOF2(494-1047). Ten days later all 10 immunized mice received an i.p. injection of SOF2(494-1047). At day 21 the immunized mice were challenged i.p. with ∼5 × 107 CFU of S. pyogenes strain T2MR. Nonimmunized control mice received an i.p. injection of ∼5 × 107 CFU. Both groups of mice that were immunized were combined since there was no difference in their rates of survival. The difference in survival between immunized and nonimmunized mice was significant (Fisher's exact test; P = 0.005).
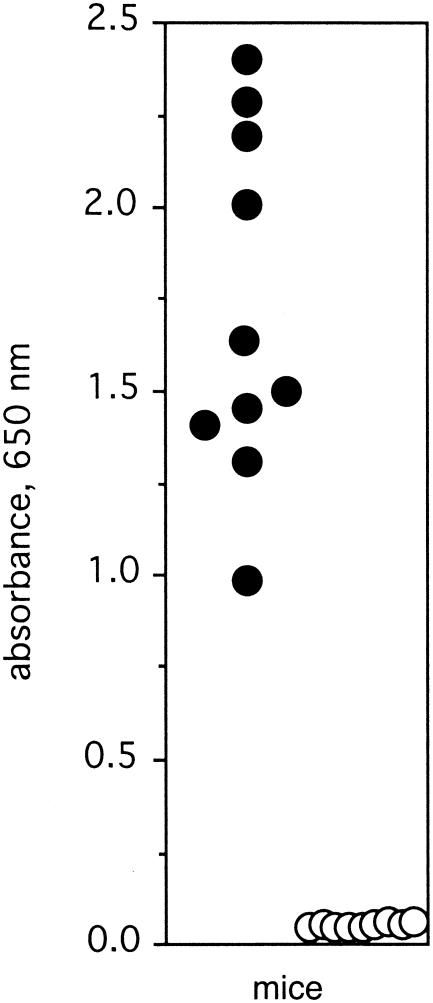
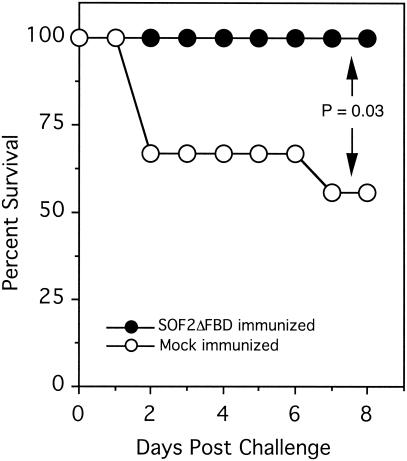
Next, we wanted to determine if the FBD of SOF was required to induce protection in mice. Ten mice were immunized with SOF2ΔFBD in CFA, and 9 mice were mock immunized with CFA. After a booster injection, blood was obtained from the tail veins of mice and tested for antibodies against SOF. The immunized mice developed significant levels of antibodies against SOF2ΔFBD, whereas the mock-immunized mice did not (Fig. 6). All of the mice were challenged i.p. with ∼1 × 107 CFU of T2MR, and the number of surviving mice was monitored daily. None of the immunized mice died, whereas four of the nine mock-immunized mice died (Fig. 7). These data provide additional evidence that SOF induces a protective immune response and that the FBD of SOF is not required for this response.
FIG. 6.
Antibody levels in mice immunized with SOF2ΔFBD. Ten mice were immunized with SOF2ΔFBD (solid circles) and 9 mice were mock immunized (open circles) as described in Materials and Methods. Serum was collected from the tail vein of each mouse, diluted 1:1,000, and tested for reactivity with SOF2ΔFBD in ELISA as described in Materials and Methods. Each circle represents a single mouse.
FIG. 7.
Survival plots demonstrating that immunization of mice with SOF2ΔFBD protects against infections from SOF-positive group A streptococci. Ten mice were subcutaneously immunized with SOF2ΔFBD and 9 mice were mock immunized as described in Materials and Methods. The mice were challenged by an i.p. injection of ∼1 × 107 CFU of S. pyogenes strain T2MR, and the number of surviving mice was determined daily. The difference in survival between SOF2ΔFBD-immunized mice and mock-immunized mice was significant (Fisher's exact test; P = 0.03).
