Abstract
This study was conducted to evaluate the immunogenicity and protective efficacy of a DNA vaccine encoding Brucella abortus Cu,Zn superoxide dismutase (SOD). Intramuscular injection of plasmid DNA carrying the SOD gene (pcDNA-SOD) into BALB/c mice elicited both humoral and cellular immune responses. Animals injected with pcDNA-SOD developed SOD-specific antibodies which exhibited a dominance of immunoglobulin G2a (IgG2a) over IgG1. In addition, the DNA vaccine elicited a T-cell-proliferative response and also induced the production of gamma interferon, but not interleukin-10 (IL-10) or IL-4, upon restimulation with either recombinant SOD or crude Brucella protein, suggesting the induction of a typical T-helper-1-dominated immune response in mice. The pcDNA-SOD (but not the control vector) induced a strong, significant level of protection in BALB/c mice against challenge with B. abortus virulent strain 2308; the level of protection was similar to the one induced by B. abortus vaccine strain RB51. Altogether, these data suggest that pcDNA-SOD is a good candidate for use in future studies of vaccination against brucellosis.
Brucella abortus, a facultative intracellular pathogen, is the etiological agent of brucellosis, a disease that affects humans and several other animal species (7). Like other facultative intracellular bacterial pathogens, resistance to B. abortus depends on acquired cell-mediated immunity (CMI) (33). The development of Th1 subset CD4+ lymphocytes that secrete gamma interferon (IFN-γ), a crucial cytokine that up-regulates the macrophage anti-Brucella activity, and the development of CD8+ T lymphocytes that are able to lyse Brucella-infected cells (20, 22) are the two main components of the protective response. Live, attenuated vaccines that can stimulate strong CMI responses are usually very effective against brucellosis. Attenuated strains such as Brucella melitensis Rev1 and B. abortus S19 and RB51 are being used to control brucellosis in domestic animals. However, there is no safe, effective vaccine available for human use; the vaccine strains used for animals are considered too virulent or unsafe for humans. A vaccine that will be noninfectious to humans but effective in stimulating a broad protective immune response is needed to control brucellosis. To develop the next generation of Brucella vaccines, several research groups are pursuing different strategies, including development of subunit vaccines (21), utilization of bacterial vectors (25), and overexpression of protective homologous antigen (32).
Another new strategy for developing safe and efficacious vaccines is immunization with plasmid DNA encoding the protective antigen. The DNA vaccines (also referred to as gene vaccines) seem to offer the best approach to activate both cellular components of the immune response. Furthermore, gene vaccine shows many advantages over the standard immunization procedure, such as no risk of infection, induction of a long-lived immune response, better stability than live attenuated vaccines, easy preparation, and low cost. The smallest vector unit comprises the antigen's gene sequence and eukaryotic regulatory elements such as a promoter and a polyadenylation signal that are functional in mammalian cells (3).
Plasmid DNA vaccination can protect against many viral, fungal, and parasitic diseases in different animal models (4, 11, 13, 30). Because of the ability of DNA vaccines to induce strong CMI responses, they can be very effective vaccines against intracellular bacteria. Most of the research conducted to date regarding DNA vaccines against bacterial diseases is focused on inducing protection against Mycobacterium tuberculosis (12, 17, 18, 19). DNA vaccines comprising the Brucella ribosomal L7/L12 gene (14) or lumazine synthase gene (31) have been demonstrated to induce significant levels of protection in the mouse model of brucellosis.
We have previously demonstrated that an 18.5-kDa periplasmic protein of B. abortus, which was later identified to be Cu,Zn superoxide dismutase (SOD), can induce a cellular immune response (23). SOD can induce protection in mice after immunization with a recombinant Escherichia coli strain expressing this antigen (25). In addition, mice immunized with purified SOD (2) or SOD synthetic peptides (29) developed a significant degree of protection against infection with the virulent strain 2308. Our objective in this study was to evaluate the protective capacity of immunization with plasmid DNA carrying the Brucella Cu,Zn SOD gene (pcDNA-SOD).
