Abstract
Pregnancy increases the risk of listeriosis, a systemic disease caused by Listeria monocytogenes. However, there is incomplete agreement on the reasons for this increased risk. We examined two features of listeriosis in gravid and nongravid female mice following intragastric (gavage) inoculation, namely, (i) disease severity (measured by lethality) and (ii) listerial infectivity (measured by liver and spleen colonization levels up to 120 h postinoculation). Two listerial strains of differing serotype (1/2a and 4nonb) were initially employed. Neither strain produced a lethal infection in nonpregnant female mice (dose range, 106 to 109 CFU/mouse), and only the 4nonb strain produced lethalities in pregnant mice (dose range, 106 to 108 CFU/mouse). The 4nonb strain also produced a higher level of liver and spleen colonization than the 1/2a strain following gavage administration. (The two strains showed similar levels of colonization if parenterally administered.) Both strains were equally capable of binding to and forming plaques upon cultured mouse enterocytes. The ability of the 4nonb strain to produce a lethal infection in pregnant animals did not correlate with an increased incidence or level of liver and spleen colonization over that in nonpregnant females. However, the lethality rate did correlate well with the rate at which embryos and their surrounding decidual covering became infected, suggesting that intrauterine infection could be responsible for the increased disease severity in the gravid females.
Listeria monocytogenes is a gram-positive bacterium that can be isolated from soil, animal feed, water, feces, and tissues from a variety of invertebrate and vertebrate animals, including humans (8). Also, L. monocytogenes is a common contaminant of foodstuffs and has been recovered from raw vegetables, milk products, fish, poultry, and meats at rates of 15 to 70% (11). Consumption of contaminated food products can result in listerial escape from the intestinal lumen and the production of a systemic disease (listeriosis). The most recent listeriosis outbreak resulted in seven deaths and the largest recall of any agricultural product in history (27.4 million pounds of processed meat [3a]). It has been known for some time (26) that cell-mediated immunity is critical in containing and eliminating the infection due, at least in part, to the ability of listeriae to survive intracellularly (reviewed in reference 42).
Pregnancy greatly increases the risk of listeriosis in virtually all susceptible animals (14, 24, 41). In humans, approximately 33% of clinically documented cases of listeriosis are in pregnant women (2), and pregnant women constitute approximately 60% of all cases (male and female) in patients aged 10 to 40 years (6). In one outbreak, 65% of those contracting listeriosis were pregnant and 63% of those pregnancies ended in fetal or neonatal death (19).
Despite the well-known association of listeriosis with pregnancy, there is incomplete agreement on how pregnancy increases disease risk (5, 45). Laboratory-based investigations have been complicated by the varying virulence of listerial strains (3), the various routes of inoculation employed, and the normal resistance of the preferred host, the mouse, to infection via the natural route, the alimentary tract (22). A better understanding of how pregnancy increases disease risk is critically important in developing prophylactic strategies or, more simply, a scientifically based risk assessment for the exposure of pregnant women to sources of Listeria. Considering that the infectious dose for humans is not known (10, 23) and considering the somewhat arbitrary criteria used to regulate Listeria levels in prepackaged foods (31) and control listeriosis outbreaks (3a), much needs to be done.
In this report, we compare the gravid female mouse to her nongravid counterpart with respect to disease severity and susceptibility to infection. Since individual listerial strains differ in their ability to establish an intestinal infection (3), we initially employed two listerial strains, namely, a serotype 4nonb and a serotype 1/2a strain. We found that both strains were completely avirulent in nonpregnant female mice following gavage inoculation and that only the 4nonb strain produced a lethal infection in pregnant animals. The lethality in pregnant animals was not correlated with an increased incidence or level of liver and spleen colonization over that for nonpregnant females but rather with the rate at which embryos and their surrounding decidual covering became infected, suggesting that intrauterine infection could be responsible for the increased disease severity.
MATERIALS AND METHODS
Bacterial strains and media.
Two strains of L. monocytogenes were employed in this study, namely, strain 10403S, a streptomycin-resistant serotype 1/2a strain (7), and strain F6214-1, a spontaneous streptomycin-resistant derivative of a serotype 4nonb strain, F6214 (a gift of B. Swaminathan). Strain F6214 has been referred to by Pine et al. (32, 33) as strain G1032. Strains 10403S and F6214-1 are both naturally nalidixic acid-resistant. Two Tn917 insertion mutants of F6214-1 (PAS341 and PAS351; constructed as described below) were also employed. All strains were routinely propagated at 37°C in brain heart infusion (BHI) broth and BHI broth supplemented with 1.2% agar. Broth-grown bacteria destined for inoculation were initially cultivated overnight with shaking. The resulting stationary-phase culture was diluted 1:50 in prewarmed fresh BHI broth, and incubation continued with shaking until an optical density at 600 nm of between 0.3 and 0.6 was reached (logarithmic phase). Bacteria were harvested by centrifugation and resuspended in phosphate-buffered saline (PBS) to give the approximate doses needed. Viable counts (CFU) were determined by plating serial 10-fold dilutions on BHI agar plates and counting the resulting colonies after 2 days of incubation at 37°C. Where indicated, antibiotics were incorporated into BHI agar at the following concentrations: streptomycin, 50 μg/ml; nalidixic acid, 40 μg/ml; erythromycin, 1 μg/ml; lincomycin, 25 μg/ml; chloramphenicol, 10 μg/ml.
LD50 determinations.
