Abstract
A mutant strain of Streptococcus uberis (AJS001) that was unable to grow in bovine milk was isolated following random insertional mutagenesis. The level of growth in milk was restored to that of the parental strain (strain 0140J) following addition of MnSO4 but not following addition of other metal ions. The mutant contained a single insertion within mtuA, a homologue of mtsA and psaA, which encode metal-binding proteins in Streptococcus pyogenes and Streptococcus pneumoniae, respectively. Strain AJS001 was unable to infect any of eight quarters on four dairy cows following intramammary challenge with 105 CFU. Bacteria were never recovered directly from milk of these animals but were detected following enrichment in Todd-Hewitt broth in three of eight milk samples obtained within 24 h of challenge. The animals showed no inflammatory response and no signs of mastitis. Three mammary quarters on two different animals simultaneously challenged with 600 CFU of the parental strain, strain 0140J, became colonized, shed high numbers of S. uberis organisms in milk, displayed a marked inflammatory response to infection, and showed overt signs of mastitis. These data indicate that mtuA was required for efficient uptake of Mn2+ during growth in bovine milk and infection of the lactating bovine mammary gland.
Bovine mastitis (inflammation of the udder) usually arises as a result of intramammary infection by bacteria (15). Over 135 infectious agents have been associated with clinically apparent episodes of mastitis (49), but the vast majority of cases are due to infection with one of five bacterial species. Those most commonly implicated are Streptococcus uberis, Streptococcus dysgalactiae, Streptococcus agalactiae, Escherichia coli, and Staphylococcus aureus (19). Mastitis is one of the most common infectious diseases of dairy cattle. In the United Kingdom, it occurs at a frequency of between 35 and 40 cases per 100 cows per year, and the annual cost is around £170 million (27). Worldwide, the disease has been estimated to cause losses of more than several billion dollars. Manifestations of the disease can range from visible abnormalities in the milk (protein aggregates or clots) to production of a secretion that is composed solely of aggregated protein in a serous fluid and often results in pain and swelling in the affected gland.
Milk from an uninfected gland contains leukocytes, including macrophages, neutrophils, and lymphocytes, typically at concentrations of less than 150,000 cells/ml. Infection usually results in an inflammatory response which leads to an increase in the number of cells, primarily due to the influx of neutrophils from the peripheral circulation (39). Milk from clinically infected quarters usually contains in excess of 2 × 106 cells/ml, more than 90% of which are neutrophils (38). The inflammatory reaction and the increase in the number of neutrophils result in a lower rate of milk production and in gross deterioration of the quality of the secretion.
The ability of mastitis-causing bacteria to grow in mammary gland secretions has been correlated with infection of this organ. Within the lactating gland S. uberis is able to replicate to around 107 to 108 CFU/ml of milk, resist the bactericidal action of neutrophils, and induce an inflammatory response (12, 13, 17, 32). During a clinical episode of disease it is not uncommon for neutrophils and bacteria to coexist within the secretion at a level of 107 CFU/ml of milk (12, 13, 17, 32). In addition to being able to infect the lactating mammary gland, S. uberis is also able to infect the nonlactating or dry gland. The susceptibility of the udder to infection with S. uberis is relatively low at the end of lactation but increases as the dry period progresses (36). The increase in susceptibility correlates directly with an increase in the ability of S. uberis to grow in dry cow secretions (9). The precise interactions between host and pathogen that allow growth in milk and other mammary gland secretions and that promote infection and disease during both the lactating and nonlactating periods have not been fully described.
It has been shown that S. uberis is able to activate bovine plasminogen through the action of the secreted protein PauA (23, 29). It has been hypothesized that activation of plasminogen to plasmin and acquisition of plasmin at the bacterial surface (33) may facilitate growth of this auxotrophic bacterium (42) in milk due to the release of peptides and amino acids from host proteins (30, 50). In support of this hypothesis, it has been shown that S. uberis can utilize plasmin-derived peptides from casein as a source of essential amino acids (42) and that immunization, which is capable of inducing a neutralizing response to PauA, can reduce colonization of a lactating gland by S. uberis following experimental challenge (32).
The ability to produce mutations at random within S. uberis has been exploited for identification of genes responsible for capsulation (48) and has enabled the role of capsule to be investigated in vivo in the target species (11). A mutant strain that was unable to utilize specific tri- and oligopeptides as sources of essential amino acids due to an insertion within oppF (encoding an ATPase associated with the oligopeptide permease operon of S. uberis) was found to grow poorly in milk compared to its isogenic parent (42). However, the effect of this lesion on the ability of S. uberis to infect the bovine mammary gland and induce mastitis has not been investigated.
In the present investigation we exploited the ability to produce mutations within S. uberis to identify a single gene essential for growth in milk and to determine the role of this gene in colonization and virulence for the lactating mammary gland.
MATERIALS AND METHODS
Bacterial strains and reagents.
S. uberis strain 0140J, originally isolated from a clinical case of bovine mastitis in the United Kingdom, was used throughout this study. The bacterium was routinely grown in Todd-Hewitt broth. Other bacteria and media used are described below.
Skim milk was produced from raw bovine milk collected aseptically from several cows in the dairy herd at the Institute for Animal Health. Milk was collected from animals that did not have intramammary infections. Following centrifugation (3,000 × g, 10 min), skim milk was removed carefully from the upper fat layer and the pellet of sedimented cells. The sterility of the skim milk was determined by plating 500 μl of milk directly onto blood agar containing 1.0% (wt/vol) esculin (ABA) and by enrichment culturing of 5 ml of the milk in an equal volume of Todd-Hewitt broth, followed by isolation of single colonies on ABA. In both cases, plates were incubated at 37°C for 18 h. Skim milk was stored at 4°C and used within 72 h.
Other bacterial strains and reagents were used as described below. Oligonucleotide primers for PCR amplification are referred to below by their designations, and the sequence of each primer is shown in Table 1.
TABLE 1.
Primers used for PCR amplification and their applications in this investigation
| Primer | Sequence | Application | Template | Annealing temp (°C) |
|---|---|---|---|---|
| P042 | 5′-GAAAATCTCATCCTCCGGGGC | Screening recombinant clones and mtuA sequencing | pCALnFLAG-rmtuA plasmids | 55 |
| P043 | 5′-GGATCTAAGCTTGAGCTCGAG | |||
| P064 | 5′-GGATCTAAGCTTGAGCTCGAG | ISS1 primers for Vectorette PCR amplification | Genomic S. uberis 0140J Vectorette libraries | 52 |
| P082 | 5′-CCAACAGCGACAATAATCACATC | |||
| P182 | 5′-CTTGGAGCTTGTTCAGTAGG | PCR amplification of mtuA open reading frame | Genomic S. uberis 0140J | 50 |
| P183 | 5′-TCCATTTCATCATTGCATAA | |||
| P204 | 5′-GAGGAGGAGAAGTGTTCAGTAGGAAATGGTAGA | Ligation-independent cloning expression of mtuA | Genomic S. uberis 0140J | 50 |
| P185 | 5′-GGAACAAGACCCGTATGTGATATTGTGCTTTATTTT |
Random mutagenesis in S. uberis.