MATERIALS AND METHODS
Animals.
Female BALB/c mice (7 to 8 weeks old; obtained from Instituto de Salud Publica, Santiago, Chile) were acclimated and randomly distributed into experimental groups. The mice were kept in conventional animal facilities and received water and food ad libitum.
Bacterial strains and growth conditions.
B. abortus virulent strain 2308 and attenuated strain RB51 were from our own culture collection; strain RB51 was originally obtained from the Virginia-Maryland Regional College of Veterinary Medicine (Virginia Polytechnic Institute and State University, Blacksburg) (27). The bacterial cells were grown under aerobic conditions in tryptose-soy broth (Difco Laboratories, Detroit, Mich.) for 72 h at 37°C. For inoculation, the bacterial suspensions were adjusted spectrophotometrically to an OD600 corresponding to 104 CFU of B. abortus strain 2308 and 2 × 108 CFU of B. abortus RB51. All experiments with live brucellae were performed in biosafety level 2 facilities. E. coli strain DH5α (Life technology, Gaithersburg, Md.) was used for producing the necessary plasmid constructs. The E. coli cultures were routinely grown at 37°C in Luria-Bertani broth or agar supplemented, when required, with 100 μg of ampicillin per ml.
Construction of Cu,Zn SOD DNA vaccine.
Recombinant plasmid pBAII-3, containing the gene for B. abortus Cu,Zn SOD (sodC) along with its own promoter, was initially obtained from a pUC9 genomic library of B. abortus strain 2308 (25). A 1.1-kb fragment containing the sodC gene and its promoter sequences were excised from the insert of pBAII-3 with EcoRI and XhoI restriction enzyme digestion and ligated into expression vector pcDNA3 downstream to the CMV promoter (Invitrogen, San Diego, Calif.). The resulting plasmid was designated pcDNA-SOD. A colony of E. coli containing pcDNA-SOD was cultured in Luria-Bertani broth containing ampicillin (100 μg/ml). Large-scale plasmid DNA isolation was performed using an EndoFree Plasmid Giga Kit (Qiagen, Valencia, Calif.), according to the manufacturer's directions. The DNA was finally resuspended in phosphate-buffered saline (PBS) at a concentration of 1,000 μg/ml. DNA concentration and purity were determined by optical density, and the A260/A280 ratio was typically greater than 1.8. The pcDNA-SOD plasmid construct was verified by restriction digestion and by sequencing the complete insert at the Universidad de Concepción Sequencing Facility.
Purification of Cu,Zn SOD.
Expression of the Brucella Cu,Zn SOD protein by E. coli DH5α (pBSSOD) has been previously described (25). A previously described method was used to extract Cu,Zn SOD from E. coli cells using 10 mM phosphate buffer (pH 7.6) containing 0.1% Triton X-100 (6). The Cu,Zn SOD was purified by applying the extract on an equilibrated anion-exchange column (HiTrapQ; Pharmacia Biotech). All of the protein except Cu,Zn SOD bound to the resin. Cu,Zn SOD present in the flowthrough was collected, absorbed with polymyxin B beads (Affi-Prep polymyxin support; Bio-Rad Laboratories, Hercules, Calif.) to remove the lipopolysaccharide, and dialyzed extensively against PBS. After determining the protein concentration by the Bradford method (5), aliquots of the purified Cu,Zn SOD were stored at −70° until used for enzyme-linked immunosorbent assay (ELISA) or for in vitro stimulation of splenocytes.
Immunization.
Mice were anesthetized with inhaled halothane and injected with 50 μg of plasmid DNA in 50 μl of PBS in each tibialis anterior muscle (100 μg of DNA/mouse) by using an insulin syringe with a 28-gauge needle. Mice were vaccinated at weeks 0, 2, and 4 with pcDNA-SOD. As negative controls, groups of mice were immunized with pcDNA3, with plasmid without SOD insert, or with PBS only. In protection experiments, mice in the positive control group were vaccinated intraperitoneally with 2 × 108 CFU of B. abortus strain RB51 in 0.2 ml of PBS.
ELISA.
