Abstract
Symbiotic Wolbachia organisms of filarial nematodes have received much attention as possible chemotherapy targets and disease-causing organisms. In order to further investigate the association between anti-Wolbachia immune responses and chronic filarial disease in humans, antibody responses to Wolbachia surface protein (WSP) were assayed in serum samples collected from 232 individuals living in Leogane, Haiti, an area where Wuchereria bancrofti infection is endemic, and from 67 North Americans with no history of lymphatic filariasis. As opposed to antifilarial antibody responses, which were largely influenced by the patient's infection status, the prevalence and levels of anti-WSP immunoglobulin G (IgG) antibodies among individuals with lymphedema or hydrocele were significantly greater than those in gender- and infection-matched individuals without disease. In at least one case, the anti-WSP IgG response was coincident with the onset of lymphedema development, and among anti-WSP-positive women with lymphedema, anti-WSP IgG levels were negatively correlated with the duration of lymphedema. The presence of anti-WSP IgG was also associated with the severity of inguinal adenopathy among men with hydrocele. In addition to the presence of anti-WSP antibodies among Haitians, 15 of 67 (22%) serum samples collected from individuals from North America, where filariasis is not endemic, were also positive for anti-WSP antibodies. In comparison to those from Haitians, anti-WSP antibodies from North Americans primarily recognized a distinct region of WSP located within the highly conserved second transmembrane domain. The results of this study demonstrate that anti-WSP antibody responses are associated with the presence of chronic filarial morbidity and not filarial infection status in humans and suggest that WSP should be further studied as a potential trigger for the development of filarial disease.
Bancroftian filariasis is a mosquito-transmitted parasitic disease of humans that has been considered to be potentially eradicable due to the inefficiency of transmission of the filarial parasites to humans and the fact that there are no zoonotic reservoir hosts of the parasite. The goals of the current global lymphatic filariasis elimination program are to (i) reduce microfilaremia levels, by using filaricidal drugs, to a level that is too low to sustain transmission of filarial parasites to humans and (ii) reduce the morbidity associated with chronic filarial disease (9). However, in order to achieve these goals, research efforts are still needed to develop better filaricidal drugs (especially macrofilaricides) and a better understanding of the etiology of chronic filarial disease. One aspect of the biology of filarial nematodes that may be exploited in the effort to advance the elimination program is the presence of a rickettsia-like endosymbiont belonging to the genus Wolbachia found inside many filarids. Recent studies of symbiotic Wolbachia organisms suggest that these bacteria may be potentially important as both chemotherapeutic targets and disease-causing organisms.
In animal models of filarial infection, treatment with antibiotics that specifically target Wolbachia decreases microfilaria loads, inhibits development of larval worms, and renders adult female worms infertile (3, 6, 16, 24). In addition, high doses of antibiotics have been shown to have adulticidal effects in Onchocerca volvulus and Brugia malayi (22, 24). Other studies have shown that inflammatory responses induced by Wolbachia endotoxin may be responsible for the systemic adverse reactions following treatment with microfilaricidal drugs (8, 20, 27). These results imply that therapy that eliminates Wolbachia may reduce the adverse reactions associated with current treatment regimens. Human trials in Ghana are currently exploring the efficacy of using doxycycline as a possible treatment for human onchocerciasis (15, 17). While the lengthy course of antibiotic therapy and the possibility of inducing antibiotic resistance may make anti-Wolbachia treatment impractical as a public health measure, such therapy may be beneficial to patients on an individual basis (i.e., as treatment for infected individuals returning from areas where filariasis is endemic).
In addition to the possible role of Wolbachia as a chemotherapy target, evidence suggests that Wolbachia antigens can stimulate host immune responses that may be associated with the development of filarial disease. In a laboratory model of onchocerciasis, Wolbachia endotoxin has been shown to mediate neutrophil infiltration and stromal haze when a worm extract including Wolbachia antigens was injected into the eyes of mice (25). Furthermore, we have shown that B. malayi-infected rhesus monkeys mount antibody responses to Wolbachia surface protein (WSP) that are temporally associated with the death of filarial worms and lymphedema development (23). Although these studies suggest that Wolbachia may be important in understanding human disease caused by filarial worms, no studies to date have reported Wolbachia-specific immune responses among human populations with lymphatic filariasis. In the present study, we have assayed antibody responses to WSP in a cohort of Haitian individuals living in an area where Wuchereria bancrofti infection is endemic. The results reported here compare anti-WSP and antifilarial antibody responses among individuals with morbidity to those of individuals without morbidity to determine whether the presence of disease, as opposed simply to infection, is associated with anti-WSP antibody responses.
MATERIALS AND METHODS
Study population.
Banked serum samples from 232 adult individuals living in Leogane, Haiti, an area where W. bancrofti infection is endemic, were selected based on serum availability for a retrospective analysis of antibody responses to WSP. In addition, 10 longitudinally collected serum specimens from one individual were available to assay anti-WSP antibody levels before and after the onset of lymphedema. Serum samples were collected over a 10-year period ranging from 1989 to 1999 and stored frozen at −20°C until use. All serum samples were collected before the initiation of the ongoing mass drug administration that is part of the lymphatic filariasis elimination program in this area. Individuals in this study were selected to represent the major parasitologic and clinical outcomes of infection seen in Leogane, Haiti. Infection status at the time of blood drawing was determined by the presence of microfilaremia and/or filarial antigenemia. Microfilaremia was assessed by filtering 1 ml of whole nocturnal blood through a Nuclepore filter, and microfilaremia was recorded as number of microfiliariae (Mf) per milliliter of blood. Antigenemia was assessed by the commercially available ICT card test or antigen enzyme-linked immunosorbent assay (ELISA). Clinical disease status was determined by physical examination at the time that the serum sample was collected. The two major clinical outcomes of infection seen in this area are hydrocele in men and lymphedema of the leg, primarily in women. In addition, serum samples from 67 North Americans with no history of filariasis were selected and assayed for anti-WSP antibody responses. All serum samples from human subjects were collected under protocols approved by the institutional review boards of the Centers for Disease Control and Prevention (CDC) and the University of Georgia.