The procedures described previously by Maguin et al. (35) were used to generate a bank of random mutants (42, 48). Transformation of S. uberis 0140J with plasmid pGh9:ISS1 was performed by using a Gene Pulser apparatus (Bio-Rad) and cuvettes with a path length of 0.1 cm. Parameters of 25 μF, 2.4 kV, and 100 Ω generated pulse time constants on the order of 2 ms. Erythromycin-resistant transformants were selected following growth at 28°C. Chromosomal integration of pGh9:ISS1 was achieved by 100-fold dilution of an overnight culture of S. uberis 0140J/pGh9:ISS1 in Todd-Hewitt broth lacking erythromycin and incubation for 3.5 h at 28°C (to an optical density at 550 nm of 0.1). The culture was then transferred to 37.5°C and incubated for a further 2.5 h, after which glycerol was added to a final concentration of 15% and aliquots were stored at −70°C.
Aliquots from the bank of random insertion mutants were diluted in Todd-Hewitt broth at 37°C and spread onto prewarmed Todd-Hewitt agar (310 ml in 220-mm square petri dishes) at a density of approximately 2,500 CFU per plate. The plates were incubated overnight at 37°C, and 8,640 single colonies were transferred into individual wells of 96-well microtiter trays containing Todd-Hewitt broth (100 μl) and glycerol (10%, vol/vol) by using a Flexys robotic workstation (Genomic Solutions, Ann Arbor, Mich.). Cultures were grown overnight at 37°C and stored at −70°C.
Selection of mutants that failed to grow in milk.
A total of 2,400 mutants were transferred from the bank into the corresponding wells of 96-well microtiter trays containing 140 μl of Todd-Hewitt broth (50% [vol/vol] in a solution containing 2.0 g of sterile glucose per liter and 1 mg of erythromycin per liter). After overnight incubation at 37°C, the cultures were replica plated into 96-well microtiter trays containing 240 μl of skim milk supplemented with erythromycin (1 mg/liter). Following overnight incubation at 37°C, bacterial growth in milk was assessed by addition of 20 μl of bromocresol purple (BDH, Poole, United Kingdom). Cultures showing reduced growth in milk (i.e., no color change in response to a lower pH) were selected for further investigation.
Curing of plasmid sequences from the insertional mutant.
The plasmid vector sequence was excised from the chromosome of selected mutant strains by permitting plasmid replication during overnight growth in a broth culture at 28°C in the absence of antibiotic selection, as described previously (35, 42, 48). Briefly, broth cultures were diluted, and plate counting was performed on Todd-Hewitt agar in the presence and absence of erythromycin (1 mg/liter) at the nonpermissive temperature (37°C). Excision of the vector sequence was confirmed by replica plating. The retention of a single copy of the IS element was verified by Southern blotting of DNA prepared from cultures derived from single erythromycin-sensitive colonies by using a probe directed against the insertion sequence. All subsequent studies were conducted with the cured derivative of AJS001.
Extraction of chromosomal DNA from S. uberis.
Genomic DNA was prepared by using a variation of the method of Hill and Leigh (18), as described previously (48). Briefly, 1.5 ml of an overnight culture was centrifuged at 10,000 × g for 2 min, and the cell pellet was washed with 500 μl of 10 mM Tris-Cl-5 mM EDTA (pH 7.8). Bacterial cell walls were disrupted by resuspension in 375 μl of 10 mM Tris-Cl-5 mM EDTA (pH 7.8) containing 30 U of mutanolysin (Sigma) per ml and 10 mg of lysozyme (Sigma) per ml and subsequent incubation at 37°C for 30 min. Total cell lysis was achieved by addition of 20 μl of a sodium dodecyl sulfate (SDS) solution (20% [wt/vol] SDS in 50 mM Tris-Cl-20 mM EDTA [pH 7.8]) and proteinase K (Sigma) to a final concentration of 150 μg/ml and incubation at 37°C for 1 h. Cell wall material was removed by precipitation following the addition of 200 μl of saturated NaCl and subsequent centrifugation at 12,000 × g for 10 min. The supernatant was extracted with phenol-chloroform, and the DNA was precipitated by addition of 2 volumes of absolute ethanol. DNA pellets were washed with 70% aqueous ethanol and air dried prior to resuspension in Tris-EDTA buffer containing 20 μg of RNase A (Sigma) per ml.
Identification and sequencing of the insertionally inactivated gene.
Sequences flanking the insertion in mutant AJS001 were identified by PCR amplification from chromosomal DNA libraries constructed with the Vectorette system (Sigma-Genosys) by using the methods described by Arnold and Hodgson (2). Genomic libraries were constructed by using chromosomal DNA from the cured derivative of AJS001 and the wild type (strain 0140J). These libraries were subjected to PCR by using primers supplied with the Vectorette kit and/or primers directed at either ISS1 (P064 or P082) or a previously generated sequence (P182 and P183) (Table 1). Sequencing reactions were performed directly with DNA amplified by PCR following purification with DNA Purification Kit II spin columns (Hybaid).
Cloning of mtuA in E. coli.
A truncated form of mtuA (′mtuA) was amplified by PCR by using primer P204 (designed to start from the cysteine residue in the LraI common prolipoprotein recognition sequence LXXC [21], as described by Pilling et al. [40]), primer P185 (50 pmol), each deoxynucleoside triphosphate at a concentration of 200 μM, and 2 U of Pfu polymerase (Stratagene) in 50 μl of PCR buffer (Stratagene). The reaction consisted of 30 cycles of denaturation for 1 min at 95°C, annealing for 1 min at 50°C, and extension for 2 min at 72°C.
To facilitate expression cloning, ligation-independent cloning sticky ends were generated at each end of the purified PCR product with a DNA Purification Kit II (Hybaid). Purified DNA (75 fmol) was incubated with Pfu DNA polymerase (1 U), dATP (1 mM), and Pfu DNA polymerase reaction buffer in a 10-μl (final volume) mixture at 72°C for 15 min and then placed on ice for 2 min. Twenty nanograms of plasmid CAL-n-FLAG (a ligation-independent cloning vector; Stratagene) was added to the insert preparation and incubated for 1 h at room temperature according to the manufacturer's instructions.