The 50% lethal dose (LD50) required to kill 50% of inoculated female CD-1 mice was determined following gavage inoculation of groups of four mice with serial 10-fold-increasing amounts of the listerial strains grown and prepared as described above. The inoculum was delivered intragastrically using a 22-gauge feeding needle in a total volume of 0.25 ml. In the case of nonpregnant females, deaths were recorded until 11 days postinoculation. In the case of pregnant females, the dose was delivered at 7.5 gestational days (gd), and deaths were recorded until 18.5 gd (near term). Enumeration of gd began after observation of a postcoital plug on the morning (0.5 gd) following an evening pairing. Pairings were carried out by placing one male with three female mice. At 18.5 gd (11 days postinoculation), surviving pregnant mice were sacrificed, and the intrauterine contents were examined for signs of embryonic infection or resorption. Viable fetuses were enumerated and weighed. In rare instances, some mice appeared distressed soon after inoculation (ca. 24 h) and died prior to 72 h postinoculation. Such behavior was most often associated with obvious injury during the insertion or removal of the feeding needle. Since it was not always possible to detect injury at inoculation, mice showing obvious signs of distress (huddling and/or piloerection) within 24 h after gavage inoculation were assumed to have been injured and were not scored. The LD50 value was calculated by the methods of Reed and Meunch (37).
Fecal, liver, spleen, and embryo infectivity.
CD-1 female mice that were intragastrically inoculated for organ infectivity studies received an average intragastric dose of ca. 3 × 107 CFU/mouse in 0.25 ml of PBS through a 22-gauge feeding needle. In the case of pregnant females, the dose was delivered at 7.5 gd. At this dose (ca. one-fifth of the LD50 [see Results]), deaths and moribund animals were seldom seen over the 120-h time course of the infection. The average parenteral (tail vein) dose (ca. 2 × 103 CFU/mouse, delivered in 0.2 ml) was arrived at from published LD50 values (18) and trial and error. As with the gavage dose, the parenteral dose seldom resulted in lethalities or moribund animals over the 120-h time course.
In all infectivity experiments, six animals were employed, and all were inoculated at the same time and housed together. Two animals were sacrificed at 24, 72, and 120 h postinoculation. The liver, spleen, and, in the case of pregnant animals, concepti (embryos surrounded by their yolk sac and maternal decidua basalis) isolated from the uterus were homogenized in a small volume of chilled PBS with a motorized Teflon grinding apparatus. Concepti were obtained through an incision along the long axis of the uterine horn and by scraping the concepti free with forceps. In addition to the tissues just mentioned, the contents of ca. 1.5 cm of the distal colon were obtained by extruding the fecal pellets with forceps and homogenization of the pellets was carried out as for the other organs. In the case of the liver and spleen, the CFU reported represent the number of listeriae found in the entire organ. The CFU reported for concepti include all concepti in the uterus except in instances in which histological examination of the embryos was performed. In those instances, concepti from one of the two uterine horns were examined histologically. Concepti from the other horn were utilized for CFU determinations (corrected for the loss of ca. half of the concepti). In all cases, CFU were determined by plating dilutions of 2% of the total liver and spleen homogenates and 3.3% of the total conceptus homogenates on BHI medium containing streptomycin and nalidixic acid (to which both strains were resistant). The addition of these antibiotics suppressed the growth of fecal flora and had a relatively minor and reproducible effect on plating efficiency (ca. 15% reduction compared to that for plain BHI). In those experiments in which a mixture of two genetically marked strains were employed, homogenates were plated on BHI containing streptomycin and nalidixic acid. The resulting colonies were then replica printed (29) onto medium containing, in addition to streptomycin and nalidixic acid, erythromycin and lincomycin.
The average log10 CFU from the colon contents, liver, spleen, and concepti of each mouse was determined. Samples in which no CFU were detected were assigned a value of 1 to serve as a placeholder for averaging log10 values (log10 1 = 0) and applying other parametric statistical tests. Infection rate (incidence) was determined by dividing the number of animals whose specified organs contained listerial CFU when sampled (at 72 and 120 h) by the number of animals inoculated.
Cell culture and in vitro methods.
Replicate cultures of MODE-K (43) mouse enterocyte monolayers in 35- by 14-mm tissue culture wells were incubated for 10 min with L. monocytogenes strain F6214-1 or 10403S at ca. 107 CFU/ml in 1 ml of Dulbecco's modified Eagle's medium (Cellgro) and 5% fetal bovine serum. MODE-K cells exhibit several characteristics of normal mouse enterocytes, including intercellular junctions, cytokeratin, villin, poly-immunoglobulin receptors, vasoactive intestinal peptide receptors, and major histocompatibility complex class I molecules (43). At the end of this incubation period, the bacterial suspension was aspirated, and the monolayers were washed two times with 1 ml of Dulbecco's PBS (pH 7.4). Following these washes, one group of replicate cultures was lysed with 0.5 ml of Triton X-100 (0.1% solution). The lysates were serially diluted and plated to determine the numbers of listeriae bound to the MODE-K enterocytes. The second group of replicate cultures was used to determine plaquing efficiencies of the two listerial strains. Immediately following the Dulbecco's PBS washing procedure, 2 ml of Dulbecco's modified Eagle's medium, 5% fetal bovine serum, and gentamicin (5 μg/ml) were added and incubation continued at 37°C in a 5% CO2 humidified incubator. Sixty-eight hours later, the monolayers were fixed and stained with LeukoStat (Fisher Diagnostics), and the listerial plaques were counted. Plaquing efficiencies were similar when overlaying was done with a liquid medium (as described here) or semisolid agarose (data not shown).
Genetic methods.