DISCUSSION
In this report, SOF is shown to evoke a protective or bactericidal immune response by three independent experiments. First, immunization with SOF evoked opsonic antibodies in rabbits. Second, purified, human antibodies against SOF opsonized and killed S. pyogenes in human blood. Third, immunization of mice with SOF protected them against death from a challenge infection by SOF-positive streptococci.
Antibodies, in general, can provide protection against infections by several different mechanisms. Antibodies may bind to an adhesin on the surface of an organism and block adhesion of the bacteria to host cells, or antibodies may neutralize the function of a virulence factor. Alternatively, antibodies may opsonize bacteria. In the present case, the possibility of an antiadhesive effect can be excluded because the mice were challenged i.p., which effectively bypasses the adherence and colonization stage of an infection. We cannot rule out the possibility that neutralization of the opacity reaction of SOF may reduce virulence. However, our findings that rabbit antisera against SOF opsonized and killed SOF-positive S. pyogenes but did not neutralize the opacity reaction of SOF suggest that the protection afforded by immunization with SOF is most likely due to opsonic antibodies and not neutralizing antibodies. Others have also reported the lack of neutralizing antibodies in the serum of SOF-immunized animals (29).
Although our data suggest that protection from infection is due to opsonic antibodies that recognize SOF, other mechanisms may come into play under different conditions. For example, if mice were challenged intranasally, then antibodies that block adhesion could be protective. Recent experiments indicate that SOF may mediate the adhesion of group A streptococci to certain types of host cells (H. Courtney, unpublished data) and raise the possibility that antibodies against SOF may be able to prevent adhesion.
Antibodies against SOF2 not only opsonized the homologous M type 2 strain but also opsonized and killed M type 4 and 28 S. pyogenes. These data indicate that SOF contains common epitopes that can induce cross-reactive opsonic antibodies that recognize SOF on the surfaces of M type 2, 4, and 28 S. pyogenes. To our knowledge, this is the first instance in which a protective antigen in M type 4 S. pyogenes has been identified. It has been previously reported that the M type 4 protein of S. pyogenes did not confer resistance to phagocytosis (24) and that antiserum against the M type 4 protein was not opsonic (6). It is noteworthy that many of the serotypes of S. pyogenes that are poorly opsonized by antisera against M proteins are SOF positive (6, 23).
The common or shared epitope(s) of SOF that evokes cross-opsonic antibodies has not yet been identified. The FBD of SOF is an obvious candidate, especially since Schulze et al. (43) found that intranasal immunization of mice with the FBD of SfbI protected against an intranasal challenge of group A streptococci. Other domains of SfbI failed to stimulate a protective response. The primary protective effect was judged to be due to immunoglobulin A antibodies against the FBD of SfbI, which blocked adhesion of the streptococci to host cells (43). We have demonstrated that the FBD of SOF is not necessary for the induction of opsonic antibodies. Thus, epitopes outside of the FBD of SOF can induce protective antibodies. Whether the FBD of SOF may also induce opsonic antibodies remains to be demonstrated.
SOF is also produced by Staphylococcus epidermidis and by group C streptococci (9, 19), which can cause infections of the respiratory tract and skin in humans. The sequence of SOF from a group C streptococcus, Streptococcus dysgalactiae, shares many domains with SOF from group A streptococci (9), and antiserum against SOF from group A streptococci cross-reacts with SOF from group C streptococci (H. Courtney, unpublished data). Thus, the usefulness of SOF as a vaccine may extend to other pathogenic species of gram-positive bacteria besides S. pyogenes. Moreover, some of the SOF-producing bacteria cause infections in both humans and animals, suggesting that SOF may also be utilized to help prevent infections in animals.
In summary, we have shown that antisera against SOF can opsonize SOF-positive streptococci in human blood and protect mice against streptococcal infections. The finding that antisera against one type of SOF can opsonize both homologous and heterologous SOF-positive serotypes of group A streptococci suggests that different serotypes of SOF contain a shared epitope(s) that evokes opsonic antibodies. The potential impact of a vaccine that targets this epitope(s) could be significant when one considers that almost one-half of all clinical isolates and 45% of invasive strains of group A streptococci in the United States are SOF positive (35). A further indication of the potential effectiveness of SOF as a vaccine candidate is provided by the finding that antiserum against SOF dramatically enhanced the opsonic efficiency of anti-M protein serum. It is envisioned that the identification of a common protective epitope(s) of SOF could lead to its incorporation in current vaccines to broaden their protective coverage and effectiveness.