One Shot TOP10 chemically competent E. coli (Invitrogen) was transformed with approximately 50 ng of the resulting construct. Transformants were selected on Luria-Bertani agar containing 100 μg of ampicillin per ml.
Plasmid DNA was prepared with a QIAprep Miniprep kit (Qiagen Ltd.) and was transformed into E. coli BL21-Gold (DE3)/pLysS (Novagen, Madison, Wis.). The presence of the desired insert in the expression host strain was confirmed by PCR by using primers P042 and P043 directed towards sequences flanking the cloning site within the pCAL-n-FLAG vector.
Expression and purification of recombinant ′MtuA-Cal-n-FLAG fusion protein.
One colony of E. coli BL21-Gold (DE3)/pLysS/pCAL-n-FLAG::′mtuA was inoculated into Luria-Bertani medium (250 ml) containing ampicillin and chloramphenicol (100 and 50 μg/ml, respectively) and grown at 37°C in an orbital incubator (100 rpm) to the logarithmic phase (approximately 3 h). Isopropyl-β-d-thiogalactopyranoside (IPTG) was added to the culture to a final concentration of 1 mM, and the culture was grown until the stationary phase was reached (3 to 6 h). Bacteria were harvested by centrifugation (15,000 × g, 5 min) and suspended in 10 ml of binding buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1 mM imidazole, 2 mM CaCl2). Bacteria were lysed by sonic disruption (Heat Systems-Ultrasonic Inc., New York, N.Y.), and the unbroken cells and debris were removed by centrifugation (15,000 × g, 5 min). The clarified cell lysate was loaded onto 2 ml (packed volume) of calmodulin affinity resin (Stratagene) that had been preequilibrated by three washes with 10 volumes of binding buffer. The final volume of the mixture was adjusted to 25 ml by adding binding buffer, and the mixture was incubated at 4°C for 18 h with constant end-over-end rotation. The loaded resin was precipitated by centrifugation (1,000 × g, 2 min), and the resin was resuspended and washed three times in 10 volumes of binding buffer. Protein was eluted from the resin in batch fashion by mixing with an equal volume of elution buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 10 mM β-mercaptoethanol, 2 mM EGTA), incubation at room temperature for 5 min, and precipitation of the resin by centrifugation (1,000 × g, 2 min). The elution procedure was repeated three times to ensure complete recovery of the bound protein, and the resulting supernatants were pooled, dialyzed at 4°C for 18 h against 200 volumes of distilled water, and stored at −20°C.
Samples (20 μl) were mixed with 2 volumes of SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer and analyzed by SDS-PAGE by using 10% polyacrylamide ready gels (Bio-Rad). Following electrophoresis, the proteins were transferred to nitrocellulose and detected by incubation of the membrane for 1 h at room temperature in a mouse anti-FLAG M2 antibody (Stratagene) diluted 1:500 in blocking solution (1% Marvel and 0.1% Tween 20 in phosphate-buffered saline [PBS]). The membrane was washed three times in washing solution (0.1% Tween 20 in PBS) before incubation for 1 h at room temperature in rabbit anti-mouse horseradish peroxidase-conjugated antibody (Sigma) diluted 1:1,000 in blocking solution. Following three additional washes, detection was carried out in developing solution (15 mg of 4-chloro-1-naphthol dissolved in 5 ml of methanol containing 15 μl of 30% hydrogen peroxide, made up to a final volume of 30 ml in PBS).
Production of antiserum to MtuA.
Antiserum directed against the MtuA protein was produced by repeated immunization of a single rabbit with the affinity-purified CAL-n-FLAG-′MtuA fusion protein. In brief, 10 to 20 μg of the fusion protein was mixed with the adjuvant Titer-max-Gold (Stratech Scientific Ltd., Cambs, United Kingdom) according to the manufacturer's instructions. The adjuvant-antigen combination was administered at a level of 5 to 10 μg per dose via the subcutaneous route at 28-day intervals on four occasions. Ten days after the final administration, the total blood volume was collected and allowed to coagulate overnight at 4°C, and serum was collected following centrifugation (5,000 × g, 10 min). The serum was stored in aliquots at −20°C and used to detect the MtuA protein in Western blots at a dilution of 1/1,000.
Production of extracts of S. uberis for detection of MtuA.
Bacteria were harvested from overnight cultures (20 ml) by centrifugation (12,000 × g, 5 min), and the pellets were washed three times in an equal volume of PBS. Washed cells were suspended in 500 μl of PBS and mixed with 170- to 180-μm-diameter glass beads (0.5 g; Braun Biotech International). Bacteria were disrupted by rapid agitation (2 min) by using a Cell Homogenizer-MSK (Braun Biotech International). During disruption, samples were cooled with liquid CO2. Following disruption, samples were kept on ice for 1 h in the presence of 1% (vol/vol) Triton X-100 (BDH).
Cell debris and unbroken cells were removed by centrifugation (12,000 × g, 5 min), and the supernatant was stored at −20°C. The proteins from 8 μl of each sample were separated by SDS-PAGE, and MtuA was located following Western blotting by using MtuA-specific rabbit antiserum and goat anti-rabbit-horseradish peroxidase conjugate, both at a dilution of 1/1,000, as described above.
Determination of bacterial growth in skim milk.
Skim milk (2 ml) was inoculated with washed bacterial cultures to a density of around 103 to 104 CFU/ml. The skim milk was supplemented, as indicated below, with components of a chemically defined medium (31). In each case, the supplement was added in a volume that did not exceed 500 μl, and the equivalent volume of the appropriate diluent was used as a nonsupplemented control. Growth was monitored at various times by estimating the number of viable bacteria following dilution of samples in saline, plating onto ABA, and incubation at 37°C for 18 h.
Challenge of lactating dairy cows with S. uberis 0140J and AJS001.
The role of MtuA in the pathogenesis of infection was determined by comparison of the virulence of strain 0140J and the virulence of mutant derivative AJS001 in an intramammary infection model in dairy cows. Bacteria were grown for 18 h at 37°C in Todd-Hewitt broth. Cells were recovered by centrifugation (10,000 × g, 10 min), suspended in pyrogen-free saline (Sigma), and diluted in the same to obtain the required cell density. Suspensions of each strain were kept on ice before they were used to challenge animals. The numbers of viable bacteria in identical aliquots of each suspension were determined both prior to and following challenge.
Five dairy cows that were 2 to 10 weeks into their first lactation were selected from the institute's dairy herd for challenge. The criteria for selection were absence of signs of mastitis, absence of bacteria in milk samples prior to challenge, no history of mastitis during the current lactation, and no evidence of intramammary infection in milk samples taken 7 and 14 days after parturition.