Construction of Tn917 insertion mutants (PAS341 and PAS351) was initiated in strain F6214-1 by the introduction of the suicide vector pTV1 (46) by electroporation (39). Plasmid pTV1 carries the Tn917 transposon, a temperature-sensitive replication origin (p194ts [15]), and the chloramphenicol acetyltransferase gene (cat) that confers chloramphenicol resistance (Camr). Tn917 insertion mutants were isolated following a temperature shift from 31 to >44°C of a liquid culture of a Camr F6214-1 electroporant, dilution, and plating onto BHI agar plates containing erythromycin and lincomycin (resistances conferred by Tn917). Patching of selected colonies onto medium containing Cm revealed that >99.9% of the isolates had lost pTV1. Replica printing of ca. 1,500 Tn917 mutants onto Columbia blood agar revealed two nonhemolytic mutants (Hly−) that were subsequently shown to each have an insertion in the hly gene (28). Gene disruption was indicated by the absence of hly amplicons characteristic of the phenotypically Hly+ strains. Amplicons were generated with oligonucleotide primers Hly P1 (5′GTCTACCAATTGCGCAACAAACTGAAG) and Hly P2 (5′GTGCCCCAGATGGAGATATTTC). An Hly+ Tn917 insertion mutant, designated PAS351, was picked at random as a potential virulent control strain (i.e., fully virulent but containing a Tn917 insertion). Subsequent LD50 determinations (not shown) and organ colonization tests using one-to-one mixtures of strain PAS351 and the parental (F6214-1) strain justified its use in this capacity (see Results). DNA hybridizations using a Tn917 probe consisting of amplicons generated from amplification of a 605-bp region of the erythromycin resistance determinant (Erm P1, 5′ ACCGTTTACGAAATTGGAACAGG, and Erm P2, 5′ AGACAATACTTGCTCATAAGTAACGGTAC) revealed that PAS341 and PAS351 each had a single and distinctly different insertion site (data not shown). The sequence of the internalin A gene (inlA) was determined from amplicons generated with primers based upon inlA from strain EGD 1/2a (13). The oligonucleotide primers InlA 5 (5′TTACAGACGCAGCTCTAGCG) and InlA 3 (5′CCATGCTTGATCATTCAAC) were used to generate a PCR product of the 5′ end of inlA. Primers InlA 2 (5′ GCGATCTTACACCATTGG) and InlA 4 (5′ACCACATCTAGCTCTTTACAC) were used to generate a PCR product of the 3′ end of inlA. For sequencing, the PCR products were purified over a Centricon-100 column. Amplicons were sequenced at the University of North Carolina—Chapel Hill automated DNA sequencing facility on an Applied Biosystems model 3100 genetic analyzer. The sequencing reactions were performed with the ABI PRISM BigDye terminator cycle sequencing ready reaction kit with AmpliTaq DNA Polymerase FS (Applied Biosystems). The PCR primers used for amplification were also used for the DNA sequencing.
Histological methods.
Embryos for histological examination were initially sampled between 48 and 120 h postinoculation. Infected embryos decomposed rapidly, and histological examination 72 h after inoculation was not informative. The individual concepti were fixed in 4% paraformaldehyde, dehydrated, and paraffin imbedded by standard histological techniques. Sections (5 μm) were routinely Gram stained in order to reveal the presence of gram-positive rods. In initial experiments, a peroxidase-staining kit (Biogenics Inc) that employed polyclonal antibody to L. monocytogenes (Difco) was used to corroborate Gram stain results.
Statistical methods.
Standard deviation of the mean was calculated with the aid of Microsoft Excel's STDEV function. Standard error was calculated as the standard deviation divided by the square root of the number of experiments. The statistical significance of mean differences was determined by using Student's t test with the aid of Microsoft Excel's TTEST function. The statistical relevance of differences between bacterial strains or between pregnant or nonpregnant animals based upon dichotomous variables (i.e., infected and not infected) was determined by using the Fisher exact probability test (see the Vassarstat web site, http://faculty.vassar.edu/lowry/webtext.html) and Simple Interactive Statistical Analysis procedures (http://home.clara.net/sisa/fisher.htm). Unless otherwise noted, the probability of error threshold was such that P < 0.05.
RESULTS
Lethality of intragastrically inoculated L. monocytogenes strains 10403S and F6214-1 in pregnant and nonpregnant mice.
In order to examine the relationship between pregnancy and listeriosis severity, we examined the effect of intragastric inoculation of two strains of L. monocytogenes. In nonpregnant females, no deaths were seen with either strain at doses ranging between ca. 106 and 109 CFU/mouse. However, in pregnant animals, strain F6214-1 had an oral LD50 of 1.5 × 108 CFU, calculated over a dose range of ca. 106 to 108 CFU/mouse, whereas no deaths were witnessed in pregnant mice over the same dose range with strain 10403S.
Colon, liver, and spleen infectivity of intragastrically inoculated 10403S and F6214-1 in pregnant and nonpregnant mice.
To better understand the central importance of both listerial strain and pregnancy in disease severity, we compared the effect of pregnancy and listerial strain on the initial stages of the infection by examining the colon contents and internal organs of pregnant and nonpregnant female mice for colonization over the first 120 h postinfection.
Mice were intragastrically inoculated with ca. 3 × 107 CFU of either 10403S or F6214-1, and the colon contents, spleen, liver, and concepti were examined for viable L. monocytogenes organisms at 24, 72, and 120 h postinoculation. L. monocytogenes was detected in the colon contents of almost all mice sampled at 24 h postinoculation (35 of 38 mice [92%]). The level of colon colonization at 24 h was similar regardless of strain or pregnancy status: for strain 10403S, pregnant and nonpregnant mice had 2.8 ± 0.3 and 3.3 ± 0.5 log10 CFU, respectively, while results for strain F6214-1 were 2.7 ± 0.3 and 2.8 ± 0.4, respectively (values are average log10 CFU in distal colon contents ± standard deviations from a minimum of three experiments; none of the values were statistically different by an unpaired two-tailed Student's t test). Internal organ colonization at 24 h was rarely observed. Consequently, the levels of internal organ colonization at 72 and 120 h postinoculation were employed to assess strain- and pregnancy-associated differences in infectivity.