ELISA.
Antifilarial immunoglobulin G1 (IgG1) and IgG4 antibody levels were determined by ELISA with a crude adult Brugia pahangi antigen extract as previously described (14). Levels of serum antibody to a recombinant WSP antigen were also determined by ELISA. The wsp gene from Wolbachia of B. malayi was cloned into the expression vector pQE41 (Qiagen, Valencia, Calif.), and the recombinant WSP protein was expressed and purified as previously described (23). The wsp genes from Wolbachia of B. malayi (EMBL accession number AJ252062) and W. bancrofti (EMBL accession number AJ252180) share 97% identity at the nucleotide level and 98% identity at the amino acid level (5). Ninety-six-well microtiter plates were coated with WSP (0.5 μg/ml) diluted in 0.1 M NaHCO3 by overnight incubation at 4°C. Following a blocking period with 0.1 M phosphate-buffered saline plus 0.3% Tween 20 (0.3% PBST), human serum samples diluted 1:50 in 0.3% PBST were added to the wells in duplicate and incubated overnight at 4°C. The next day, plates were washed four times with 0.3% PBST and then incubated with a biotinylated mouse anti-human IgG secondary antibody (1:500; Zymed, South San Francisco, Calif.) for 2 h at room temperature. Following another wash step, plates were incubated with streptavidin-alkaline phosphatase (1:500; GibcoBRL, Grand Island, N.Y.) for 1 h at room temperature and subsequently developed by the addition of 0.1% p-nitrophenylphosphate-3 mM MgCl2-10% diethanolamine (pH ∼9.8). The optical density of each well was determined with a Molecular Devices UVmax microplate reader. Plates were allowed to develop to an optical density at 405 nm of 1 for the highest point on the standard curve (see below).
Each plate contained a standard curve consisting of twofold serial dilutions (1:20 to 1:2,560) of a human serum sample determined to have a high antibody titer to WSP (data not shown). The highest point on the standard curve was assigned a value of 1,280 arbitrary units, and unit values for unknown serum samples were determined by comparison to the standard curve. Determinations for duplicate serum samples with a coefficient of variation of ≥15% were repeated. In addition to the standard curve, each plate contained three Haitian serum samples that were determined to be negative by Western blotting (data not shown). A cutoff for determining a positive anti-WSP response was determined independently for each plate by the mean unit values of the negative controls plus three standard deviations.
Epitope mapping.
Twenty-six biotinylated peptides were chemically synthesized to cover the entire predicted amino acid sequence of the B. malayi WSP protein (minus the N-terminal signal sequence). Peptides were synthesized as 18-mers that overlapped by nine amino acids. Peptides were solubilized according to the manufacturer's instructions, and 100 ng of each peptide (diluted in 0.05% PBST) was added to an individual well of streptavidin-coated microtiter plates in duplicate and incubated overnight at 4°C. The next day, microtiter plates were washed and blocked with a 1% casein-0.3% PBST solution. Serum samples that were determined to be positive for anti-WSP IgG antibodies by ELISA (n = 77) were assayed to determine which linear epitopes of the WSP protein were recognized. Because most IgG antibodies to WSP were of the IgG1 subclass (data not shown), we assayed IgG1 responses to the overlapping peptides. Human serum samples diluted 1:50 in 1% casein-0.3% PBST were added to each plate and incubated overnight at 4°C. Serum antibodies that recognized WSP peptides were detected by the addition of a secondary mouse anti-human IgG1 antibody (1:2,000; provided by V. Tsang, CDC) followed by an alkaline phosphatase-labeled rabbit anti-mouse IgG antibody (1:1,000; Zymed). Plates were then developed by the addition of 0.1% p-nitrophenylphosphate-3 mM MgCl2-10% diethanolamine (pH ∼9.8) as described above.
Statistical analysis.
Statistical analyses to assess differences in anti-WSP antibody responses among the different groups were performed with EpiInfo version 6.03 software (CDC). The χ2 test was used to compare difference in the seroprevalence of antibodies between groups, and the nonparametric Kruskal-Wallis H test was used to compare differences in median antibody levels between groups. A significant difference was defined as a P value of <0.05.
RESULTS
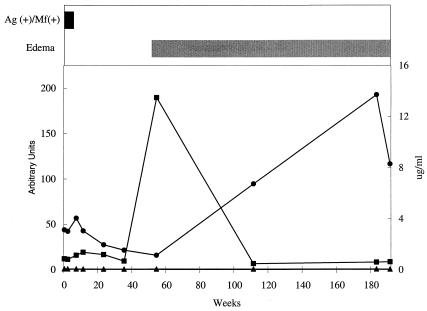
As part of our previous studies using B. malayi-infected rhesus monkeys, we saw a temporal association between the development of anti-WSP antibody responses and lymphedema development (23). In our studies in Leogane, we have few sets of longitudinal serum specimens from incident lymphedema cases. As an initial approach to investigate the potential association between antibody responses to WSP and chronic filarial disease in humans, we assayed longitudinal serum samples from one individual that were collected before and after the onset of disease. At the time that the first serum sample was collected, this individual was a 54-year-old male living in Leogane, Haiti, with a microfilaremia of 100 Mf/ml of blood. This individual was enrolled in a treatment study and treated with diethylcarbamazine. After treatment, he became amicrofilaremic, and approximately 1 year after treatment, he developed unilateral lymphedema of the leg. All serum samples collected before the onset of lymphedema were negative for anti-WSP IgG; however, there was a significant but transient increase in anti-WSP IgG coincident with the onset of lymphedema (Fig. 1). Antifilarial IgG1 antibody levels began to increase after the development of lymphedema and peaked at approximately 2 years after lymphedema development. There was no evidence of antifilarial IgG4 antibodies in any of the serum samples from this patient (Fig. 1). This result prompted us to conduct a retrospective analysis of antibody responses to WSP in patients living in an area where lymphatic filariasis is endemic to further investigate the association between anti-WSP IgG and filarial disease.