The animals were challenged in their mammary quarters by infusion of 1 ml of pyrogen-free saline (Sigma) containing S. uberis. The challenge was administered through the teat canal by using a sterile canula and syringe. Two animals were challenged in a total of three quarters with 6.0 ×102 CFU of strain 0140J, and an additional four animals were challenged in a total of eight quarters with 1.0 × 105 CFU of strain AJS001.
Following challenge, the animals were milked and inspected twice daily (at 0700 and 1600) for clinical signs of mastitis (clotted and discolored milk and/or swollen or tender udder quarters). Milk samples were taken and analyzed for bacteria and somatic cells, as described below.
Analysis of milk samples.
The number of viable bacteria present was estimated by direct plating of 1 ml and 100 μl of each milk sample onto ABA. Samples were also diluted in saline, and 50 μl of each dilution was plated directly onto ABA. In addition, milk samples (5 ml) were enriched for bacteria by mixing them with Todd-Hewitt broth (10 ml) and incubating them at 37°C for 24 h, after which cultures were plated directly for isolation of single colonies onto ABA. In each case, the presence and/or number of S. uberis cells was determined, and the genotypes of the isolates recovered were determined by comparing the restriction fragment length polymorphism of chromosomal DNA (18) and amplification of the mtuA locus, as described below.
The numbers of somatic cells present in milk samples were determined by using a Coulter Counter (Beckman Coulter, Ltd.) as described previously (12, 13, 17, 32).
Amplification of mtuA by PCR.
Individual colonies obtained from milk samples of challenged animals were transferred into Todd-Hewitt broth (30 μl), and cultures were grown at 37°C for 2 h. Samples (5 μl) were removed and placed in sterile Eppendorf tubes with 21.8 μl of sterile, deionized water and 5 μl of 10× PCR buffer (Promega). The tubes were placed in a boiling water bath for 5 min, after which they were cooled on ice for at least 2 min.
Fifty-microliter portions of oligonucleotide primers P182 and P183 (Table 1) were added to each mixture along with deoxynucleoside triphosphates (each at a final concentration of 200 μM) and 2 U of Taq polymerase (Promega). The volume of the mixture was adjusted to 50 μl by addition of sterile, deionized water.
The mtuA gene was amplified by PCR; the reaction consisted of 30 cycles of denaturation at 95°C for 1 min, annealing at 50°C for 1 min, and extension at 72°C for 2 min. The products of the PCR were separated by electrophoresis through 1% agarose and visualized by staining with ethidium bromide, followed by UV transillumination.
Nucleotide sequence accession number.
The DNA sequence of the 1.635-kb fragment containing mtuA and the flanking sequence has been deposited in the GenBank database under accession number AJ539135.
RESULTS
Screening random insertional mutants for the ability to grow in raw bovine milk.
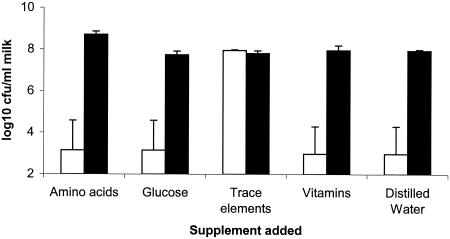
Strain AJS001 was one of three mutants isolated for their inability to grow in bovine milk following replica plating of 2,400 random insertional mutants of S. uberis from Todd-Hewitt broth. In contrast to the parental strain, the number of AJS001 cells did not increase following incubation in bovine milk for 24 h at 37°C. The ability of this mutant to grow in milk was restored by addition of trace elements but not by addition of other components from a chemically defined medium (31) able to support the growth of S. uberis (Fig. 1).
FIG. 1.
Growth of S. uberis in skim milk containing supplements from a chemically defined medium. The data are the geometric means of the numbers of bacteria detected following inoculation of skim milk containing different supplements with 103 to 104 CFU of either S. uberis 0140J (solid bars) (n = 2) or AJS001 (open bars) (n = 2) and incubation at 37°C for 24 h. The error bars indicate standard errors. Supplements were added from 10× stock solutions, and distilled water was added as a control. Glucose was added to a final concentration of 10 g/liter. The amino acid supplement contained Glu (30 mg/ml); Arg, Trp, Tyr, and His, each at a concentration of 20 mg/ml; and Phe, Met, Ile, Cys, Leu, and Val, each at a concentration of 10 mg/ml. All the amino acids were in the l configuration. The trace element supplement contained MgSO4, (2 g/liter), FeSO4 (100 mg/liter), and MnSO4 (100 mg/liter). The vitamin supplement contained riboflavin (4 mg/liter), thiamine (4 mg/liter), folate (1 mg/liter), p-aminobenzoate (1 mg/liter), pantothenate (8 mg/liter), pyridoxamine (8 mg/liter), biotin (0.1 mg/liter), and niacinamide (20 mg/liter).
Identification of components required for growth of mutant AJS001 in milk.
Growth of AJS001 in milk was restored only by addition of Mn2+; addition of other trace elements (Zn2+, Cu2+, Fe2+, Mg2+) did not stimulate bacterial growth (Fig. 2). The final cell density of AJS001 following growth in bovine milk was shown to be dependent on the amount of Mn2+ present. The maximal cell densities were obtained in the presence of 1.5 to 4 μM MnSO4. In contrast, growth of the wild-type strain was not affected by the presence of exogenous Mn2+ (Fig. 3). The rates of growth and the final cell densities of the mutant and parental strains in milk containing 4 μM Mn2+ were similar in three milk samples from three different animals (data not shown), indicating that acquisition of Mn2+ was not growth limiting for the mtuA mutant when the ion was present at this concentration.
FIG. 2.
Growth of S. uberis in skim milk containing trace metals. The data are the geometric means of the number of S. uberis 0140J CFU (solid bars) (n = 2) or AJS001 CFU (open bars) (n = 2) detected in skim milk following incubation at 37°C for 24 h. The error bars indicate standard errors. The mean number of bacteria at zero time is shown (T = 0). Each metal ion was added at a concentration of 4 μM as the sulfate salt, and distilled water was added as a diluent control.
FIG. 3.
Growth of S. uberis in skim milk containing different concentrations of Mn2+. The data are the geometric means of the number of S. uberis 0140J CFU (solid bars) (n = 3) or AJS001 CFU (open bars) (n = 5) detected in skim milk containing MnSO4 at different concentrations following incubation at 37°C for 24 h. The error bars indicate standard errors. Both strains were inoculated into milk to a density of ∼103 CFU/ml. S. uberis strain 0140J was investigated only in milk samples containing 0 and 4 μM MnSO4.
Identification of the insertionally inactivated gene in AJS001 by PCR from genomic DNA libraries.