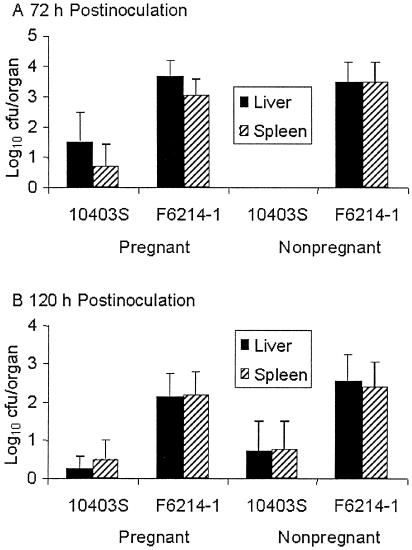
We found that animals inoculated with strain F6214-1 had a significantly higher level of liver and spleen colonization than those inoculated with strain 10403S, regardless of pregnancy status and time sampled (Fig. 1A and B). Also, the livers and spleens of pregnant mice were statistically no more apt to be colonized at a higher level than those of nonpregnant mice when comparisons were matched by infecting strain. This was true regardless of the time sampled (Fig. 1A and B). The low level of strain 10403S colonization was particularly noticeable in nonpregnant animals at 72 h (refer to Fig. 1A), but this low level was not statistically distinguishable from the level recorded for pregnant animals.
FIG. 1.
Liver and spleen colonization levels of strains 10403S and F6214-1 in pregnant and nonpregnant intragastrically inoculated groups of mice sacrificed at 72 (A) and 120 (B) h postinoculation. Columns indicate the average log10 CFU colonization levels, and error bars indicate standard errors of the mean. Averages were obtained from a minimum of three experiments, each involving at least two mice at each time point. Analysis for mean variation employed Student's t test in an unpaired, two-tailed test assuming equal variance. Statistically relevant differences are described in the text.
Since all mice were capable of being intestinally colonized by strain 10403S (refer to the colon colonization data described above), the attenuation exhibited by this strain appeared to be at some later stage in the infection. If the defect was in intestinal escape, then parenteral inoculation should result in comparable levels of internal organ colonization by both strains.
Liver and spleen infectivity of parenterally inoculated 10403S and F6214-1 in pregnant and nonpregnant mice.
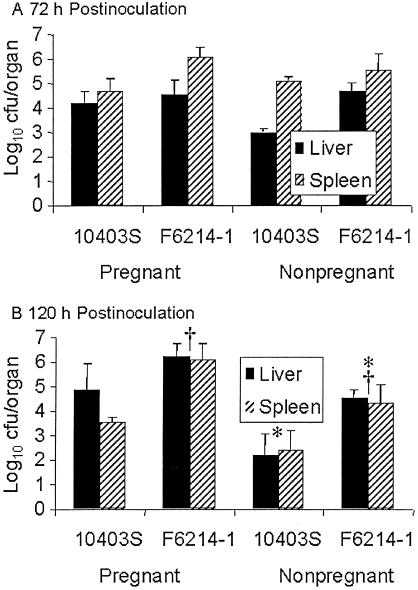
Parenteral administration (tail vein injection) of ca. 2 × 103 CFU of strain 10403S or strain F6214-1 resulted in the infection of the livers and spleens of virtually all mice sampled from 24 to 120 h postinoculation, regardless of listerial strain or pregnancy status. In contrast to the gavage inoculation, the two strains did not show, in general, a statistically discernible difference in liver and spleen colonization levels (Fig. 2A and B). There was only one instance in which F6214-1 had significantly higher liver and spleen CFU than 10403S: this was in the comparison of nonpregnant animals at 120 h postinfection (denoted by an asterisk in Fig. 2B). As with the intragastrically inoculated animals, pregnancy did not influence, in general, the level of liver and spleen colonization. In only one instance was there a statistically significant difference in the level of liver and spleen colonization between a pregnant and nonpregnant animal: this was at the measurement taken at 120 h in animals infected with strain F6214-1 (denoted by a cross in Fig. 2B).
FIG. 2.
Liver and spleen colonization levels of strains 10403S and F6214-1 in pregnant and nonpregnant parenterally inoculated groups of mice sacrificed at 72 (A) and 120 (B) h postinoculation. Columns indicate the average log10 CFU colonization levels, and error bars indicate standard errors of the mean. Averages were obtained from a minimum of two experiments, each involving at least two mice at each time point. Analysis for mean variation employed Student's t test in an unpaired, two-tailed test assuming equal variance. Statistically relevant differences referred to in the text are denoted with an asterisk (*) or cross (†).
In vitro mouse enterocyte binding and plaque formation.
The foregoing results indicated that 10403S could be less effective in escaping the intestinal lumen than F6214-1. To see if this putative defect could be at the level of enterocyte binding or enterocyte infectivity, we compared the two strains in a mouse enterocyte cell line (MODE-K) for their ability to (i) bind to and (ii) form plaques on this cell line. We found that the two strains were not significantly different in either respect. Results for binding efficiency (number of bacteria bound relative to CFU added to a monolayer) for strains 10403S and F6214-1 were 1.9 ± 0.4 and 1.4 ± 0.2, respectively, and results for plaquing efficiency (number of plaques per bacterium bound) were 2.8 ± 1.6 and 3.4 ± 0.8, respectively (results for binding and plaquing efficiencies are multiplied by 102 and 105, respectively, for ease of comparison). No strain-associated differences were revealed by a two-tailed Student's t test of paired data sets.
In one further test, we examined the predicted primary sequence of internalin A from each strain. In at least one serotype 1/2a strain (EGD [22]), internalin A contributes to the specificity of the bacterial interaction with cells of the intestinal tract (22). If the internalin from 10403S (a serotype 1/2a strain) and F6214-1 were identical, then internalin A could not be responsible for the difference in infectivity of the two strains when delivered intragastrically. The DNA sequenced from both inlA alleles (accession numbers AY228471 [strain10403S] and AY228472 [strain F6214-1]) revealed a number of predicted amino acid differences and thus left open the possibility that inlA allelic differences could be at least in part responsible for the infectivity differences.