Acknowledgments
This study was supported by research funds from the U.S. Department of Veterans Affairs and from the U.S. Public Health Service, grant AI-10085 (J.B.D.).
We express our appreciation for the expert technical assistance of Yi Li and Edna Chiang.
Editor: F. C. Fang
REFERENCES
- 1.Batzloff, M., W. Hayman, M. Davies, M. Zeng, S. Pruksakorn, E. Brandt, and M. F. Good. 2003. Protection against group A streptococcus by immunization with J8-diphtheria toxoid: contribution of J8- and diphtheria toxoid-specific antibodies to protection. J. Infect. Dis. 187:1598-1608. [DOI] [PubMed] [Google Scholar]
- 2.Beachey, E. H., M. Bronze, J. B. Dale, W. Kraus, T. Poirier, and S. Sargent. 1988. Protective and autoimmune epitopes of streptococcal M proteins. Vaccine 6:192-196. [DOI] [PubMed] [Google Scholar]
- 3.Beall, B., G. Gherardi, M. Lovgren, R. Facklam, B. Forwick, and G. Tyrrell. 2000. emm and sof gene sequence variation in relation to serological typing of opacity-factor positive group A streptococci. Microbiology 146:1195-1209. [DOI] [PubMed] [Google Scholar]
- 4.Bessen, D., and V. A. Fischetti. 1988. Influence of intranasal immunization with synthetic peptides corresponding to conserved epitopes of M protein on mucosal colonization by group A streptococci. Infect. Immun. 56:2666-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodner, S., S. Morshed, and B. Peterson. 2001. The question of PANDAS in adults. Biol. Psychiatry 49:807-810. [DOI] [PubMed] [Google Scholar]
- 6.Brandt, E., T. Teh, W. Relf, R. Hobb, and M. F. Good. 2000. Protective and nonprotective epitopes from Australian aboriginal isolates and reference strains of group A streptococci. Infect. Immun. 68:6587-6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bronze, M., H. S. Courtney, and J. B. Dale. 1992. Epitopes of group A streptococcal M protein that evoke cross-protective local immune response. J. Immunol. 148:888-893. [PubMed] [Google Scholar]
- 8.Courtney, H. S., D. L. Hasty, and J. B. Dale. 2002. Molecular mechanisms of adhesion, colonization, and invasion of group A streptococci. Ann. Med. 34:77-87. [DOI] [PubMed] [Google Scholar]
- 9.Courtney, H. S., D. L. Hasty, Y. Li, H. C. Chiang, J. L. Thacker, and J. B. Dale. 1999. Serum opacity factor is a major fibronectin-binding protein and virulence determinant of M type 2 Streptococcus pyogenes. Mol. Microbiol. 32:89-98. [DOI] [PubMed] [Google Scholar]
- 10.Courtney, H. S., J. B. Dale, and D. L. Hasty. 2002. Mapping the fibrinogen-binding domain of serum opacity factor of group A streptococci. Curr. Microbiol. 44:236-240. [DOI] [PubMed] [Google Scholar]
- 11.Cu, G., S. Mezzano, J. Bannan, and J. B. Zabriskie. 1998. Immunohistochemical and serological evidence for the role of streptococcal proteinase in acute post-streptococcal glomerulonephritis. Kidney Int. 54:819-826. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham, M. W. 1996. Streptococci and rheumatic fever, p. 13-66. In N. R. Rose and H. Friedman (ed.), Microorganisms and autoimmune disease. Plenum Publishing Corp., New York, N.Y.