A DNA product with a size of approximately 1.3 kb flanking the point of insertion in the chromosome of AJS001 was amplified from Vectorette library AJS001/ClaI. Analysis of this sequence revealed that it had high levels of homology (77.5 and 75.8%) to mtsA and psaA of Streptococcus pyogenes (accession no. AF180521) and Streptococcus pneumoniae (accession no. AF055088), respectively. The products of psaA and mtsA are both putative lipoproteins capable of binding and are involved in the active uptake of divalent metal ions through ABC transporter systems.
Further sequence data were obtained by chromosome walking by using primers designed based on a previously derived sequence (Table 1). The entire sequence obtained (1,635 nucleotides) was shown to exhibit homology (71.4%) to the mts locus of S. pyogenes. The sequence homology included homology over the whole mtsA gene and over the first 300 nucleotides of the mtsB gene (Fig. 4). The size of the open reading frame containing the insertion in AJS001 was 932 nucleotides. This entire open reading frame showed homology to mtsA (76%) and psaA (72%). The gene identified in S. uberis was designated mtuA (metal transporter uberis A). The mtuA open reading frame was predicted to encode a sequence of 310 amino acids, and residues 1 to 20 of this sequence corresponded to a putative leader peptide sequence. A predicted cleavage site between residues 20 and 21 was identified by the LxACy cleavage motif for signal peptidase II.
FIG. 4.
mtu ABC transporter operon of S. uberis and other LraI loci. The open reading frames whose designations end withA encode solute-binding lipoproteins; the open reading frames whose designations end with B encode ATP-binding proteins; and the open reading frames whose designations end with C encode hydrophobic membrane proteins. psaD encodes a peroxidase not encoded at comparable S. uberis or S. pyogenes loci. Expression of the mtu operon is thought to be coordinated by a metallorepressor protein homologue (Regl.) located immediately upstream of mtuA. The open arrows indicate directions of transcription, and putative hairpin loops are shown between open reading frames. The location and orientation of ISS1 in S. uberis AJS001 are also shown.
Comparison of these sequence data with the data released from the S. uberis genome project (http://www.sanger.ac.uk/Projects/S_uberis/) showed that mtuA lies within a putative ABC transporter operon similar to that found in S. pyogenes (20) and distinct from that in S. pneumoniae, Streptococcus parasanguis, Streptococcus gordonii (25), or Streptococcus mutans (24) (Fig. 4).
Sequence analysis of the inactivated gene (mtuA::ISS1) revealed that the insertion sequence element interrupted the original sequence between nucleotides 607 and 608. The predicted translation product of the mutated gene was shown to contain 209 amino acids corresponding to amino acids 1 to 202 of MtuA and an additional seven residues (G, F, C, C, K, V, F) encoded by ISS1 prior to a termination codon.
Expression of MtuA by wild-type and insertionally inactivated MtuA− mutant AJS001.
Western blotting of proteins from whole-cell lysates of strain 0140J revealed a single protein band at around 37 kDa, corresponding to the expected size of MtuA (calculated mass based on the translated amino acid sequence, 34.6 kDa). No equivalent protein was detected in lysates from the mtuA mutant strain AJS001 (Fig. 5). Sequence analysis revealed that the insertion element within the mtuA gene of AJS001 may result in synthesis of a truncated MtuA protein with a predicted mass of 23.7 kDa; however, no protein of a corresponding size was detected by immunoblotting (Fig. 5).
FIG. 5.
SDS-PAGE and Western blot analyses of whole-cell lysates of S. uberis, showing detection of MtuA. Western blotting with antiserum raised against the CAL-n-FLAG-′MtuA fusion protein (A) and SDS-PAGE (B) of whole-cell lysates from S. uberis revealed a single reactive protein band at around 37 kDa (arrow) in strain 0140J (lane 2 in panel A) and no cross-reactive proteins in strain AJS001 (lane 3 in panel A) resulting from matched sample loadings (lanes 2 and 3 in panel B). Lane 1 contained molecular weight markers, and the mass of each marker protein (in kilodaltons) is indicated on the left.
Infectivity and virulence of 0140J and AJS001 following experimental challenge in the bovine mammary gland.
A pilot experiment in which AJS001 was administered at a dose of 6.0 × 102 CFU revealed that this strain was likely to be considerably less virulent than the parental strain. To confirm this, two mammary quarters on each of four dairy cows were challenged with 105 CFU of AJS001, and three quarters on an additional two animals were challenged with 6.0 × 102 CFU of the parental strain.
Mammary quarters challenged with strain 0140J shed S. uberis at detectable levels in milk from the first milking postchallenge (approximately 14 h), and the infection persisted (>106 CFU/ml of milk) until the fifth milking (Fig. 6). At this time, intervention with antibiotic therapy was required to cure the infection and alleviate the signs of disease. In contrast, following challenge with the isogenic mtuA mutant, bacteria were not detected by direct plating of milk (<1 CFU/ml of milk) but were detected in samples (5 ml) obtained from three of the eight challenged quarters (from two animals) following enrichment culture (Fig. 6). In all cases, bacteria were shown by restriction fragment length polymorphism analysis (18) to have the correct strain lineage (data not shown). In addition, isolates obtained from animals challenged with strain 0140J were shown by PCR to yield a product corresponding to the intact mtuA gene (900 bp), and isolates obtained from animals challenged with AJS001 were shown to yield a product corresponding to insertionally inactivated mtuA::ISS1 (1,700 bp).
FIG. 6.
Inflammatory response and bacterial recovery following experimental challenge of the bovine mammary gland with S. uberis. The data are the geometric means of the number of somatic cells (A) or bacteria (B) detected in milk samples obtained before (milking 0) and after (milkings 1 to 7) challenge with S. uberis 0140J (solid bars) (n = 3) or AJS001 (open bars) (n = 8). Following challenge with strain AJS001, bacteria were detected in three of eight challenged quarters on two animals following enrichment culture (asterisks).
Mammary quarters challenged with strain 0140J exhibited an inflammatory response to infection. This was measured by an increase in the influx of neutrophils into the milk (Fig. 6). The influx of inflammatory cells in response to infection was typical of that seen previously following challenge with this strain (12, 13, 17, 32) and peaked at between 107 and 108 cells/ml of milk. The cell counts for milk from quarters challenged with 0140J were significantly higher (P < 0.001) than those obtained from quarters challenged with AJS001 from 24 h (second milking) postchallenge (Fig. 6). The cell counts for milk from quarters of animals challenged with strain AJS001 did not differ significantly from those observed prior to challenge (Fig. 6), and all counts were less than 3 × 105 cells/ml of milk for the duration of the experiment.