FIG. 1.
Composite graph showing a temporal association between anti-WSP IgG responses and the onset of lymphedema. Longitudinal serum samples were collected from a 54-year-old male before and after the onset of lymphedema. This individual was treated with diethylcarbamazine at week zero and developed unilateral lymphedema of the leg approximately 1 year posttreatment. Horizontal bars represent the period of time during which this individual was Ag+ Mf+ (black bar) and experienced lymphedema (gray bar). Line graphs represent anti-WSP IgG levels (squares), given in arbitrary units on the left axis, and antifilarial IgG1 (circles) and antifilarial IgG4 (triangles) levels, given in micrograms per milliliter on the right axis.
We selected 232 serum samples collected from individuals living in Leogane, Haiti, and 67 serum samples collected from North Americans with no history of lymphatic filariasis to assay for the presence of anti-WSP IgG. Demographic and parasitologic characteristics of the study groups and anti-WSP seroprevalence data are shown in Table 1. Individuals with lymphedema or hydrocele were significantly more likely to be seropositive for anti-WSP IgG (anti-WSP+) than asymptomatic antigen-positive and microfilaria-positive (Ag+ Mf+) (P = 0.007 and P = 0.034, respectively), asymptomatic Ag− Mf− (P = 0.010 and P = 0.004, respectively) and North American (P = 0.011 and P = 0.004, respectively) individuals. Antibodies to WSP were also detected in 10 of 18 (56%) asymptomatic individuals who were Ag+ Mf−. Despite the small number of individuals in this group, they were also significantly more likely to be anti-WSP+ than asymptomatic Ag+ Mf+ (P = 0.034), asymptomatic Ag− Mf− (P = 0.006), and North American (P = 0.006) individuals. However, median anti-WSP antibody levels did not differ significantly between any of these groups (data not shown). Due to the uncertainty as to whether individuals who were Ag+ Mf− harbored microfilaria at levels too low to be detected, had recently cleared infections with adult worms, or had single-sex filarial infections and to the lack of statistically significant associations, these individuals were excluded from further analysis. There were no statistically significant differences in gender (41 versus 36% male; P = 0.50), median age (35 versus 31 years old; P = 0.11), or antigen or microfilaria prevalence (41 versus 43% Ag+ Mf+; P = 0.70) between anti-WSP+ and anti-WSP− individuals from Haiti, respectively. However, anti-WSP+ individuals did have a slightly higher median antifilarial IgG1 level than anti-WSP− individuals (2.2 versus 1.7 μg/ml; P = 0.048). Because of the differences in the pathogenesis of filarial lymphedema and hydrocele, disease-specific anti-WSP antibody results were analyzed separately for these two outcomes.
TABLE 1.
Comparison of anti-WSP antibody responses among the groups
| Population | Group | n | Median age (yr) | Age range (yr) | % M/Fa | WSP+b
|
|
|---|---|---|---|---|---|---|---|
| No. | % | ||||||
| Haitian | Symptomatic | ||||||
| Lymphedema | 44 | 37 | 17-65 | 5/95 | 20 | 45 | |
| Hydrocele | 57 | 36 | 17-65 | 100/0 | 27 | 47 | |
| Asymptomatic | |||||||
| Ag+ Mf+ | 60 | 28 | 17-65 | 35/65 | 17 | 28 | |
| Ag− Mf− | 53 | 30 | 17-65 | 13/87 | 11 | 21 | |
| Ag+ Mf− | 18 | 27 | 22-59 | 31/69 | 10 | 56 | |
| North American | Asymptomatic, Ag−, Mf− | 67 | Unkc | Unk | Unk | 15 | 22 |
M, male; F, female.
P = 0.0009 by χ2 test for significant differences among groups.
Unk, unknown.
Anti-WSP antibody responses among lymphedema patients.
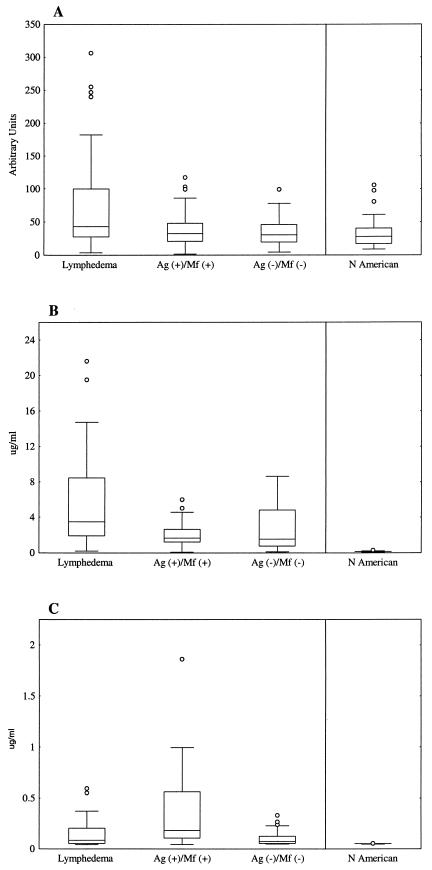
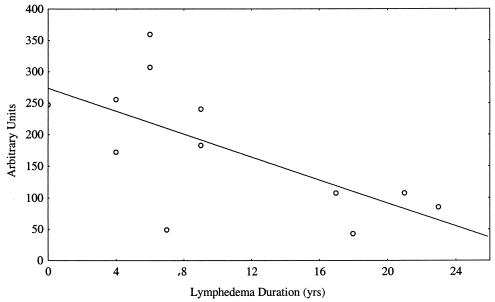
Of 44 individuals with lymphedema, 42 (95%) were female, 2 (5%) were Ag+ Mf+, and 2 (5%) were Ag+ Mf−. Because the vast majority of patients with lymphedema were Ag− Mf− females, anti-WSP antibody results for these 40 women were compared to those for women in the other groups who had no evidence of lymphedema. The median anti-WSP antibody level was significantly higher for women with lymphedema than for asymptomatic Ag+ Mf+ women (P = 0.011), asymptomatic Ag− Mf− women (P = 0.009), and North American individuals (P = 0.0001) (Fig. 2A). Therefore, women with lymphedema had significantly higher levels of serum anti-WSP IgG than gender- and infection-matched individuals without lymphedema. Although the median ages of these groups of women differed, there was no correlation between anti-WSP IgG levels and age. Women with lymphedema also had a significantly higher median antifilarial IgG1 level than asymptomatic Ag+ Mf+ women (P = 0.0002) and asymptomatic Ag− Mf− women (P = 0.0043) (Fig. 2B). In contrast, antifilarial IgG4 levels were highest among asymptomatic Ag+ Mf+ women (Fig. 2C). Among the 20 women in this study with lymphedema who were anti-WSP+, sufficient data regarding the duration of time since the onset of lymphedema were available for 12 (60%). Anti-WSP antibody levels were inversely correlated with lymphedema duration (P = 0.02) (Fig. 3).