The presence of serum immunoglobulin G (IgG), IgG1, and IgG2a isotypes with specificity to Cu,Zn SOD was determined by indirect ELISA. The purified recombinant Cu,Zn SOD was diluted to 3 μg/ml in carbonate buffer (pH 9.6) and used to coat the wells of a polystyrene plate (100 μl/well; Nunc-Immuno plate with MaxiSorp surface). After overnight incubation at 4°C, plates were washed four times in wash buffer (Tris-buffered saline [pH 7.4] with 0.05% Tween 20) and blocked with 5% gelatin in Tris-buffered saline for 2 h at 37°C and then incubated with serial dilution of the sera for 3 h at room temperature and washed four times. Isotype-specific rabbit anti-mouse horseradish peroxidase conjugates (ICN Biomedicals, Inc., Aurora, Ohio) were added (50 μl/well) at an appropriate dilution. After 30 min of incubation at room temperature, plates were washed four times, and 100 μl of substrate solution (200 μmol of o-phenylenediamine and .04% H2O2) was added to each well. After 20 min of incubation at room temperature, the enzyme reaction was stopped by addition of 100 μl of 0.18 M sulfuric acid/well, and the absorbance of the developed color was measured at 492 nm. The cutoff value for the assay was calculated as the mean specific optical density plus standard deviation (SD) for 10 sera from nonimmunized mice assayed at a dilution of 1:40 (31). The titer of each serum was calculated as the reciprocal of the highest serum dilution yielding a specific optical density higher than the cutoff value.
Splenocyte cultures and lymphocyte proliferation.
Mice were sacrificed, and their spleens were removed under aseptic conditions. Single-cell suspensions were prepared from the spleens according to the standard procedure (25), and the red blood cells were lysed with ACK solution (150 mM NH4Cl, 1 mM KHCO3, 0.1 mM N2 EDTA [pH 7.3]). Splenocytes were cultured at 37°C with 5% CO2 in a 96-well flat-bottom plate at a concentration of 4 × 105 viable cells/well in the presence of 0.4 μg of crude B. abortus RB51 proteins, an extract obtained from bacteria subjected to hypertonic salt solution (1 M NaCl with 0.1 M sodium citrate) and sonication (24), 0.08 μg of purified SOD, 0.25 μg of concanavalin A per well, or no additives (unstimulated control). RPMI 1640 medium (Sigma) supplemented with 2 mM l-glutamine, 10% heat-inactivated fetal calf serum (GIBCO BRL), and 50 μl of penicillin-streptomycin was used for culturing the splenocytes. The cells were cultured for 3 days and pulsed for 8 h with 0.4 μCi of thymidine (50 Ci/mmol; Amersham, London, United Kingdom) per well, and the radioactivity incorporated in the DNA was measured in a liquid scintillation counter. Cell proliferation was expressed as mean counts per minute obtained from triplicate cultures obtained from a cell pool of each group. In addition, a stimulation index was calculated for each experimental group by dividing the counts per minute of cells with antigen by the counts per minute of cells without antigen.
Cytokine ELISAs.
For detection of cytokines, culture supernatants of spleen cells were collected after 48 h of antigen stimulation and tested for the presence of the cytokines by antigen-capture ELISA using OptEIA Set Mouse IFN-γ, IL-4, and IL-10 (BD Biosciences, San Diego, Calif.). All assays were performed in triplicate. The concentration of IFN-γ, IL-4, and IL-10 in the culture supernatants was calculated by using a linear-regression equation obtained from the absorbance values of the standards.
Protection experiments.
The protection experiments were performed as described previously (25). Briefly, 5 weeks after vaccination (day 60), six mice from each group were challenged by intraperitoneal injection of 104 CFU of B. abortus 2308. Two weeks later, the infected mice were sacrificed, their spleens were macerated, and dilutions were plated to determine the number of Brucella CFU per spleen. This experiment was repeated three times, and results from the pooled data are shown. Statistical analyses were performed with Student's paired t test. Log10 units of protection were obtained by subtracting the mean log10 CFU for the experimental group from the mean log10 CFU of the corresponding control group.