Mammary quarters challenged with strain 0140J developed overt clinical signs of disease (discolored and clotted milk accompanied by swollen udder quarters) that were readily detected from the fifth milking after challenge. Antibiotic therapy was required to resolve the infection and alleviate the signs of disease. In contrast, none of the eight quarters challenged with strain AJS001 showed any sign of infection or disease, and none required antibiotic therapy up to 10 days postchallenge (the duration of the experiment).
DISCUSSION
Mutant strain AJS001 contained a single genetic lesion within mtuA, a homologue of mtsA and psaA of S. pyogenes and S. pneumoniae, respectively. The phenotypic manifestation of this lesion was shown to be the inability of the organism to grow in raw bovine milk in the absence of exogenous ionic manganese. Addition of MnSO4 to milk at a final concentration of 4 μM resulted in growth at a rate and to a final cell density similar to those seen for the parental strain, strain 0140J. Subsequent studies (data not shown) identified three further mutant clones within the S. uberis 0140J mutant bank, each with a single ISS1 lesion which mapped to a different point within mtuA. The phenotypes of these strains matched that of AJS001 in terms of the inability to grow in raw bovine milk. Similarly, addition of exogenous manganese to milk enabled growth rates and final yields equivalent to those of wild-type strain 0140J to be achieved by all mtuA mutant clones, thereby strongly suggesting that the lesions observed in AJS001 and additional mutants and not additional unidentified mutations were the cause of the phenotype observed.
The observation that AJS001 is able to grow both in conventional bacteriological media, such as Todd-Hewitt broth, and in milk in the presence of exogenous ionic manganese indicates that S. uberis is able to acquire manganese from its surroundings. Our data indicate that during growth in milk S. uberis is dependent upon a different uptake system that requires the mtuA gene product. Bovine milk contains 10 to 50 μg (184 to 919 nmol) of manganese per liter (46). However, manganese has been shown to be associated with protein complexes in milk (16, 34), which may limit the concentration of free manganese.
The homologous genes, mtsA and psaA, are known to encode metal-binding proteins. In the different species, these genes are contained in operons encoding ABC transporter systems capable of transporting divalent metal ions. Such operons typically contain three structural genes encoding a metal-binding lipoprotein, a transmembrane protein, and an ATPase. The operon in S. pneumoniae is similar to the operons described for other species, including ssa in S. sanguis (14), sca in S. gordonii (1), and fim in S. parasanguis (10). The order of genes within these operons differs from that detected in S. uberis, which more closely resembles the arrangement found in S. pyogenes (20).
The genes encoding the metal-binding lipoproteins have been grouped into a family of genes whose products are collectively called the lipoprotein receptor-associated antigens (lraI genes) (22); MtuA of S. uberis fits within this group of proteins. Solute-binding proteins were previously categorized into eight discrete families described by Tam and Saier (45). The LraI family of proteins was recently added to cluster 9 (7), an emerging but discrete group of bacterial proteins that fell outside the terms of reference for the eight families previously described. Structural studies performed on pneumococcal surface antigen PsaA revealed the absence of a central hinge region thought to facilitate conformational changes essential to the functions of other ABC-type binding proteins (28). This suggested that a novel mode of solute uptake and release was likely to be adopted by LraI family members, possibly defined by the interaction with membrane permeases and the location at the outer face of the cell membrane (28).
MtuA has sequence characteristics common to other LraI proteins, exhibiting 75% amino acid identity with pneumococcal PsaA and 81% identity with metal-binding lipoprotein homologues from Streptococcus equi and Streptococcus zooepidemicus and MtsA from S. pyogenes. The PsaA structure (PDB accession no. 1PSZ), determined to 2.0 Å (28, 40), consists of two (β/α)4 domains linked together by a single long helix. The metal-binding cavity is formed between the two domains and consists of residues His67, His139, Glu205, and Asp280. Lawrence et al. (28) stated that the metal ion in their structure of PsaA is buried 5.7 Å beneath the molecular surface and the four coordinating residues are also entirely buried. This situation appears to be readily duplicated in structural models of MtuA (data not shown). Corresponding metal-binding residues in S. uberis MtuA are identical in nature, but their positions are all advanced one place in the full MtuA linear amino acid sequence (His68, His140, Glu206, and Asp281). Both conserved Asp-Pro-His metal-binding motifs present in PsaA (28) are duplicated in the N-terminal portion of MtuA. An additional residue, Glu255MtuA, is conserved, and its PsaA equivalent (Glu254) is thought to be a bridge between strands and to stabilize the orientation of metal-binding residue Glu205PsaA (28).
Lipoproteins expressed by the lra operons have been proposed to mediate bacterial coaggregation (1, 26), adhesion to host cells (4), adhesion to salivary components (37), and adhesion to fibrin (6). Further characterization has shown that these surface-expressed lipoproteins are extracytoplasmic and capable of binding metal ions (7, 8). The mtuA gene product of S. uberis similarly appears to be associated with accumulation of manganese ions during growth in bovine milk. The similarity of MtuA to other LraI proteins offers the possibility that structural motifs associated with metal binding and transport may provide targets for rational drug design and effective therapeutic agents to control growth of streptococcal species, including the growth of S. uberis in the bovine mammary gland.
With the exception of iron transport, little is known about the molecular aspects of bacterial transport of trace metals. The Adc Zn2+ transporter in S. pneumoniae has also been proposed to be able to transport Mn2+ (8). Data have suggested that scaA and scaC of S. gordonii are necessary for activity of a high-affinity manganese uptake system that is inhibited by zinc (25). Another Mn2+ transporter has also been suggested in S. gordonii (25), which is proton motive force dependent; this system mediates the uptake of Mn2+ under Mn2+-replete conditions and is not significantly inhibited by Zn2+. Uptake of Mn2+ by the cyanobacterium Synechocystis occurs via a high-affinity ABC transporter and also via a lower-affinity system (3). Saccharomyces cerevisiae contains at least two Mn2+ transport systems with different affinities for Mn2+ (43).
Mutations that block expression of PsaA do not affect growth of S. pneumoniae in vitro, but they have been shown to completely eliminate virulence in a mouse model (41). Further investigation has revealed that the PsaA mutant displays reduced adherence to eukaryotic cells, suggesting that like other LraI protein family members, PsaA functions in bacterial adherence (41). However, it has been hypothesized that PsaA is involved in the regulation of adherence rather than acting as an adhesin (8).
S. pneumoniae and S. parasanguis lraI mutants are attenuated in virulence when they are examined in animal models (4, 6), and immunization with the PsaA lipoprotein confers protective immunity against S. pneumoniae in mice and rats (5, 44). In S. parasanguis, mutation of fimA causes a loss of virulence for endocarditis in a rat model (6). FimA has also been shown to be a promising immunogen for preventing S. parasanguis-induced endocarditis in the same model (47).