Correlation between conceptus infectivity and disease severity.
Whereas disease severity (as measured by LD50) and liver and spleen infectivity both supported the more virulent nature of strain F6214-1 over that of 10403S, the reason that a pregnant animal was more likely to die from an infection with F6214-1 than her nonpregnant counterpart was not as clear: both pregnant and nonpregnant animals appeared equally susceptible to F6214-1 infection in terms of initial liver and spleen colonization levels (refer to Fig. 1). Also, infection rates (for liver and spleen) over the first 120 h postinoculation were similar (17 of 26 for nonpregnant animals [65%] and 24 of 38 for pregnant animals [63%]). Finally, both pregnant and nonpregnant animals appeared equally resistant to a listeriolysin O (LLO)-negative mutant, PAS341 (F6214-1 [hly::Tn917]), with the mutant being undetectable in mixed infections in any liver, spleen, or conceptus sample from any mouse examined (n = 22) at any time point, regardless of oral or parenteral routes of inoculation.
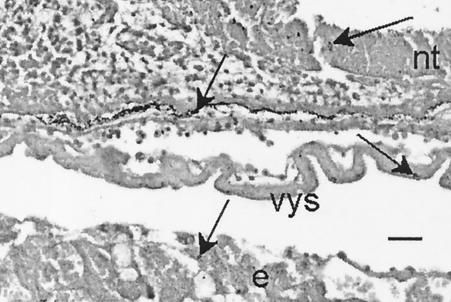
The most likely explanation for the increased disease severity with gravid animals was provided by an examination of the concepti. While conceptus infection was not as common as liver and spleen infection (see below), routine microbiological examination of harvested concepti revealed bacterial numbers often comparable to those of liver and spleen (data not shown). When concepti were examined histologically, bacteria were present in the embryo, the yolk sack, and the surrounding decidual tissue (Fig. 3).
FIG. 3.
Gram-stained section through a 9.5-gd conceptus (ca. 48 h after intragastric inoculation). Arrows point to foci of listerial infection in the neural tube (nt), the vitelline membrane (vys), and the endometrial decidual layer (e). Bar, 40 μm.
Several lines of evidence suggested that concepti were relatively difficult to infect but that conceptus infection occurring within 120 h postinoculation predicted maternal mortality. (Maternal deaths, if they occurred, did not typically occur within the 120-h time frame employed for the infectivity studies.) First, embryo infection was never observed in any mouse in the absence of liver and/or spleen colonization, regardless of inoculation route or listerial strain employed. (This suggested that concepti were not more readily infected than the liver or spleen.) Second, conceptus colonization following gavage inoculation was noted in only a single instance in the case of the less virulent strain, 10403S (13 mice examined), compared to for strain F6214-1 13 of 38 mice examined. Third, inoculation (parenteral or gavage) of pregnant mice with a one-to-one mixture of a genetically marked but fully virulent strain of F6214-1 (PAS351) and F6214-1 produced mice in which the infected concepti were significantly more likely than liver or spleen to be colonized with just one of the infecting strains (Table 1). Finally, in pregnant mice infected with F6214-1, the proportion of mice with infected concepti (when organs were examined at 72 or 120 h postinfection) correlated most closely with the proportion of pregnant mice that died when a similar dose was administered and the infection was allowed to proceed to death or to near term (18.5 gd) (Table 2).
TABLE 1.
Distribution of strains F6214-1 and insertion mutant PAS351 following inoculation of a one-to-one mixture of the strains into groups of pregnant mice
| Route | No. of organs infected with one strain/total no. of organs infecteda
|
||
|---|---|---|---|
| Liver | Spleen | Conceptusc | |
| Parenteral | 0/6 | 1/6 | 3/4 |
| Gavageb | 0/4 | 0/4 | 2/3 |
Groups of six mice were inoculated with ca. 2 × 103 CFU (parenteral) or 3 × 107 CFU (gavage). Two mice from each group were sacrificed at 24, 72, and 120 h postinoculation. The denominator indicates the number of mice that had CFU detected in the organ listed at the time of sacrifice. The numerator indicates the number of those organs that had only one of the inoculated strains rather than both.
Results were compiled from two separate experiments. Fisher's exact analysis of the incidence of single-strain infection (combining incidences from intragastric and parenteral routes) revealed that the conceptus was significantly more likely to be colonized by a single strain than was the liver or the spleen.
In the five conceptus samples colonized with only one strain, strains F6214-1 and PAS351 were isolated at approximately equal frequencies (i.e., two conceptus samples had only F6214-1 and three had only PAS351).
TABLE 2.
Proportion of organs infected following gavage administration of strain F6214-1 (ca. 3 × 107 cfu/mouse) compared to the proportion of mice dying when inoculated at a similar dose
| Proportion (%) of mice dyinga | Proportion (%) of organs infected at 72 and 120 h postinoculation in:
|
||
|---|---|---|---|
| Liver | Spleen | Conceptus | |
| 4/11 (36%) | 26/38 (68%) | 24/37 (65%) | 13/38 (34%) |
The proportion of mice dying was obtained from LD50 measurements. Fisher's exact analysis revealed that none of the infection rates for liver, spleen, and conceptus were necessarily independent of death rate.
DISCUSSION
The results herein presented indicate that both listerial strain and pregnancy status can affect the severity of murine listeriosis when the inoculum is administered via gavage. Whereas neither strain was virulent for nonpregnant mice, the serotype 4nonb strain (F6214-1) produced a more severe infection (as indicated by LD50 results) and was more highly infective (as measured by CFU in the liver and spleen over the first 120 h postinoculation) than the 1/2a serotype strain (10403S). The ability of strain F6214-1 to produce a lethal infection in gravid females was not correlated with a higher incidence or level of liver and spleen colonization over nongravid females during the first 120 h postinoculation but rather with the incidence of conceptus infection. To our knowledge, this is the first study to incorporate conventionally reared, outbred pregnant animals, a natural route of infection, and an examination of the virulence characteristics of two different listerial strains inoculated at a comparatively early stage in pregnancy.