RESULTS
Immune response of mice vaccinated with pcDNA-SOD.
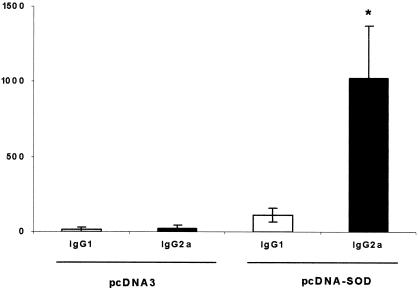
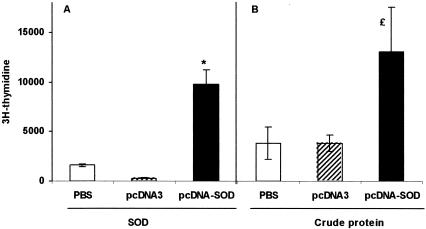
Titers of anti-SOD antibodies were measured by ELISA in serum from mice immunized with pcDNA-SOD, with the expression vector alone (pcDNA3), or control PBS. Mice immunized with pcDNA-SOD gave a rapid IgG response by week 2 following vaccination (titers ranged from 80 to 160) and the peak titers (range, 2,560 to 5,120) were detected at day 60 postvaccination (end of experiment). None of the animals inoculated with pcDNA3 showed specific anti-SOD antibodies (data not shown). Subisotype analysis of these antibodies (IgG1 and IgG2a) indicated that the anti-SOD antibodies detected in pcDNA-SOD-immunized mice were predominantly IgG2a at day 60 postvaccination. The specific IgG2a titers (range, 640 to 1,280) were higher than the specific IgG1 titers (range, 80 to 160) (IgG2a/IgG1 ratio = 9.1) (Fig. 1). To examine the CMI response to Brucella recombinant SOD (rSOD) protein and crude B. abortus proteins, the proliferation response and cytokine profile of spleen cells from mice immunized with pcDNA-SOD, pcDNA3, and PBS were determined. As demonstrated in Fig. 2A, lymphocytes from mice immunized with pcDNA-SOD had a significant T-cell proliferation response to rSOD protein (P < 0.01) with a stimulation index of 14.95. On the other hand (Fig. 2B), when splenocytes from mice immunized with pcDNA-SOD were stimulated in vitro with crude Brucella proteins they showed proliferation, but it was not statistically significant from control groups (P > 0.1). With respect to cytokine profile, supernatants of spleen cell cultures from pcDNA-SOD-immunized animals contained high levels of IFN-γ compared to the negative control groups. The rSOD protein and crude Brucella proteins significantly induced the production of IFN-γ in cells from pcDNA-SOD-immunized mice (P < 0.05 in both groups) (Fig. 3A), and only a low level of IL-10 was detected in all groups (Fig. 3B). In addition, no IL-4 was detected in any of the culture supernatants of splenocytes stimulated with specific antigens (data not shown). Splenocytes from all groups of mice, including the saline-inoculated group, produced a similar level of IFN-γ, IL-4, and IL-10 upon stimulation with concanavalin A (data not shown). Taken together, our results indicate that immunization with pcDNA-SOD induces a specific Th1-type immune response in mice.
FIG. 1.
Antibody isotype profile of mice inoculated with pcDNA-SOD or pcDNA3. Mice (five per group) were inoculated intramuscularly with 100 μg of pcDNA-SOD or pcDNA3. Sixty days after immunization, serum was collected and antibody titers (shown on the y axis) were evaluated by ELISA. Data are representative of two separate experiments. Each bar represents the mean titer ± SD (error bars) of antibodies in five animals. *, significantly different from titer of IgG1 in mice immunized with pcDNA-SOD (P < 0.003).
FIG. 2.