FIG.2.
Anti-WSP IgG levels are associated with the presence of lymphedema. Box and whisker plots show anti-WSP IgG (A), antifilarial IgG1 (B), and antifilarial IgG4 (C) antibody data from women in this study and North Americans. Horizontal lines represent the 25th, 50th, and 75th percentiles of anti-WSP IgG responses, given in arbitrary units, and antifilarial responses, given in micrograms per milliliter. Vertical lines represent the nonoutlier minimum and maximum responses for each group, and circles represent outliers.
FIG. 3.
Correlation between anti-WSP IgG levels and lymphedema duration among anti-WSP+ women with lymphedema. Correlation (r = 0.66) was determined by linear regression analysis.
Many studies have suggested a role for secondary bacterial infections in the progression of lymphedema development, thus raising the question of the possible association between host immune responsiveness to WSP and to other bacterial antigens. As part of a previous study in our lab (2), immune responses of patients with lymphedema to various bacterial antigens were assayed. Among 25 Ag− Mf− women with lymphedema who were included in both this study and the previous study and for whom antibody data for WSP and other bacterial antigens were available, there were no differences in median IgG responses to Pseudomonas exotoxin (41 versus 17 arbitrary units; P = 0.28), Staphylococcus enterotoxin B (202 versus 329 arbitrary units; P = 0.19), Streptococcus group A antigen (78 versus 62 arbitrary units; P = 0.55), or streptolysin O (131 versus 142 arbitrary units; P = 0.83) between individuals who were anti-WSP+ and anti-WSP−, respectively. In addition, there was no association between WSP positivity and the period of time since a patient's last acute attack of adenolymphangitis (7 versus 5.5 months; P = 0.86) or the number of acute attacks experienced in the previous 18 months (1.7 ± 1.5 versus 2.1 ± 1.6; P = 0.54).
Anti-WSP antibody responses among hydrocele patients.
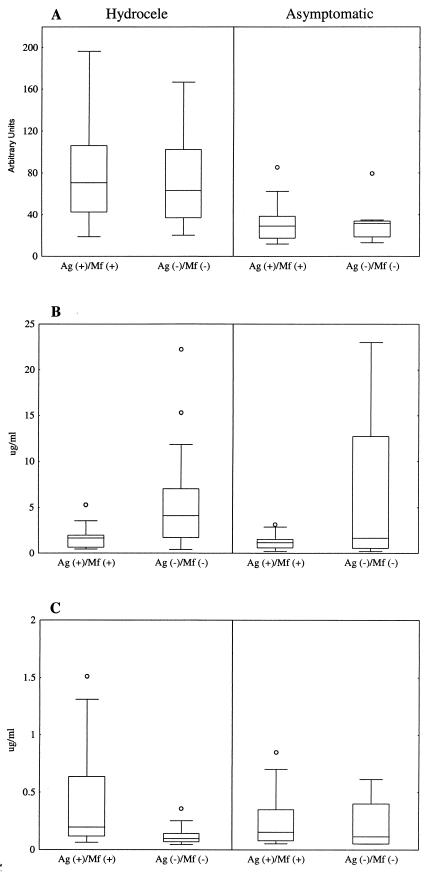
Unlike patients with lymphedema, men with hydrocele form a heterogeneous group in which the percentage of men actively infected with filarial worms parallels the prevalence of microfilaremia seen in the community. Of 57 men in this study with hydrocele, 22 were Ag+ Mf+, 23 were Ag− Mf−, and 12 were Ag+ Mf−. Median anti-WSP antibody levels of men with hydrocele were significantly greater than those of infection-matched men without hydrocele (Fig. 4A). There were no differences in the median ages of any of the groups shown in Fig. 4; therefore, men with hydrocele had significantly higher levels of anti-WSP IgG than age- and infection-matched men without hydrocele. Consistent with a previous report (1), antifilarial antibody responses among men in this study with hydrocele were influenced by infection status. Men with hydrocele who were Ag+ Mf+ had a significantly lower median antifilarial IgG1 response (P = 0.002) (Fig. 4B) and a significantly higher median antifilarial IgG4 response (P = 0.001) (Fig. 4C) than men with hydrocele who were Ag− Mf−. Although not statistically significant, the same trend was seen in men without hydrocele.
FIG.4.
Anti-WSP IgG levels are associated with the presence of hydrocele. Box and whisker plots show anti-WSP (A), antifilarial IgG1 (B), and antifilarial IgG4 (C) antibody data from men in this study stratified by infection status. Individuals with hydrocele are shown on the left, and individuals without hydrocele are shown on the right. Horizontal lines represent the 25th, 50th, and 75th percentiles of anti-WSP IgG responses, given in arbitrary units, and antifilarial responses, given in micrograms per milliliter. Vertical lines represent the nonoutlier minimum and maximum responses for each group, and circles represent outliers.