Strain AJS001 has a single insertion within mtuA, fails to produce a detectable gene product, and is unable to grow in raw bovine milk in the absence of exogenous Mn2+. Following challenge this organism was also found to be unable to infect the bovine mammary gland. This is in direct contrast to the parental strain, strain 0140J, which was shown in this experiment and has been reported on several other occasions (12, 13, 17, 32) to infect the bovine mammary gland and cause clinical mastitis. Strain AJS001 was detected in milk obtained from only three of eight challenged quarters and then only following enrichment; hence, its failure to generate a proliferating infection is most likely due to its inability to grow in bovine milk within the mammary gland. It has been postulated that bacteria that are able to infect the mammary gland and cause mastitis need to be able to grow in bovine milk; however, this has never previously been demonstrated experimentally. The data presented here therefore provide evidence that the acquisition of manganese via the function of the mtuA gene product and the predicted associated ABC transporter is essential for S. uberis growth in milk in vitro and for growth in vivo. In the absence of this transporter system S. uberis is unable to colonize and cannot infect the bovine mammary gland.
Editor: A. D. O'Brien
REFERENCES
- 1.Andersen, R. N., N. Ganeshkumar, and P. E. Kolenbrander. 1993. Cloning of the Streptococcus gordonii PK488 gene, encoding an adhesin which mediates coaggregation with Actinomyces naeslundii PK606. Infect. Immun. 61:981-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold, C., and I. J. Hodgson. 1991. Vectorette PCR: a novel approach to genomic walking. PCR Methods Appl. 1:39-42. [DOI] [PubMed] [Google Scholar]
- 3.Bartsevich, V. V., and H. B. Pakrasi. 1995. Molecular identification of an ABC transporter complex for manganese: analysis of a cyanobacterial mutant strain impaired in the photosynthetic oxygen evolution process. EMBO J. 14:1845-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry, A. M., and J. C. Paton. 1996. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect. Immun. 64:5255-5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briles, D. E., R. C. Tart, E. Swiatlo, J. P. Dillard, P. Smith, K. A. Benton, B. A. Ralph, A. Brooks-Walter, M. J. Crain, S. K. Hollingshead, and L. S. McDaniel. 1998. Pneumococcal diversity: considerations for new vaccine strategies with emphasis on pneumococcal surface protein A (PspA). Clin. Microbiol. Rev. 11:645-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnette-Curley, D., V. Wells, H. Viscount, C. L. Munro, J. C. Fenno, P. Fives-Taylor, and F. L. Macrina. 1995. FimA, a major virulence factor associated with Streptococcus parasanguis endocarditis. Infect. Immun. 63:4669-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dintilhac, A., G. Alloing, C. Granadel, and J. P. Claverys. 1997. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol. Microbiol. 25:727-739. [DOI] [PubMed] [Google Scholar]
- 8.Dintilhac, A., and J. P. Claverys. 1997. The adc locus, which affects competence for genetic transformation in Streptococcus pneumoniae, encodes an ABC transporter with a putative lipoprotein homologous to a family of streptococcal adhesins. Res. Microbiol. 148:119-131. [DOI] [PubMed] [Google Scholar]
- 9.Dutt, K. W., R. J. Eberhart, and R. A. Wilson. 1986. In vitro growth of mastitis pathogens in mammary secretions of the dry and peripartum periods. J. Dairy Sci. 69:2408-2415. [DOI] [PubMed] [Google Scholar]
- 10.Fenno, J. C., A. Shaikh, G. Spatafora, and P. Fives-Taylor. 1995. The fimA locus of Streptococcus parasanguis encodes an ATP-binding membrane transport system. Mol. Microbiol. 15:849-863. [DOI] [PubMed] [Google Scholar]
- 11.Field, T. R., P. N. Ward, L. H. Pedersen, and J. A. Leigh. 2003. The hyaluronic acid capsule of Streptococcus uberis is not required for the development of infection and clinical mastitis. Infect. Immun. 71:132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finch, J. M., A. W. Hill, T. R. Field, and J. A. Leigh. 1994. Local vaccination with killed Streptococcus uberis protects the bovine mammary gland against experimental intramammary challenge with the homologous strain. Infect. Immun. 62:3599-3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finch, J. M., A. Winter, A. W. Walton, and J. A. Leigh. 1997. Further studies on the efficacy of a live vaccine against mastitis caused by Streptococcus uberis. Vaccine 15:1138-1143. [DOI] [PubMed] [Google Scholar]
- 14.Ganeshkumar, N., P. M. Hannam, P. E. Kolenbrander, and B. C. McBride. 1991. Nucleotide sequence of a gene coding for a saliva-binding protein (SsaB) from Streptococcus sanguis 12 and possible role of the protein in coaggregation with actinomyces. Infect. Immun. 59:1093-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidry, A. J. 1985. Mastitis, p. 237-262. In B. L. Larson (ed.), Lactation. Iowa State University Press, Ames, Iowa.