Lymphocyte proliferation assay. BALB/c mice were immunized with saline, pcDNA3, or pcDNA-SOD. T-cell proliferation response was measured at 60 days after the immunization. Splenocytes from each group (4 × 105 cells per well) were prepared and stimulated in vitro with purified recombinant SOD (0.8 μg/ml) (A) and CBP (4 μg/ml) as antigens. Each value is the average counts per minute of triplicate cultures of cells ± SD (error bars) obtained from a pool of five mice in each group. Symbols: *, P < 0.01 compared with value for saline control mice; £, P ≥ 0.1 compared with value for saline control mice.
FIG. 3.
Quantitative ELISA analysis of IFN-γ (A) and IL-10 (B) secreted by lymphocytes upon in vitro stimulation with different antigens. Spleen cells (4 × 106/ml) from mice inoculated with pcDNA3 or pcDNA-SOD were stimulated with RPMI 1640, rSOD (0.8 μg/ml), or CBPs (4 μg/ml) for 48 h. Each bar represents the geometric mean ± SD (error bars) of the responses in spleen cells from five individual mice. *, statistically significant differences compared to RPMI 1640 (P ≤ 0.005).
Efficacy of pcDNA-SOD immunization in generating protective immunity against B. abortus 2308.
Protection experiments were carried out by challenging vaccinated and control mice by intraperitoneal injection of virulent B. abortus 2308, and the level of infection was evaluated by determining the CFU in their spleens. The results indicate that immunization with pcDNA-SOD resulted in a significantly higher degree of protection (2.16 log increase in protection) compared to the unimmunized control groups (P < 0.0005). To compare the extent to which mice could be protected, vaccination with live B. abortus strain RB51 induced 2.22 log protection. No significant difference in the number of CFU was seen between groups injected with pcDNA3 and PBS (Table 1). These results indicate that pcDNA-SOD vaccine afforded a significant degree of protection against Brucella infection.
TABLE 1.
Protection of mice against challenge with B. abortus 2308 after immunization with DNA vaccine coding for Cu,Zn SODa
| Vaccine (dose) | Log10 CFU of B. abortus 2308 in spleen (mean ± SD) | Log10 units of protection |
|---|---|---|
| Saline control | 5.99 ± 0.24 | 0 |
| RB51 live (2 × 108 CFU) | 3.77 ± 0.42b | 2.22 |
| pcDNA-SOD (100 μg) | 3.83 ± 0.29c | 2.16 |
| pcDNA3 (100 μg) | 6.01 ± 0.65 | 0 |
Mice were challenged i.p. with 104 CFU of strain 2308 2 weeks prior to sacrifice.
P < 0.005 compared to the control groups.
P < 0.0005 compared to the control groups.
DISCUSSION
Vaccination continues to be the most successful procedure for preventing infectious diseases in animals and humans. The development of new-generation vaccine systems to prevent brucellosis is needed to overcome the disadvantages of the currently used live vaccines. It is well-established that protection against infection by an intracellular pathogen, including Brucella, requires the generation of a Th1-type immune response over time (10, 20).
The improvement of the methods for cloning and purifying proteins has led to the use of purified recombinant proteins as acellular vaccines in experimental trials. These preparations, as well as synthetic peptides, are more convenient to use than attenuated vaccines but are not able to confer a high degree of protection or induce a strong CMI response (21, 29). The reduced effectiveness of the acellular vaccines might be related to inadequate processing and presentation of the antigen. Immunization with plasmid DNA coding for the antigen of interest represents a novel and promising method in vaccine research and development. A number of studies have demonstrated that after naked DNA immunization, the antigen is naturally processed and presented on major histocompatibility complex class I and class II molecules, inducing a broad range of immune responses including antibody production, CD8+ cytotoxic T cells (CTLs), and CD4+ T helper cell activation (8, 9, 26, 30).
There is sufficient evidence that the complete protein or certain epitopes of Brucella Cu,Zn SOD can induce protective immunity in mice (25, 29). In this study, we evaluated the capacity of Cu,Zn SOD DNA vaccine to elicit an immune response and protective immunity in BALB/c mice.