Sufficient data were available for 28 of 57 (49%) men with hydrocele to make comparisons between anti-WSP antibody responses and clinical observations. Among these 28 men, WSP seropositivity was associated with the degree of inguinal adenopathy (P = 0.036) (Table 2). Although not statistically significant, a similar association was also seen between the percentage of men who were anti-WSP+ and the presence of inguinal lymph node tenderness (P = 0.071). In addition, men who were anti-WSP+ had a greater median hydrocele volume (241 versus 79 ml); however, this difference was not statistically significant (P = 0.21).
TABLE 2.
Association between anti-WSP antibody responses and clinical findings in men with hydrocele
| Clinical findinga | n | WSP+
|
|
|---|---|---|---|
| No. | % | ||
| Inguinal Adenopathy | |||
| None | 16 | 8 | 50 |
| Mild | 5 | 3 | 60 |
| Moderate | 7 | 7 | 100 |
| Inguinal Tenderness | |||
| No | 23 | 13 | 57 |
| Yes | 5 | 5 | 100 |
P = 0.036 and P = 0.071 by χ2 test for overall differences between groups for inguinal adenopathy and inguinal lymph node tenderness, respectively.
Epitope mapping.
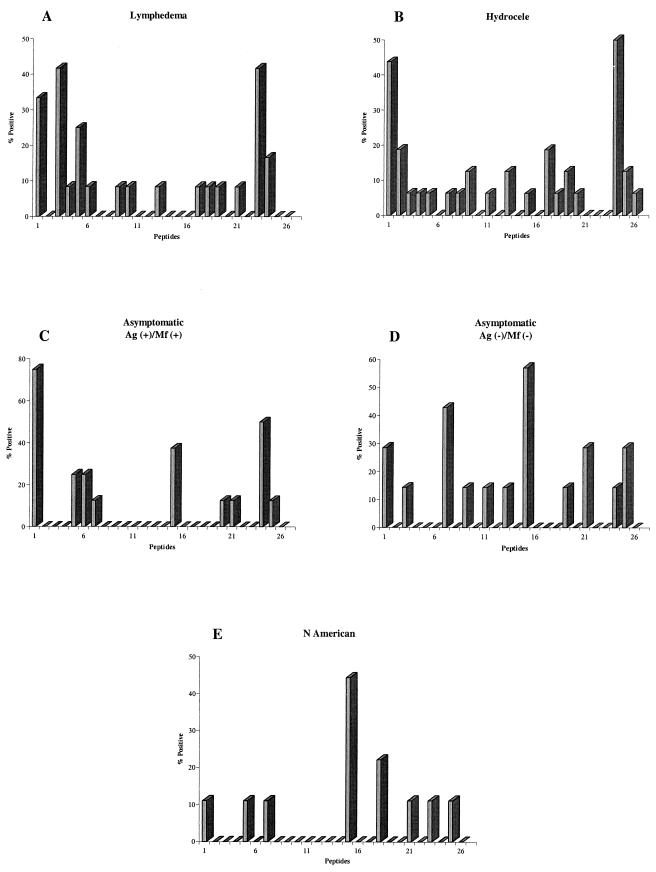
Because 22% of serum samples collected from North Americans with no history of lymphatic filariasis were anti-WSP+, we attempted to determine whether there was anything unique about these responses that could possibly explain immune reactivity to WSP in areas where the infection is not endemic. Our approach was to determine whether individuals living in an area where lymphatic filariasis is endemic recognized different regions of the WSP protein than North Americans, by using 26 linear peptides that span the entire region of our recombinant WSP. Of 77 individuals in this study who were anti-WSP+, 52 (68%) had detectable serum IgG1 antibodies to at least one of the 26 WSP peptides, and positive individuals responded to an average of 2.3 ± 1.2 peptides (mean ± standard deviation). There were no differences in the percentage of individuals positive for at least one peptide or the average number of peptides recognized between any of the groups. The decreased sensitivity of the epitope mapping compared to the ELISA with recombinant WSP may be attributable to the specificity of this assay for IgG1 as opposed to total IgG or to the recognition of conformationally determined epitopes in the ELISA with recombinant WSP. In fact, when serum samples were preabsorbed with individual peptides and then assayed for antibodies to recombinant WSP, we found that antibodies to the linear epitopes accounted for only a fraction of the total antibodies (data not shown). However, there were still differences in antibodies to the linear peptides between Haitian and North American individuals. The percentage of individuals in each group who were positive for a particular peptide is shown in Fig. 5. Anti-WSP+ individuals with lymphedema or hydrocele primarily recognized peptides located at either the N- or C-terminal region of the protein. Lymphedema patients responded primarily to peptides 1, 3, 5, and 23, and hydrocele patients responded primarily to peptides 1 and 24 (Fig. 5A and B). The majority of anti-WSP+ asymptomatic microfilaremic individuals also responded to peptides 1 and 24; however, 38% of these individuals also responded to peptide 15 (Fig. 5C). Twenty-nine percent of anti-WSP+ normal individuals from the area of endemicity responded to peptides 1, 21, and 25, located at the N- and C-terminal regions of the protein. In addition, 43 and 57% of these individuals also responded to peptides 7 and 15, respectively (Fig. 5D). In comparison, anti-WSP+ North Americans showed a strikingly different pattern of reactivity. These individuals primarily responded to peptide 15, and they showed little recognition of peptides located at the N- and C-terminal regions of the WSP protein (Fig. 5E). Predictions of structural motifs within WSP have repeatedly suggested the presence of two transmembrane domains (amino acids 112 to 127 and 137 to 150) (7, 19). The first 13 amino acids of peptide 15 (TPYVGVGLGVAYI) lie within the second transmembrane domain predicted by Jiggins et al. (19).
FIG. 5.
