Abstract
Following infection with the protozoan parasite Leishmania major, C57BL/6 mice develop a small lesion that heals spontaneously. Resistance to infection is associated with the development of CD4+ Th1 cells producing gamma interferon (IFN-γ) and tumor necrosis factor (TNF), which synergize in activating macrophages to their microbicidal state. We show here that C57BL/6 mice lacking both TNF and Fas ligand (FasL) (gld TNF−/− mice) infected with L. major neither resolved their lesions nor controlled Leishmania replication despite the development of a strong Th1 response. Comparable inducible nitric oxide synthase (iNOS) activities were detected in lesions of TNF−/−, gld TNF−/−, and gld mice, but only gld and gld TNF−/− mice failed to control parasite replication. Parasite numbers were high in gld mice and even more elevated in gld TNF−/− mice, suggesting that, in addition to iNOS, the Fas/FasL pathway is required for successful control of parasite replication and that TNF contributes only a small part to this process. Furthermore, FasL was shown to synergize with IFN-γ for the induction of leishmanicidal activity within macrophages infected with L. major in vitro. Interestingly, TNF−/− mice maintained large lesion size throughout infection, despite being able to largely control parasite numbers. Thus, IFN-γ, FasL, and iNOS appear to be essential for the complete control of parasite replication, while the contribution of TNF is more important in controlling inflammation at the site of parasite inoculation.
In the experimental murine model of infection with the intracellular protozoan Leishmania major, susceptibility to infection has been correlated with the development of lesions associated with a Th2 type of immune response, while healing of lesions in resistant mice has been correlated with the development of a Th1 type of immune response (reviewed in reference 27). The resolution of lesions has been shown to involve several factors contributing to the killing of L. major within macrophages. The most efficient mechanism of parasite destruction involves the production of gamma interferon (IFN-γ) by CD4+ Th1 cells, which induces the synthesis of inducible nitric oxide synthase (iNOS), generating the production of reactive nitrogen radicals toxic for the intracellular parasite (reviewed in reference 4). Tumor necrosis factor (TNF) has also been shown to be involved in parasite killing in a process that depends on NO production, and endogenously produced TNF has been reported to be necessary for NO production by macrophages in vitro (3, 12, 17, 33).
The importance of TNF in the control of infection with Leishmania has been studied extensively, but its exact role remains unclear. Mice resistant to infection with L. major were shown to produce significant amounts of TNF in their draining lymph nodes during the course of infection, while no TNF was detectable in susceptible animals (35). Injections of recombinant human TNF (rhTNF) into either resistant or susceptible strains of mice had a therapeutic effect on the course of infection, with reduced parasite numbers at the site of inoculation, while administration of monoclonal antibody (MAb) directed against TNF exacerbated the course of infection, with a 10-fold increase in parasite numbers 21 days after infection (35). In a model using transgenic mice expressing high levels of soluble hTNF receptor p55 (hTNFRp55) fusion protein, high-expressor mice on a C57BL/6-resistant genetic background developed severe lesions that ultimately ulcerated (11). Mice genetically deficient for TNFR1 (TNFRp55−/−) developed a Th1 response and showed a transient increase in parasite burden but eventually cleared parasites from their lesions (15, 24, 36). Surprisingly, C57BL/6 mice lacking the TNF gene developed a Th1 response but were not able to control parasite dissemination and succumbed to infection (38). Taken together, these results show that although TNF is involved in lesion healing, its precise role in the control of parasite replication remains to be determined.
The Fas/Fas ligand (FasL) system is another pathway reported to be involved in the full resolution of L. major lesions. MRL/lpr and B6.lpr mice, which are deficient in Fas (CD95) expression, failed to achieve complete resolution of their lesions even in the presence of a strong Th1 response, and they only partially controlled parasite replication (6, 13). In another study, B6.lpr mice were observed to have a delay in lesion resolution (15). Mice deficient for functional FasL (gld), due to a point mutation resulting in a nonfunctional form of FasL on the cell surface (20, 31), also failed to heal their lesions and did not successfully control parasite multiplication despite the development of a Th1 type of response (6). It was further shown that early after infection, extensive cell death occurred within the draining lymph nodes of mice resistant to L. major, while apoptosis was minimal at the same time of infection in the draining lymph nodes of susceptible BALB/c strains (7). The Fas/FasL pathway was also required for the cytotoxicity exerted by CD4+ Th1 cells in several in vitro studies (14, 26, 30, 39), and activated CD4+ Th1 cells have been shown to express FasL while Th2 cells were FasL negative (26, 40).
Thus, in the absence of functional TNF or Fas/FasL systems, but in the presence of a Th1 response, mice from a resistant genetic background fail to completely resolve their lesions upon infection with L. major and delay control or fail to control parasite growth within these lesions.
In order to investigate the respective contributions of TNF and FasL in the control of parasite replication and lesion development after infection with L. major, we infected B6.gld × B6.TNF−/− (gld TNF−/−) mice on the C57BL/6 resistant background and compared the course of disease, the primary response within the site of parasite inoculation, and the developing CD4+-T-cell response with that observed in mice deficient in a functional FasL or TNF pathway. The results obtained show that the control of parasite replication within the site of parasite inoculation requires not only iNOS and IFN-γ but also functional FasL, while the contribution of TNF is minimal.