Injection of plasmid DNA containing the Cu,Zn SOD gene sequence elicited specific humoral and cellular immune responses in BALB/c mice. Two weeks after the first immunization, we found a weak titer of specific IgG in mice immunized with the plasmid pcDNA-SOD. By the end of the experiment this response was five times higher. Several previously described DNA vaccines to Brucella induced antibody titers higher than the pcDNA-SOD employed in this work (1, 16, 31). This difference may be attributed to several factors, including the amount of SOD protein expressed, development of a preferential CMI (32) or the structure of the SOD protein. Velikovsky et al. (31) concluded that the polymeric structure of the Brucella lumazine synthase protein induces a humoral response higher than that detected in the case of SOD. In general, SOD-specific antibodies are not detected in mice (15) or cattle vaccinated with strain RB51 (28). However, mice do develop specific antibodies when immunized with RB51 overexpressing SOD (32).
The induction of a T-cell immune response after DNA immunization was evaluated by measuring lymphocyte proliferation and cytokine production after in vitro stimulation of splenic cells with purified recombinant SOD or crude Brucella protein (CBP). Both SOD and CBP induced a high T-cell-proliferative response (Fig. 2) and high levels of IFN-γ (Fig. 3A). Low levels of IL-10 and no detectable level of IL-4 (data not shown) were present in the supernatants. These results indicate that immunization with the pcDNA-SOD plasmid induces a Th1 cellular response. The predominance of IgG2a over IgG1 also supported this conclusion.
On the basis of these data we have started to analyze the protective efficacy of the pcDNA-SOD vaccine against a B. abortus challenge. A significant finding of this study was that the protection achieved with pcDNA-SOD was comparable to the protection conferred by the live RB51 vaccine. Animals vaccinated with pcDNA-SOD showed a log protection of 2.16. This is a quite promising result and is in agreement with some previously reported efficacies of Brucella DNA vaccines encoding the L7-L12 ribosomal and lumazine synthase proteins (14, 31). One of the subpopulations of T cells stimulated by DNA vaccines is CD8+ CTLs, which were slightly enhanced in the pcCNA-SOD-vaccinated mice when compared to the levels of CD4+ T cells (data not shown). The pcDNA-SOD vaccination also stimulated CD8+ T cells to produce more IFN-γ compared to that of CD4+ cells (data not shown). This finding is highly relevant to the protection observed in this study since previous studies have demonstrated that CTLs are critical for protection against B. abortus (20).
In conclusion, we have shown that inoculation of plasmid DNA containing the Cu,Zn SOD gene leads to the elicitation of both antibody and CMI responses of Th-1 type, and confers protection against B. abortus challenge. Further studies are required to delineate the role of different T-cell types in the protection induced by vaccination with pcDNA-SOD.
Acknowledgments
This work was supported by grant 1010851 from the Fondo Nacional de Investigación Científica y Tecnologica (FONDECYT), Santiago, Chile.
Editor: J. D. Clements
REFERENCES
- 1.Al-Mariri, A., A. Tibor, P. Mertens, X. De Bole, P. Michel, J. Godfroid, K. Walravens, and J.-J. Letesson. 2001. Induction of immune response in BALB/c mice with a DNA vaccine encoding bacterioferritin or P39 of Brucella spp. Infect. Immun. 69:6264-6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae, J. 1999. Generation of baculovirus-Brucella abortus heat shock protein recombinants; mouse immune responses against the recombinants, and B. abortus superoxide dismutase and L7/L12 recombinant proteins. Ph.D thesis. Virginia Polytechnic Institute and State University, Blacksburg.
- 3.Bona, C. A., and A. Bot. 2000. Genetic vaccination, p. 11-23. In A. Bot and C. A. Bona (ed.), Genetic immunization. Kluwer Academic/Plenum Publishers, New York, N.Y.