Linear epitopes of WSP recognized by anti-WSP+ individuals with lymphedema (A) (n = 12) or hydrocele (B) (n = 16), asymptomatic Ag+ Mf+ individuals (C) (n = 8), asymptomatic Ag− Mf− individuals (D) (n = 7), and North Americans (E) (n = 9). Results are shown as the percentage of individuals recognizing at least one peptide who are positive for each of the 26 overlapping WSP peptides.
DISCUSSION
The chronic manifestations of severe lymphatic filariasis are dominated by the clinical syndromes of lymphedema and hydrocele. Although both disease manifestations are characterized by accumulation of fluid in the affected part of the body, there is considerable debate about the underlying etiology and the pathologic mechanisms for these two clinical manifestations. While both manifestations are likely to share some aspects of their pathophysiologic processes, lymphedema is thought to be the outcome of a complicated interplay of parasitologic factors, host genetic factors, and secondary bacterial infections (21). In contrast, the pathology of hydrocele has been considered to be almost entirely caused by the adult worm (12). Additionally, host inflammatory responses stimulated by parasite antigens are thought to contribute to the development of disease (13); however, immune responses to adult worm extracts in these two groups are strongly associated with the patient's infection status. Although patients with lymphedema display the greatest levels of antifilarial immunity (2), similar types of responses are also seen in asymptomatic Ag− Mf− individuals (10). In addition, antifilarial immune responses among men with hydrocele are more closely associated with the patient's infection status than with the presence of disease (1). As a result, specific parasite factors that trigger the inflammatory responses associated with disease development in these individuals have not yet been identified. In this report, we show that patients with either lymphedema or hydrocele were significantly more likely to mount antibody responses to WSP than gender- and infection-matched individuals without disease. In fact, men with hydrocele had remarkably similar seroprevalences and intensities of anti-WSP IgG responses independent of microfilaria or antigen status (Fig. 4A). These results demonstrate that anti-WSP antibody responses are associated with the presence of chronic filarial disease and not simply filarial infection status in humans.
Further support for the association between anti-WSP antibody responses and filarial disease is provided by the associations between anti-WSP IgG and clinical markers of disease noted in this study. Consistent with our previous results with B. malayi-infected rhesus monkeys (23), in the one case where longitudinal responses were assayed before and after the onset of disease, a transient peak in anti-WSP IgG was seen at the onset of lymphedema (Fig. 1). Similarly, in cross-sectional data, there was an inverse correlation between anti-WSP IgG levels and lymphedema duration among anti-WSP+ individuals (Fig. 3). Also, among men with hydrocele, anti-WSP+ men were more likely to have moderate degrees of inguinal adenopathy and inguinal lymph node tenderness than anti-WSP− men. Because the lymphatic vessels of the spermatic cord (the primary site of adult W. bancrofti in men) drain into the abdominal lymph nodes rather than the inguinal lymph nodes, this observation is unlikely to represent a hydrocele-specific response. Therefore, similar complaints may be expected among men without hydrocele following worm death. Unfortunately, data concerning inguinal adenopathy and lymph node tenderness were available only for men with hydrocele. Nonetheless, the temporal association between anti-WSP antibody responses and filarial disease development and the association with inguinal adenopathy and tenderness suggest that Wolbachia should be further studied as a potential trigger for development of filarial disease.
One other factor that has repeatedly been suggested to play an important role in the progression of filarial disease is lymphatic damage caused by secondary bacterial infections. Recurrent bacterial infections that manifest as acute dermatolymphangioadenitis have been shown to contribute to the development of chronic lymphedema (11). In addition, patients with lymphedema have been shown to display heightened immune reactivity to bacterial antigens (especially streptolysin O) compared to infection-matched individuals without disease (2). This raises the question of the possible association between anti-Wolbachia immune responses and immune responses directed at other bacteria. In this study, we found there was no difference in levels of serum antibody to various bacterial antigens between anti-WSP+ and anti-WSP− women with lymphedema. Therefore, we believe that anti-WSP reactivity is not caused by cross-reaction with other bacterial infections that occur commonly among persons with lymphedema. Instead, it is likely that Wolbachia bacteria are recognized by the host immune system during the initial pathologic events following worm death and that secondary bacterial infections contribute to the progression of disease development only after these events lead to lymphatic stasis and an inability to clear invading organisms. To the extent that filarial pathology is associated with a shift in host response from a Th2- to a Th1-type immune response, an interesting hypothesis is that immune reactivity to Wolbachia may trigger this shift in host response to a Th1-like immune response to both filarial and nonfilarial antigens. This heightened inflammatory reactivity to bacterial antigens then may be associated with increased lymphatic damage and skin pathology as disease progresses.
Despite the associations between antibody reactivity to WSP and severe filarial disease noted in this study, only 45% of lymphedema patients and 47% of hydrocele patients were anti-WSP+. In the absence of longitudinal data, it is unclear whether any of the anti-WSP− individuals had ever mounted antibody responses to WSP. Longitudinal data from humans (Fig. 1) and monkeys (23) demonstrate the importance of longitudinal data in analyzing antibody responses to WSP. In both cases, peaks in anti-WSP IgG were temporally associated with the onset of clinically apparent disease, and detectable levels of anti-WSP IgG were transient. However, longitudinal specimens from humans who develop filarial disease are difficult to obtain, and as a result, it may be impossible to determine the true patterns of antibody responses to WSP by using only cross-sectional data. An alternative approach may be to assay for cell-mediated immune responses to WSP. A critical component of the cell-mediated immune system is the production of memory T cells that can be stimulated with antigens in vitro to mount immune responses similar to those which they would mount in vivo. Considering the importance of T cells in the production of an effective antibody response (i.e., switching of the constant region of the heavy chain to produce IgG isotype antibodies), individuals who mount antibody responses to WSP would be expected to also display cell-mediated immunity to WSP. These experiments would have the added benefit of determining whether WSP could stimulate inflammatory-type immune responses that may serve as a potential trigger for the development of disease.