In agreement with a number of previous studies, we found that pregnant mice were much more susceptible to lethal infection by listeria than nonpregnant female mice (1, 20, 21, 40). In fact, we witnessed no deaths of nonpregnant animals even at relatively high intragastric doses (ca. 109 CFU). With pregnant animals, we found that doses as low as 106 CFU produced some mortality with the virulent F6214-1 strain. When animals were dosed at ca. 109 CFU and above, the response of pregnant animals to F6214-1became erratic, with fewer animals dying at the higher dose than is seen with doses of 1 and 2 orders of magnitude lower. This result, although presently unexplained, led us to suspect that in doses much higher than 108 CFU, we were triggering some host reflex (e.g., increased peristalsis, gastric reflux) not produced by the lower doses. In support of this interpretation, we saw no average increase in intestinal CFU 24 h postinoculation when mice were dosed at 109 CFU/mouse compared to those of 107 CFU/mouse and more mouse-to-mouse variation in colonization level than when the dose was ca. 107 CFU (data not shown). As a consequence of the foregoing, doses ranging from 106 to 108 CFU/mouse were utilized for LD50 determinations.
In the infectivity studies, virtually all animals that were intragastrically inoculated showed intestinal colonization when examined at 24 h. However, strain F6214-1 was significantly more effective in producing infections of the liver and spleen. From this result, we suspected that strain 10403S had some defect in intestinal escape. This conclusion was supported by parenteral inoculation studies, where the two strains were indistinguishable in terms of levels of liver and spleen colonization at most of the time points examined. In vitro tests with a mouse-derived enterocyte cell line (MODE-K) failed to show a difference between the strains in terms of their abilities to bind to and form plaques upon this cell line. Future manipulation of the MODE-K cell line (e.g., polarization) with the aim of better mimicking the morphological features of the mouse gut may be warranted. However, another report (33) has indicated a lack of correlation between cytopathic effects in cultured cells (Caco-2) and virulence in some L. monocytogenes strains, particularly strain F6214 (referred to as strain G1032 in the citation). This may indicate a lack of relevance of these immortalized lines in reliably mimicking relevant features of the in vivo infection.
The lack of virulence of strain 10403S via the alimentary tract supported results of others, most recently Lecuit et al. (22), who showed that the internalin expressed by a related L. monocytogenes serotype 1/2a strain (EGD) does not bind to E-cadherin on mouse epithelial cells and that virulence is increased if the proper E cadherin is supplied in a transgenic mouse. We suspect that our 4nonb strain (F6214-1) is better matched to the mouse intestine. It is unclear if this better match is facilitated by a more effective interaction between mouse E cadherin and the F6214-1 internalin (although this is possible, since we found that the inlA DNA sequence predicted a number of amino acid differences) or some other feature(s) of the F6214-1 infectious process. Our results with strain F6214-1 disagreed somewhat with those of Pine et al., (32) who witnessed lethality in nonpregnant mice following intragastric inoculation with essentially the same strain that we employed. However, there were significant differences in experimental protocols. For example, the mice we employed were mature, fully immunocompetent, and outbred. Recent experiments by Czuprynski et al. (9), using two inbred lines of mice, indicate that mouse strain is a significant factor in determining listerial susceptibility via the intragastric route.
We found that pregnancy did not increase liver and spleen infectivity of either listerial strain delivered via gavage: average bacterial loads over the first 120 h postinoculation were similar in both pregnant and nonpregnant animals. Even when delivered parenterally, it was only at the latest time tested that liver and spleen CFU were seen to differ between pregnant and nonpregnant females with the more virulent F6214-1 strain. We conclude that pregnancy did not predispose females to listerial infection (at least at the 7.5 gd we examined). Rather, it seemed that the infection, once acquired, was more severe. This conclusion is similar to that formed by several other investigators (20, 21, 34) but is contrary to one other report that indicated higher initial CFU in the livers and spleens of pregnant mice (40). Our results are also contrary to reports that show lower initial CFU in livers and (or) spleens of pregnant animals (17, 25, 44). In most cases just cited, listeria and host genetic backgrounds differed from ours, as did inoculation route, dose, tissues collected, gd of inoculation (later in all cases), and times postinoculation at which mice were sampled. These factors all likely contribute to some of the disagreement. Indeed, we documented here how listerial strain and infectious route could influence results.
Reports that cells of the decidual membrane were restricted in their ability to prevent infection of the embryo (16, 27, 35, 36) initially led us to test a Hly− (LLO-deficient) mutant to see if it could more readily cause disease in a pregnant animal. In agreement with the studies of McKay and Lu (27), who specifically examined decidual tissue of pregnant animals following parenteral inoculation, we found the Hly− mutant to be completely attenuated regardless of the route of inoculation or the pregnancy status of the animal. In fact, the intragastrically inoculated Hly− mutant did not persist in the colon for more than 24 h. The inability of Hly− mutants to survive intracellularly in cultured cells is often cited as the reason for attenuation in vivo (22). However, our present results and one earlier report (38) indicate that the defect is detectable much earlier in the infectious process.