- 4.Boyer, J. D., K. E. Ugen, B. Wang, M. Agadjanyan, L. Gilbert, M. L. Bagarazzi, M. Chattergoon, P. Frost, A. Javadiana, W. V. Williams, Y. rafaeli, R. B. Ciccarelli, D. McCallus, L. Coney, and D. B. Weiner. 1997. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. Nat. Med. 3:526-532. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Bricker, B. J., L. B. Tabatabai, B. A. Judge, B. L. Deyoe, and J. E. Mayfield. 1990. Cloning, expression, and occurrence of the Brucella Cu-Zn superoxide dismutase. Infect. Immun. 58:2935-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbel, M. J. 1997. Brucellosis: an overview. Emerg. Infect. Dis. 3:213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnelly, J. J., J. B. Ulmer, J. W. Shiver, and M. A. Liu. 1997. DNA vaccines. Annu. Rev. Immunol. 15:617-648. [DOI] [PubMed] [Google Scholar]
- 9.Feltquate, D. M., S. Heaney, R. G. Webster, and H. L. Robinson. 1997. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J. Immunol. 158:2278-2284. [PubMed] [Google Scholar]
- 10.Golding, B., D. E. Scott, O. Scharf, L. Huang, M. Zaitseva, C. Lapham, N. Eller, and H. Golding. 2001. Immunity and protection against Brucella abortus. Microbes Infect. 3:43-48. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman, S. L., D. L. Doolan, M. Sedegah, R. Wang, L. F. Scheller, A. Kumar, W. R. Weiss, T. P. Le, D. M. Klinman, P. Hobart, J. A. Norman, and R. C. Hedstrom. 1997. Toward clinical trials of DNA vaccines against malaria. Immunol. Cell. Biol. 75:376-381. [DOI] [PubMed] [Google Scholar]
- 12.Huygen, K., J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, R. R. Deck, C. M. DeWitt, I. M. Orne, S. Baldwin, C. D’Souza, A. Drowart, E. Lozes, P. Vandenbussche, J. P. Van Voore, M. A. Liu, and J. B. Ulmer. 1996. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 2:893-898. [DOI] [PubMed] [Google Scholar]
- 13.Jiang, C., D. M. Magee, and R. A. Cox. 1999. Coadministration of interleukin 12 expression vector with antigen expressing cDNA enhances induction of protective immunity against Coccidioides immitis. Infect. Immun. 67:5848-5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurar, E., and G. A. Splitter. 1997. Nucleic acid vaccination of Brucella abortus ribosomal L7/L12 gene elicits immune response. Vaccine 15:1851-1857. [DOI] [PubMed] [Google Scholar]
- 15.Latimer, E., J. Simmers, N. Sriranganathan, R. M. Roop, G. G. Schurig, and S. M. Boyle. 1992. Brucella abortus deficient in copper/zinc superoxide dismutase is virulent in BALB/c mice. Microb. Pathog. 12:105-113. [DOI] [PubMed] [Google Scholar]
- 16.Leclero, S., J. S. Harms, G. M. Rosinha, V. Azevedo, and S. C. Oliveira. 2002. Induction of a Th1-type of immune response but not protective immunity by intramuscular DNA immunisation with Brucella abortus GroEL heat-shock gene. J. Med. Microbiol. 51:20-26. [DOI] [PubMed] [Google Scholar]
- 17.Lowrie, D. B., R. E. Tascon, M. J. Colston, and C. L. Silva. 1994. Towards a DNA vaccine against tuberculosis. Vaccine 12:1537-1540. [DOI] [PubMed] [Google Scholar]
- 18.Lowrie, D. B., C. L. Silva, and R. E. Tascon. 1997. DNA vaccines against tuberculosis. Immunol. Cell. Biol. 75:591-594. [DOI] [PubMed] [Google Scholar]
- 19.Lozes, E., K. Huygen, J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, P. Vanderbussche, J. P. Van Voore, A. Drowart, J. B. Ulmer, and M. A. Liu. 1997. Immunogenicity and efficacy of a tuberculosis DNA vaccine encoding the components of the secreted antigen 85 complex. Vaccine 15:830-833. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira, S. C., and G. A. Splitter. 1995. CD8+ type 1 CD4hi CD4RBlo T lymphocytes control intracellular Brucella abortus infection as demonstrated in major histocompatibility complex class I- and class II-deficient mice. Eur. J. Immunol. 25:2551-2557. [DOI] [PubMed] [Google Scholar]