In addition to detecting anti-WSP IgG in serum samples from Haitian individuals living in an area where lymphatic filariasis is endemic, we found that 22% of serum samples from North Americans with no history of lymphatic filariasis were also anti-WSP+. Based on this observation, there are at least three hypotheses that may explain why some North Americans are anti-WSP+. The first hypothesis, and perhaps the most difficult to test, is that some degree of cross-reactivity between WSP and unidentified bacterial antigens exists in human populations in areas where the infection is not endemic. We consider this hypothesis unlikely given lack of an association between WSP and other bacterial antigens and the observation that WSP epitopes recognized by North Americans in this study do not have significant homology to non-Wolbachia antigens when compared by BLAST search (data not shown). However, carefully controlled experiments would be needed to rule this hypothesis out. Alternatively, exposure to other Wolbachia-containing filarial nematodes may elicit an anti-WSP antibody response. Dirofilaria and Mansonella spp. are endemic in many regions of North America, and anti-WSP antibody responses have been observed in Dirofilaria immitis-infected cats and humans (4, 26). Finally, a third hypothesis is that Wolbachia of filarial worms does not represent the only means of human exposure to Wolbachia antigen(s). In this study, we found that the WSP epitopes primarily recognized by Haitians with lymphedema and hydrocele were concentrated at the amino- and carboxy-terminal ends of the protein, while the first 13 amino acids of the epitope that was primarily recognized by North Americans were located in the second transmembrane domain predicted by Jiggins et al. (19). Interestingly, mathematical predictions based on the ratio of synonymous and nonsynonymous amino acid substitutions suggest that the transmembrane regions of WSP are not under positive selective pressure in either arthropod or nematode Wolbachia (19). These results suggest that the transmembrane regions of WSP are likely to have the greatest degree of sequence conservation between different strains of Wolbachia. In addition to filarial worms, Wolbachia bacteria also reside in a number of other invertebrates distributed throughout the world that are known to have contact with humans (18, 28). Although it has been reported that human exposure to Wolbachia antigens from arthropods does not occur (29), this hypothesis has not been empirically tested. In light of the observation that anti-WSP IgG can be detected in human subjects in areas where the infection is not endemic, this hypothesis deserves further consideration.
The recognition of WSP by the human immune system and the association between antibody responses to WSP and chronic filarial disease raise the question of whether Wolbachia may play a causative role in the development of filarial disease. Because Wolbachia bacteria are located inside the filarial worm, it is likely that Wolbachia antigens will come into contact with components of the host immune system only if they are released following worm death. Interestingly, a critical factor in the development of both lymphedema and hydrocele seems to be the death of the adult worm. Therefore, release of Wolbachia following worm death would put these bacteria in a potential environment in which Wolbachia-specific immune responses may trigger the initial events in the development of chronic filarial disease. Further studies to determine whether Wolbachia may play a causative role in the development of filarial disease should focus on the localized immune responses to Wolbachia following worm death. Analysis of histologic specimens collected following worm death may help determine whether Wolbachia organisms are released following worm death and whether Wolbachia organisms or their antigens come into contact with components of the human immune system. In addition, an examination of cell-mediated immunity to Wolbachia antigens may help determine whether Wolbachia may contribute to the localized inflammation that is associated with disease.
Acknowledgments
This work was supported, in part, by CDC's Working Group for Infectious Causes of Chronic Diseases.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Addiss, D. G., K. A. Dimock, M. L. Eberhard, and P. J. Lammie. 1995. Clinical, parasitologic, and immunologic observations of patients with hydrocele and elephantiasis in an area with endemic lymphatic filariasis. J Infect. Dis. 171:755-758. [DOI] [PubMed] [Google Scholar]
- 2.Baird, J. B., J. L. Charles, T. G. Streit, J. M. Roberts, D. G. Addiss, and P. J. Lammie. 2002. Reactivity to bacterial, fungal, and parasite antigens in patients with lymphedema and elephantiasis. Am. J. Trop. Med. Hyg. 66:163-169. [DOI] [PubMed] [Google Scholar]
- 3.Bandi, C., J. W. McCall, C. Genchi, S. Corona, L. Venco, and L. Sacchi. 1999. Effects of tetracycline on the filarial worms Brugia pahangi and Dirofilaria immitis and their bacterial endosymbionts Wolbachia. Int. J. Parasitol. 29:357-364. [DOI] [PubMed] [Google Scholar]
- 4.Bazzocchi, C., F. Ceciliani, J. W. McCall, I. Ricci, C. Genchi, and C. Bandi. 2000. Antigenic role of the endosymbionts of filarial nematodes: IgG response against the Wolbachia surface protein in cats infected with Dirofilaria immitis. Proc. R. Soc. London B 267:2511-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazzocchi, C., W. Jamnongluk, S. L. O'Neill, T. J. Anderson, C. Genchi, and C. Bandi. 2000. wsp gene sequences from the Wolbachia of filarial nematodes. Curr. Microbiol. 41:96-100. [DOI] [PubMed] [Google Scholar]