As found by Redline and Lu (34) with parenteral inoculations at 14 gd, we observed that the lethality rate in intragastrically inoculated pregnant animals best correlated with the conceptus infection rate. We found no support for the notion that concepti were particularly prone to infection. In fact, concepti appeared to constitute an infective bottleneck: in a mixed infection of equally virulent listerial strains, concepti were most often infected by only one of the strains. Whereas the concepti may be relatively difficult to infect, we feel it likely, as do others (4, 16), that the nature of the fetoplacental boundary impairs maternal ability to respond to an intrauterine infection (hence the ability of a single infecting microorganism to expand). This impairment may be due to immunological constraints at the maternal fetal boundary (16) and/or because mice, unlike some other animals (e.g., humans), seldom expel dead embryos. Rather, they retain and resorb them.
In conclusion, we feel that the pregnant mouse, if paired with a strain of L. monocytogenes of appropriate virulence, can be used as a model to better understand why pregnant females are more prone to listeriosis. For example, questions about how gestational stage affects susceptibility in humans (12, 45) can be approached in the mouse, whose hemochorial placenta (30) resembles that of the human. In addition, the mouse may provide a better understanding of the embryological consequences of infection; mouse embryological development is well described (30), and we have shown here how maternal predisposition is affected even comparatively early in gestation when embryological development is progressing most rapidly.
Acknowledgments
We express our gratitude to Lise Barley-Maloney and Steve Simkins for performing the mouse intragastric and intravenous injections, respectively. Also, we thank Craig Altier for a critical reading of the manuscript.
This work was supported by grants from the National Institutes of Health and the state of North Carolina.
Editor: J. T. Barbieri
REFERENCES
- 1.Abram, M., and M. Doric. 1997. Primary Listeria monocytogenes infection in gestating mice. Folia Microbiol. 42:65-71. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, D. 1995. Listeria monocytogenes, p. 1880-1885. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 4th ed. Churchill Livingstone, New York, N.Y.
- 3.Barbour, A. H., A. Rampling, and C. E. Hormaeche. 2001. Variation in the infectivity of Listeria monocytogenes isolates following intragastric inoculation of mice. Infect. Immun. 69:4657-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a. Centers for Disease Control and Prevention. 2001. Public health dispatch: outbreak of listeriosis—northeastern United States, 2002. Morb. Mortal. Wly. Rep. 51:950-951. [PubMed] [Google Scholar]
- 4.Chao, K-H., Y-S. Yang, H-N. Ho, S-U. Chen, H-F. Chen, J.-J. Dai, S-C. Huang, and T. J. Gill III. 1995. Decidual natural killer cytotoxicity decreased in normal pregnancy but not in anembryonic pregnancy and recurrent spontaneous abortion. Am. J. Reprod. Immunol. 34:274-280. [DOI] [PubMed] [Google Scholar]
- 5.Chaouat, G., and E. Menu. 1997. Maternal T cell reactivity in pregnancy? Curr. Top. Microbiol. Immunol. 222:103-126. [DOI] [PubMed] [Google Scholar]
- 6.Ciesielski, C. A., A. W. Hightower, S. K. Parsons, and C. V. Broome. 1988. Listeriosis in the United States: 1980-1982. Arch. Intern. Med. 148:1416-1419. [PubMed] [Google Scholar]
- 7.Conlan, J. W. 1997. Neutrophils and tumor necrosis factor-α are important for controlling early gastrointestinal stages of experimental murine listeriosis. J. Med. Microbiol. 46:239-250. [DOI] [PubMed] [Google Scholar]
- 8.Cooper, J., and R. D. Walker. 1998. Listeriosis. Vet. Clin. North Am. Food Anim. Pract. 14:113-125. [DOI] [PubMed] [Google Scholar]
- 9.Czuprynski, C. J., N. G. Faith, and H. Steinberg. 2003. A/J mice are susceptible and C57BL/6 mice are resistant to Listeria monocytogenes infection by intragastric inoculation. Infect. Immun. 71:682-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farber, J. M., E. Daley, F. Coates, N. Beausoleil, and J. Fournier. 1991. Feeding trial of Listeria monocytogenes with a nonhuman primate model. J. Clin. Microbiol. 29:2606-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flaxman, S. M., and P. W. Sherman. 2000. Morning sickness: a mechanism for protecting mother and embryo. Q. Rev. Biol. 75:113-148. [DOI] [PubMed] [Google Scholar]
- 13.Gaillard, J. L., P. Berche, C. Frehel, E. Gouin, and P. F. Cossart. 1991. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell 65:1127-1141. [DOI] [PubMed] [Google Scholar]
- 14.Gellin, B. G., C. V. Broome, W. F. Bibb, R. E. Weaver, S. Gaventa, L. Mascola, et al. 1991. The epidemiology of listeriosis in the United States—1986. Am. J. Epidemiol. 133:392-401. [DOI] [PubMed] [Google Scholar]
- 15.Gryczan, T. J., J. Hahn, S. Contente, and D. Dubnau. 1982. Replication and incompatibility properties of plasmid pE194 in Bacillus subtilis. J. Bacteriol. 152:722-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guleria, I., and J. W. Pollard. 2000. The trophoblast is a component of the innate immune system during pregnancy. Nat. Med. 6:589-593. [DOI] [PubMed] [Google Scholar]
- 17.Hamada, M., A. Kuriowa, T. Matsumo, K. Nomoto, and K. Takega. 1981. Modification of protective mechanisms against Listeria monocytogenes during pregnancy. J. Clin. Lab. Immunol. 6:169-173. [PubMed] [Google Scholar]
- 18.Havell, E. A., G. L. Spitalny, and P. J. Patel. 1982. Enhanced production of murine interferon γ by T cells generated in response to bacterial infection. J. Exp. Med. 156:112-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James, S. M., S. L. Fannin, B. A. Agee. 1985. Listeriosis outbreak associated with Mexican-style cheese, California. Morb. Mortal. Wkly. Rep. 34:357-360. [PubMed] [Google Scholar]