- 16.Herald, C. T., J. R. Brunner, and S. T. Bass. 1957. A spectrographic analysis of two protein fractions of the fat-globule membrane of normal cow's milk. J. Dairy Sci. 40:446. [Google Scholar]
- 17.Hill, A. W., J. M. Finch, T. R. Field, and J. A. Leigh. 1994. Immune modification of the pathogenesis of Streptococcus uberis mastitis in the dairy cow. FEMS Immunol. Med. Microbiol. 8:109-117. [DOI] [PubMed] [Google Scholar]
- 18.Hill, A. W., and J. A. Leigh. 1989. DNA fingerprinting of Streptococcus uberis: a useful tool for epidemiology of bovine mastitis. Epidemiol. Infect. 103:165-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hillerton, J. E., M. F. Shearn, R. M. Teverson, S. Langridge, and J. M. Booth. 1993. Effect of pre-milking teat dipping on clinical mastitis on dairy farms in England. J. Dairy Res. 60:31-41. [DOI] [PubMed] [Google Scholar]
- 20.Janulczyk, R., J. Pallon, and L. Bjorck. 1999. Identification and characterization of a Streptococcus pyogenes ABC transporter with multiple specificity for metal cations. Mol. Microbiol. 34:596-606. [DOI] [PubMed] [Google Scholar]
- 21.Jenkinson, H. F. 1992. Adherence, coaggregation, and hydrophobicity of Streptococcus gordonii associated with expression of cell surface lipoproteins. Infect. Immun. 60:1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkinson, H. F. 1994. Cell surface protein receptors in oral streptococci. FEMS Microbiol. Lett. 121:133-140. [DOI] [PubMed] [Google Scholar]
- 23.Johnsen, L. B., K. Poulsen, M. Kilian, and T. E. Petersen. 1999. Purification and cloning of a streptokinase from Streptococcus uberis. Infect. Immun. 67:1072-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitten, T., C. L. Munro, S. M. Michalek, and F. L. Macrina. 2000. Genetic characterization of a Streptococcus mutans LraI family operon and role in virulence. Infect. Immun. 68:4441-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolenbrander, P. E., R. N. Andersen, R. A. Baker, and H. F. Jenkinson. 1998. The adhesion-associated sca operon in Streptococcus gordonii encodes an inducible high-affinity ABC transporter for Mn2+ uptake. J. Bacteriol. 180:290-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolenbrander, P. E., R. N. Andersen, and N. Ganeshkumar. 1994. Nucleotide sequence of the Streptococcus gordonii PK488 coaggregation adhesin gene, scaA, and ATP-binding cassette. Infect. Immun. 62:4469-4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kossaibati, M. A., and R. J. Esslemont. 1997. The costs of production diseases in dairy herds in England. Vet. J. 154:41-51. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence, M. C., P. A. Pilling, V. C. Epa, A. M. Berry, A. D. Ogunniyi, and J. C. Paton. 1998. The crystal structure of pneumococcal surface antigen PsaA reveals a metal-binding site and a novel structure for a putative ABC-type binding protein. Structure 6:1553-1561. [DOI] [PubMed] [Google Scholar]
- 29.Leigh, J. A. 1994. Purification of a plasminogen activator from Streptococcus uberis. FEMS Microbiol. Lett. 118:153-158. [DOI] [PubMed] [Google Scholar]
- 30.Leigh, J. A. 1999. Streptococcus uberis: a permanent barrier to the control of bovine mastitis? Vet. J. 157:225-238. [DOI] [PubMed] [Google Scholar]
- 31.Leigh, J. A., and T. R. Field. 1994. Streptococcus uberis resists the bactericidal action of bovine neutrophils despite the presence of bound immunoglobulin. Infect. Immun. 62:1854-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leigh, J. A., J. M. Finch, T. R. Field, N. C. Real, A. Winter, A. W. Walton, and S. M. Hodgkinson. 1999. Vaccination with the plasminogen activator from Streptococcus uberis induces an inhibitory response and protects against experimental infection in the dairy cow. Vaccine 17:851-857. [DOI] [PubMed] [Google Scholar]
- 33.Lincoln, R. A., and J. A. Leigh. 1998. Characterization of the interaction of bovine plasmin with Streptococcus uberis. J. Appl. Microbiol. 84:1104-1110. [DOI] [PubMed] [Google Scholar]
- 34.Ling, E. R., S. K. Kon, and J. W. G. Porter. 1961. The composition of milk, p. 217-218. In S. K. Kon and A. T. Cowie (ed.), Milk: the mammary gland and its secretion, vol. 2. Academic Press, New York, N.Y.
- 35.Maguin, E., H. Prevost, S. D. Ehrlich, and A. Gruss. 1996. Efficient insertional mutagenesis in lactococci and other gram-positive bacteria. J. Bacteriol. 178:931-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonald, J. S., and A. J. Anderson. 1981. Experimental infection of bovine mammary glands with Streptococcus uberis during the nonlactating period. Am. J. Vet. Res. 42:465-467. [PubMed] [Google Scholar]
- 37.Oligino, L., and P. Fives-Taylor. 1993. Overexpression and purification of a fimbria-associated adhesin of Streptococcus parasanguis. Infect. Immun. 61:1016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paape, M. J., W. P. Wergin, A. J. Guidry, and W. D. Schultze. 1981. Phagocytic defense of the ruminant mammary gland. Adv. Exp. Med. Biol. 137:555-578. [PubMed] [Google Scholar]
- 39.Persson, K., I. Larsson, and C. Hallen Sandgren. 1993. Effects of certain inflammatory mediators on bovine neutrophil migration in vivo and in vitro. Vet. Immunol. Immunopathol. 37:99-112. [DOI] [PubMed] [Google Scholar]
- 40.Pilling, P. A., M. C. Lawrence, A. M. Berry, A. D. Ogunniyi, R. A. Lock, and J. C. Paton. 1998. Expression, purification and preliminary X-ray crystallographic analysis of PsaA, a putative metal-transporter protein of Streptococcus pneumoniae. Acta Crystallogr. D Biol. Crystallogr. 54:1464-1466. [DOI] [PubMed] [Google Scholar]
- 41.Sampson, J. S., Z. Furlow, A. M. Whitney, D. Williams, R. Facklam, and G. M. Carlone. 1997. Limited diversity of Streptococcus pneumoniae psaA among pneumococcal vaccine serotypes. Infect. Immun. 65:1967-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith, A. J., A. J. Kitt, P. N. Ward, and J. A. Leigh. 2002. Isolation and characterization of a mutant strain of Streptococcus uberis, which fails to utilize a plasmin derived beta-casein peptide for the acquisition of methionine. J. Appl. Microbiol. 93:631-639. [DOI] [PubMed] [Google Scholar]
- 43.Supek, F., L. Supekova, H. Nelson, and N. Nelson. 1996. A yeast manganese transporter related to the macrophage protein involved in conferring resistance to mycobacteria. Proc. Natl. Acad. Sci. USA 93:5105-5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talkington, D. F., B. G. Brown, J. A. Tharpe, A. Koenig, and H. Russell. 1996. Protection of mice against fatal pneumococcal challenge by immunization with pneumococcal surface adhesin A (PsaA). Microb. Pathog. 21:17-22. [DOI] [PubMed] [Google Scholar]
- 45.Tam, R., and M. H. Saier. 1993. Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol. Rev. 57:320-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas, J. W. 1970. Metabolism of iron and manganese. J. Dairy Sci. 53:1107-1123. [DOI] [PubMed] [Google Scholar]
- 47.Viscount, H. B., C. L. Munro, D. Burnette-Curley, D. L. Peterson, and F. L. Macrina. 1997. Immunization with FimA protects against Streptococcus parasanguis endocarditis in rats. Infect. Immun. 65:994-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ward, P. N., T. R. Field, W. G. Ditcham, E. Maguin, and J. A. Leigh. 2001. Identification and disruption of two discrete loci encoding hyaluronic acid capsule biosynthesis genes hasA, hasB, and hasC in Streptococcus uberis. Infect. Immun. 69:392-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watts, J. L. 1988. Etiological agents of bovine mastitis. Vet. Microbiol. 16:41-66. [DOI] [PubMed] [Google Scholar]
- 50.Yancey, R. J., Jr. 1999. Vaccines and diagnostic methods for bovine mastitis: fact and fiction. Adv. Vet. Med. 41:257-273. [DOI] [PubMed] [Google Scholar]