- 13.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:89-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dale, J. B., and E. H. Beachey. 1987. Human cytotoxic T lymphocytes evoked by group A streptococcal M proteins. J. Exp. Med. 166:1825-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dale, J. B., and E. H. Beachey. 1986. Sequence of myosin cross-reactive epitopes of streptococcal M protein. J. Exp. Med. 164:1785-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dale, J. B., E. Chiang, S Liu, H. Courtney, and D. Hasty. 1999. New protective antigen of group A streptococci. J. Clin. Investig. 103:1261-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dale, J. B., M. Simmons, E. C. Chiang, and E. Y. Chiang. 1996. Recombinant octavalent group A streptococcal M protein vaccine. Vaccine 14:944-948. [DOI] [PubMed] [Google Scholar]
- 18.Dale, J. B., R. W. Baird, H. S. Courtney, D. L. Hasty, and M. S. Bronze. 1994. Passive protection of mice against group A streptococcal pharyngeal infection by lipoteichoic acid. J. Infect. Dis. 169:319-323. [DOI] [PubMed] [Google Scholar]
- 19.El-Tayeb, S., and E. Nasr. 1977. Serum opacity factor of Staphylococcus epidermidis. Infect. Immun. 15:335-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischetti, V. A. 1989. Streptococcal M protein: molecular design and biological behavior. Clin. Microbiol. Rev. 2:285-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillen, C., R. Towers, D. McMillan, A. Delvecchio, K. Sriprakash, B. Currie, B. Kreikemeyer, G. Chhatwal, and M. Walker. 2002. Immunological response mounted by aboriginal Australians living in the northern territory of Australia against Streptococcus pyogenes serum opacity factor. Microbiology 148:169-178. [DOI] [PubMed] [Google Scholar]
- 22.Guzman, C., S. Talay, G. Molinari, E. Medina, and G. Chhatwal. 1999. Protective immune response against Streptococcus pyogenes in mice after intransal vaccination with the fibronectin-binding protein SfbI. J. Infect. Dis. 179:901-906. [DOI] [PubMed] [Google Scholar]
- 23.Hu, M. C., M. Walls, S. Stroop, M. Reddish, B. Beall, and J. B. Dale. 2002. Immunogenicity of a 26-valent group A streptococcal vaccine. Infect. Immun. 70:2171-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Husmann, L., J. Scott, G. Lindahl, and L. Stenberg. 1995. Expression of Arp protein, a member of the M protein family, is not sufficient to inhibit phagocytosis of Streptococcus pyogenes. Infect. Immun. 63:345-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji, Y., B. Carlson, A. Kondagunta, and P. Cleary. 1997. Intransal immunization with C5a peptidase prevents nasopharyngeal colonization of mice by the group A streptococcus. Infect. Immun. 65:2080-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapur, V., J. Maffei, R. Greer, L. Li, G. Adams, and J. Musser. 1994. Vaccination with streptococcal extracellular cysteine protease (interleukin-1 beta convertase) protects mice against challenge with heterologous group A streptococci. Microb. Pathog. 16:443-450. [DOI] [PubMed] [Google Scholar]
- 27.Kawabata, S., E. Kunitmo, Y. Terao, I. Nakagawa, K. Kikuchi, K. Totsuka, and S. Hamada. 2001. Systemic and mucosal immunizations with fibronectin-binding protein FBP54 induce protective immune responses against Streptococcus pyogenes challenge in mice. Infect. Immun. 69:924-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreikemeyer, B., S. Talay, and G. S. Chhatwal. 1995. Characterization of a novel fibronectin-binding surface protein in group A streptococci. Mol. Microbiol. 17:137-145. [DOI] [PubMed] [Google Scholar]
- 29.Kreikemeyer, B., D. R. Martin, and G. S. Chhatwal. 1999. SfbII protein, a fibronectin binding surface protein of group A streptococci, is a serum opacity factor with high serotype-specific apolipoproteinase activity. FEMS Microbiol. Lett. 178:305-311. [DOI] [PubMed] [Google Scholar]
- 30.Lancefield, R. C. 1959. Persistence of type-specific antibodies in man following infection with group A streptococci. J. Exp. Med. 110:271-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCormick, J. K., T. Tripp, S. Olmsted, Y. Matsuka, P. Gahr, D. Ohlendorf, and P. M. Schlievert. 2000. Development of streptococcal pyrogenic exotoxin C vaccine toxoids that are protective in the rabbit model of toxic shock syndrome. J. Immunol. 165:2306-2312. [DOI] [PubMed] [Google Scholar]