- 20.Klink, M., and W. Rudnicka. 1995. Listeria monocytogenes infection in pregnant mice: abnormalities in the function of non-accessory light density dendritic cells. FEMS Immunol. Med. Microbiol. 12:143-152. [DOI] [PubMed] [Google Scholar]
- 21.Lammerding, A. M., K. A. Glass, A. Gendron-Fitzpatrick, and M. P. Doyle. 1992. Determination of virulence of different strains of Listeria monocytogenes and Listeria innocua by oral inoculation of pregnant mice. Appl. Environ. Microbiol. 58:3991-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lecuit, M., S. Vandormael-Pournin, J. Lefort, M. Huerre, P. Gounon, C. Dupuy, C. Babinet, and P. Cossart. 2001. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 292:1722-1725. [DOI] [PubMed] [Google Scholar]
- 23.Lorber, B. 1997. Listeriosis. Clin. Infect. Dis. 24:1-11. [DOI] [PubMed] [Google Scholar]
- 24.Low, J. C., and W. Donachie. 1997. A review of Listeria monocytogenes and listeriosis. Vet. J. 153:9-29. [DOI] [PubMed] [Google Scholar]
- 25.Luft, B. J., and J. S. Remington. 1982. Effect of pregnancy on resistance to Listeria monocytogenes and Toxoplasma gondii infections in mice. Infect. Immun. 38:1164-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackaness, G. B. 1962. Cellular resistance to infection. J. Exp. Med. 116:381-406. [PubMed] [Google Scholar]
- 27.McKay, D. B., and C. Y. Lu. 1991. Listeriolysin as a virulence factor in Listeria monocytogenes infection of neonatal mice and murine decidual tissue. Infect. Immun. 59:4286-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mengaud, J., M.-F. Vicente, J. Chenevert. J. M. Pereira, C. Geoffroy, B. Gicquel-Sanzey, F. Baquero, J.-C. Perez-Diaz, and P. Cossart. 1988. Expression in Escherichia coli and sequence analysis of the listeriolysin O determinant of Listeria monocytogenes. Infect. Immun. 56:766-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Noden, D. M., and A. De Lahunta. 1985. Extraembryonic membranes and placentation, p. 47-69. In G. Stammathis and V. M. Vaughn (ed.), The embryology of domestic animals: developmental mechanisms and malformations. Williams & Wilkins, Baltimore, Md.
- 31.Notermans, S., J. Dufrenne, P. Teunis, and T. Chackraborty. 1998. Studies on the risk assessment of Listeria monocytogenes. J. Food Prot. 61:244-248. [DOI] [PubMed] [Google Scholar]
- 32.Pine, L., G. B. Malcom, and B. D. Plikaytis. 1990. Listeria monocytogenes intragastric and intraperitoneal approximate 50% lethal doses for mice are comparable, but death occurs earlier by intragastric feeding. Infect. Immun. 58:2940-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pine, L., S. Kathariou, F. Quinn, V. George, J. D. Wenger, and R. E. Weaver. 1991. Cytopathogenic effects in enterocytelike Caco-2 cells differentiate virulent from avirulent Listeria strains. J. Clin. Microbiol. 29:990-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redline, R. W., and C. Y. Lu. 1987. Role of local immunosuppression in murine fetoplacental listeriosis. J. Clin. Investig. 79:1234-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Redline, R. W., and C. Y. Lu. 1988. Specific defects in the anti-listerial immune response in discrete regions of the murine uterus and placenta account for susceptibility to infection. J. Immunol. 140:3947-3955. [PubMed] [Google Scholar]
- 36.Redline, R. W., C. M. Shea, V. E. Papaioannou, and C. Y. Lu. 1988. Defective anti-listerial responses in deciduoma of pseudopregnant mice. Am. J. Pathol. 133:485-497. [PMC free article] [PubMed] [Google Scholar]
- 37.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent end points. Am. J. Hyg. 27:293-299. [Google Scholar]
- 38.Roll, J. T., and C. J. Czuprynski. 1990. Hemolysin is required for extraintestinal dissemination of Listeria monocytogenes in intragastrically inoculated mice. Infect. Immun. 58:3147-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sekizaki, T., T. Tanoue, M. Osaki, Y. Shimoji, S. Tsubaki, and S. Takai. 1998. Improved electroporation of Rhodococcus equi. J. Vet. Med. Sci. 60:277-279. [DOI] [PubMed] [Google Scholar]
- 40.Shinomiya, N., S. Tsuru, M. Taniguchi, H. Fujisawa, M. Ikeda, Y. Zinnaka, and K. Nomoto. 1986. Immune protective mechanisms during pregnancy. I. Cell-mediated immunity against Listeria monocytogenes in pregnant mice. Immunology 59:373-378. [PMC free article] [PubMed] [Google Scholar]
- 41.Silver, H. M. 1998. Listeriosis during pregnancy. Obstet. Gynecol. Surv. 53:737-740. [DOI] [PubMed] [Google Scholar]
- 42.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vidal, K., I. Grosjean, J. E. Revillard, C. Gespach, and D. Kaiserlain. 1993. Immortalization of mouse intestinal epithelial cells by the SV-40 T gene. Phenotypic and immune characterization of the MODE-K cell line. J. Immunol. Methods 166:63-73. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe, Y., M. Mitsuyama, M. Sano, H. Nakano, and K. Nomoto. 1986. Enhanced resistance against Listeria monocytogenes at an early phase of primary infection in pregnant mice: activation of macrophages during pregnancy. Infect. Immun. 52:730-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinberg, E. D. 1984. Pregnancy-associated depression of cell-mediated immunity. Rev. Infect. Dis. 6:814-831. [DOI] [PubMed] [Google Scholar]
- 46.Youngman, P. J., J. B. Perkins, and R. Losick. 1983. Genetic transposition and insertional mutagenesis in Bacillus subtilis with Streptococcus faecalis transposon Tn917. Proc. Natl. Acad. Sci. USA 80:2305-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]