- 6.Bosshardt, S. C., J. W. McCall, S. U. Coleman, K. L. Jones, T. A. Petit, and T. R. Klei. 1993. Prophylactic activity of tetracycline against Brugia pahangi infection in jirds (Meriones unguiculatus). J. Parasitol. 79:775-777. [PubMed] [Google Scholar]
- 7.Braig, H. R., W. Zhou, S. L. Dobson, and S. L. O'Neill. 1998. Cloning and characterization of a gene encoding the major surface protein of the bacterial endosymbiont Wolbachia pipientis. J. Bacteriol. 180:2373-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brattig, N. W., U. Rathjens, M. Ernst, F. Geisinger, A. Renz, and F. W. Tischendorf. 2000. Lipopolysaccharide-like molecules derived from Wolbachia endobacteria of the filaria Onchocerca volvulus are candidate mediators in the sequence of inflammatory and antiinflammatory responses of human monocytes. Microbes Infect. 2:1147-1157. [DOI] [PubMed] [Google Scholar]
- 9.Cox, F. E. 2000. Elimination of lymphatic filariasis as a public health problem. Parasitol. Today 16:135.. [DOI] [PubMed] [Google Scholar]
- 10.Dimock, K. A., M. L. Eberhard, and P. J. Lammie. 1996. Th1-like antifilarial immune responses predominate in antigen-negative persons. Infect. Immun. 64:2962-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreyer, G., Z. Medeiros, M. J. Netto, N. C. Leal, L. G. de Castro, and W. F. Piessens. 1999. Acute attacks in the extremities of persons living in an area endemic for bancroftian filariasis: differentiation of two syndromes. Trans. R. Soc. Trop. Med. Hyg. 93:413-417. [DOI] [PubMed] [Google Scholar]
- 12.Dreyer, G., J. Noroes, J. Figueredo-Silva, and W. F. Piessens. 2000. Pathogenesis of lymphatic disease in bancroftian filariasis: a clinical perspective. Parasitol. Today 16:544-548. [DOI] [PubMed] [Google Scholar]
- 13.Freedman, D. O. 1998. Immune dynamics in the pathogenesis of human lymphatic filariasis. Parasitol. Today 14:229-234. [DOI] [PubMed] [Google Scholar]
- 14.Hitch, W. L., A. W. Hightower, M. L. Eberhard, and P. J. Lammie. 1991. Analysis of isotype-specific antifilarial antibody levels in a Haitian pediatric population. Am. J. Trop. Med. Hyg. 44:161-167. [DOI] [PubMed] [Google Scholar]
- 15.Hoerauf, A., S. Mand, O. Adjei, B. Fleischer, and D. W. Buttner. 2001. Depletion of Wolbachia endobacteria in Onchocerca volvulus by doxycycline and microfilaridermia after ivermectin treatment. Lancet 357:1415-1416. [DOI] [PubMed] [Google Scholar]
- 16.Hoerauf, A., K. Nissen-Pahle, C. Schmetz, K. Henkle-Duhrsen, M. L. Blaxter, D. W. Buttner, M. Y. Gallin, K. M. Al-Qaoud, R. Lucius, and B. Fleischer. 1999. Tetracycline therapy targets intracellular bacteria in the filarial nematode Litomosoides sigmodontis and results in filarial infertility. J. Clin. Investig. 103:11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoerauf, A., L. Volkmann, C. Hamelmann, O. Adjei, I. B. Autenrieth, B. Fleischer, and D. W. Buttner. 2000. Endosymbiotic bacteria in worms as targets for a novel chemotherapy in filariasis. Lancet 355:1242-1243. [DOI] [PubMed] [Google Scholar]
- 18.Jeyaprakash, A., and M. A. Hoy. 2000. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol. Biol. 9:393-405. [DOI] [PubMed] [Google Scholar]
- 19.Jiggins, F. M., G. D. Hurst, and Z. Yang. 2002. Host-symbiont conflicts: positive selection on an outer membrane protein of parasitic but not mutualistic Rickettsiaceae. Mol. Biol. Evol. 19:1341-1349. [DOI] [PubMed] [Google Scholar]
- 20.Keiser, P. B., S. M. Reynolds, K. Awadzi, E. A. Ottesen, M. J. Taylor, and T. B. Nutman. 2002. Bacterial endosymbionts of Onchocerca volvulus in the pathogenesis of posttreatment reactions. J. Infect. Dis. 185:805-811. [DOI] [PubMed] [Google Scholar]
- 21.Lammie, P. J., K. T. Cuenco, and G. A. Punkosdy. 2002. The pathogenesis of filarial lymphedema: is it the worm or is it the host? Ann. N.Y. Acad. Sci. 979:131-142. [DOI] [PubMed] [Google Scholar]
- 22.Langworthy, N. G., A. Renz, U. Mackenstedt, K. Henkle-Duhrsen, M. B. de Bronsvoort, V. N. Tanya, M. J. Donnelly, and A. J. Trees. 2000. Macrofilaricidal activity of tetracycline against the filarial nematode Onchocerca ochengi: elimination of Wolbachia precedes worm death and suggests a dependent relationship. Proc. R. Soc. London B 267:1063-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Punkosdy, G. A., V. A. Dennis, B. L. Lasater, G. Tzertzinis, J. M. Foster, and P. J. Lammie. 2001. Detection of serum IgG antibodies specific for Wolbachia surface protein in rhesus monkeys infected with Brugia malayi. J. Infect. Dis. 184:385-389. [DOI] [PubMed] [Google Scholar]
- 24.Rao, R., and G. J. Well. 2002. In vitro effects of antibiotics on Brugia malayi worm survival and reproduction. J. Parasitol. 88:605-611. [DOI] [PubMed] [Google Scholar]
- 25.Saint Andre, A., N. M. Blackwell, L. R. Hall, A. Hoerauf, N. W. Brattig, L. Volkmann, M. J. Taylor, L. Ford, A. G. Hise, J. H. Lass, E. Diaconu, and E. Pearlman. 2002. The role of endosymbiotic Wolbachia bacteria in the pathogenesis of river blindness. Science 295:1892-1895. [DOI] [PubMed] [Google Scholar]
- 26.Simon, F., G. Prieto, R. Morchon, C. Bazzocchi, C. Bandi, and C. Genchi. 2003. Immunoglobulin G antibodies against the endosymbionts of filarial nematodes (Wolbachia) in patients with pulmonary dirofilariasis. Clin. Diagn. Lab. Immunol. 10:180-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor, M. J., H. F. Cross, and K. Bilo. 2000. Inflammatory responses induced by the filarial nematode Brugia malayi are mediated by lipopolysaccharide-like activity from endosymbiotic Wolbachia bacteria. J. Exp. Med. 191:1429-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Werren, J. H., and D. M. Windsor. 2000. Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proc. R. Soc. London B 267:1277-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmer, C. 2001. Wolbachia. A tale of sex and survival. Science 292:1093-1095. [DOI] [PubMed] [Google Scholar]