- 21.Oliveira, S. C., and G. A. Splitter. 1996. Immunization of mice with recombinant L7/L12 ribosomal protein confers protection against Brucella abortus infection. Vaccine 14:959-962. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira, S. C., J. S. Harms, E. L. Rech, R. S. Rodarte, A. L. Bocca, A. M. Goes, and G. A. Splitter. 1998. The role of T cell subsets and cytokines in the regulation of an intracellular bacterial infection. Braz. J. Med. Biol. Res. 32:77-84. [DOI] [PubMed] [Google Scholar]
- 23.Oñate, A., and H. Folch. 1995. 18.5 Kd protein. An interesting antigen of Brucella. Arch. Med. Vet. 27:93-102. [Google Scholar]
- 24.Oñate, A., E. Andrews, A. Beltran, G. Eller, G. Schurig, and H. Folch. 2000. Frequent exposure of mice to crude Brucella abortus proteins down-regulates immune response. J. Vet. Med. B 47:677-682. [DOI] [PubMed] [Google Scholar]
- 25.Oñate, A. A., R. Vemulapalli, E. Andrews, G. G. Schuring, S. Boyle, and H. Folch. 1999. Vaccination with live Escherichia coli expressing Brucella abortus Cu/Zn superoxide dismutase protects mice against virulent B. abortus. Infect. Immun. 67:986-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pertmer, T. M., M. D. Eisenbraun, D. McCabe, S. K. Prayaga, D. H. Fuller, and J. R. Haynes. 1995. Gene gun-based nucleic acid immunization: elicitation of humoral and cytotoxic T lymphocyte response following epidermal delivery of nanogram quantities of DNA. Vaccine 13:1427-1430. [DOI] [PubMed] [Google Scholar]
- 27.Schurig, G. G., R. M. Roop II, T. Bagchi, S. Boyle, D. Buhrman, and N. Sriranganathan. 1991. Biological properties of RB51; a stable rough strain of Brucella abortus. Vet. Microbiol. 28:171-188. [DOI] [PubMed] [Google Scholar]
- 28.Stevens, M. G., L. B. Tabatabai, S. C. Olsen, and N. F. Cheville. 1994. Immune responses to superoxide dismutase and synthetic peptides of superoxide dismutase in cattle vaccinated with Brucella abortus strain 19 or RB51. Vet. Microbiol. 41:383-389. [DOI] [PubMed] [Google Scholar]
- 29.Tabatabai, L. B., and G. W. Pugh, Jr. 1994. Modulation of immune responses in Balb/c mice vaccinated with Brucella abortus Cu-Zn superoxide dismutase synthetic peptide vaccine. Vaccine 12:919-924. [DOI] [PubMed] [Google Scholar]
- 30.Ulmer, J. B., J. J. Donnelly, S. E., Parker, G. H. Rhodes, P. L. Felgner, V. J. Dwarki, S. H. Gromkowski, R. R. Deck, C. M. DeWitt, A. Friedman, L. A. Hawe, K. R. Leander, D, Martinez, H. C. Perry, J. W. Shriver, D. L. Montgomery, and M. A. Liu. 1993. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259:1745-1749. [DOI] [PubMed] [Google Scholar]
- 31.Velikovsky, C. A., J. Cassataro, G. H. Giambartolomei, F. A. Goldbaum, S. Estein, R. A. Bodwen, L. Bruno, C. A. Fossati, and M. Spitz. 2002. A DNA vaccine encoding lumazine synthase from Brucella abortus induce protective immunity in BALB/c mice. Infect. Immun. 70:2507-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vemulapalli, R., Y. He, S. Cravero, N. Sriranganathan, S. M. Boyle, and G. G. Schurig. 2000. Overexpression of protective antigen as a novel approach to enhance vaccine efficacy of Brucella abortus strain RB51. Infect. Immun. 68:3286-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhan, Y., J. Yang, and C. Cheers. 1993. Cytokines response of T-cell subsets from Brucella abortus-infected mice to soluble Brucella proteins. Infect. Immun. 61:2841-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]