- 32.McLellan, D. G., E. Chiang, H. Courtney, D. Hasty, S. Wei, M. Hu, M. Walls, J. Bloom, and J. Dale. 2001. Spa contributes to the virulence of type 18 group A streptococci. Infect. Immun. 69:2943-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medina, E., S. Talay, G. Chhatwal, and C. Guzman. 1998. Fibronectin-binding protein I of Streptococcus pyogenes is a promising adjuvant for antigens delivered by mucosal route. Eur. J. Immunol. 28:1069-1077. [DOI] [PubMed] [Google Scholar]
- 34.Murphy, M. L., and M. E. Pichero. 2002. Prospective identification and treatment of children with pediatric autoimmune neuropsychiatric disorder associated with group A streptococcal infection (PANDAS). Arch. Pediatr. Adolesc. Med. 156:356-361. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien, K., B. Beall, N. Barret, P. Cieslak, A. Reingold, M. Farley, R. Danila, E. Zell, R. Facklam, B. Schwartz, and A. Schuchat. 2002. Epidemiology of invasive group A streptococcus disease in the United States, 1995-1999. Clin. Infect. Dis. 35:268-276. [DOI] [PubMed] [Google Scholar]
- 36.Olive, C., M. Batzloff, A. Horvath, A. Wong, T. Clair, P. Yarwood, I. Toth, and M. F. Good. 2002. A lipid core peptide construct containing a conserved region determinant of group A streptococcal M protein elicits heterologous opsonic antibodies. Infect. Immun. 70:2734-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olive, C., T. Clair, P. Yarwood, and M. F. Good. 2002. Protection of mice from group A streptococcal infection by intransasal immunization with a peptide vaccine that contains a conserved M protein B cell epitope and lacks a T cell autoepitope. Vaccine 20:2816-2825. [DOI] [PubMed] [Google Scholar]
- 38.Pruksakorn, S., A. Galbraith, R. Houghten, and M. F. Good. 1992. Conserved T and B cell epitopes on the M protein of group A streptococci: induction of bactericidal antibodies. J. Immunol. 149:2729-2735. [PubMed] [Google Scholar]
- 39.Rakonjac, J., J. Robbins, and V. Fischetti. 1995. DNA sequence of the serum opacity factor of group A streptococci: identification of a fibronectin-binding repeat domain. Infect. Immun. 63:622-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roggiani, M., J. Stoehr, S. Olmsted, Y. Matsuka, S. Pillai, D. Ohlendorf, and P. Schlievert. 2000. Toxoids of streptococcal pyrogenic exotoxin A are protective in rabbit models of streptococcal toxic shock syndrome. Infect. Immun. 68:5011-5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salvadori, L. G., M. Blake, M. McCarty, J. Tai, and J. B. Zabriskie. 1995. Group A streptococcus-liposome ELISA antibody titers to group A polysaccharide and opsonophagocytic capabilities of the antibodies. J. Infect. Dis. 171:593-600. [DOI] [PubMed] [Google Scholar]
- 42.Saravani, G., and D. Martin. 1990. Opacity factor from group A streptococci is an apoproteinase. FEMS Microbiol. Lett. 68:35-40. [DOI] [PubMed] [Google Scholar]
- 43.Schulze, K., E. Medina, S. Talay, R. Towers, G. Chhatwal, and C. Guzman. 2001. Characterization of the domain of fibronectin-binding protein I of Streptococcus pyogenes responsible for elicitation of a protective immune response. Infect. Immun. 69:622-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stalhammar-Carlemalm, M., T. Areschoug, C. Larsson, and G. Lindahl. 1999. The R28 protein of Streptococcus pyogenes is related to several group B streptococcal surface proteins, confers protective immunity and promotes binding to human epithelial cells. Mol. Microbiol. 33:208-219. [DOI] [PubMed] [Google Scholar]
- 45.Swedo, S. E. 2002. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS). Mol. Psychiatry. 7:S24-S25. [DOI] [PubMed] [Google Scholar]
- 46.Ward, H. K., and G. V. Rudd. 1938. Studies on haemolytic streptococci from human sources. Aust. J. Exp. Biol. Med. Sci. 16:181-192. [Google Scholar]
- 47.Widdowson, J., W. Maxted, and D. Grant. 1970. The production of opacity in serum by group A streptococci and its relation with the presence of M antigen. J. Gen. Microbiol. 61:343-353. [DOI] [PubMed] [Google Scholar]