MATERIALS AND METHODS
Mice.
BALB/c and C57BL/6 mice were obtained from Harlan (Horst, The Netherlands) and housed in the pathogen-free facility of the Swiss Institute for Experimental Cancer Research (ISREC), Epalinges, Switzerland. B6.gld (FasL mutant) and B6.lpr mice were obtained from Jackson Laboratory and bred at the ISREC animal facility. B6.TNF−/− mice (23) were obtained from M. W. Marino (Ludwig Institute for Cancer Research, New York, N.Y.). B6.gld × B6.TNF−/− (gld TNF−/−) mice, a gift from J. Tschopp, Institute of Biochemistry, Epalinges, Switzerland, were obtained by crossing B6.TNF−/− and B6.gld mice and subsequent interbreeding of the F1 generation at the ISREC facility. The double-mutant genotype was checked by PCR specific for the FasL point mutation and TNF deficiency (16). B6 × 129-TNFRp55, B6 × 129-TNFRp55p75, and B6 × 129 mice were gifts from H. Bluethmann, F. Hoffmann La Roche, Basel, Switzerland. C57BL/6 and BALB/c transgenic mice expressing high levels of TNFRp55 have been described previously (11). Animal research complied with the Vaud Veterinary Service guidelines.
Parasites.
L. major (LV39 MRHO/Sv/59/P strain) was maintained in vivo and grown in vitro as previously described (18). Mice were injected in the hind footpads with 3 × 106 stationary-phase promastigotes in a final volume of 50 μl.
Detection of cytokines.
Mice from each group were infected with 3 × 106 stationary-phase L. major promastigotes and sacrificed at selected time points after infection. Their draining lymph nodes were isolated and processed for intracellular fluorescence-activated cell sorter analysis or 5 × 106 lymph node cells were restimulated in vitro in the presence of UV-irradiated L. major promastigotes. Supernatants were collected after 72 h of culture. IFN-γ in the supernatants was measured by enzyme-linked immunosorbent assay, and interleukin-4 (IL-4) was detected by bioassay using the CTL44 cell line (gift of P. Erb, University of Basel) as previously described (10, 29). The limit of detection was 10 IU/ml for IFN-γ and 20 pg/ml for IL-4.
For intracellular staining, lymph node cells were isolated, homogenized, and washed in Dulbecco's modified Eagle's medium. The cells were stimulated for 4 h with phorbol myristate acetate (50 ng/ml) and ionomycin (500 ng/ml) in the presence of brefeldin A (1 μg/ml). The cells were then collected and stained for surface markers (αCD3-fluorescein isothiocyanate [FITC], αCD4-FITC, αB220-FITC, αNK1.1, and αCD-FITC [all from Pharmingen]). Thirty minutes later, the cells were fixed and incubated in saponin buffer containing a MAb against the Fc receptor (2.4G2) and stained for 20 min with phycoerythrin (PE)-conjugated anti-cytokine antibody (anti-IFN-γ−PE, 2.5 μg/ml, or anti-IL-4- PE, 5 μg/ml). The cells were then analyzed by flow cytometry (FACScan; Becton Dickinson, Mountain View, Calif.). Each experiment contained lymph nodes pooled from three different mice per group.
Quantitation of parasites.
The number of parasites per lesion was evaluated by limiting-dilution analysis (34). The estimation of the frequency was calculated using the program Estimfree based on the Taswell method (32). The sensitivity of the technique is 10 parasites/g of footpads.
Determination of iNOS activity and nitrites.
NOS activity was assessed as previously described (19). Briefly, footpad tissues were weighed and homogenized in 0.04% Tween 80- saline solution (125 mg of tissue/ml of buffer). Crude supernatant was obtained by centrifugation of the homogenate at 10,000 × g for 5 min. iNOS activity was measured by the conversion of radioactive l-[14C]arginine to l-[14C]citrulline using the NOS Detect assay kit (Stratagene, La Jolla, Calif.).
For determination of nitrite production by bone marrow macrophages (BM Mφ) in vitro, BM Mφ were incubated with 2 ng of lipopolysaccharide (LPS)/ml (from Escherichia coli serotype 055-B5) (Sigma, St. Louis, Mo.) and increasing concentrations of IFN-γ (R&D, Minneapolis, Minn.). Culture supernatants of macrophages were analyzed for their contents of nitrite (NO2−) by the Griess reaction as previously described (9). The detection limit was 1 μM.
Macrophages.
BM Mφ were obtained by in vitro differentiation in RPMI medium supplemented with 20% horse serum and 30% supernatant from L929 cells as a source of macrophage colony-stimulating factor. After 7 days of culture, nonadherent cells were removed, and the remaining adherent macrophages were detached and cultured in RPMI supplemented with 10% heat-inactivated fetal calf serum, l-glutamine (216 mg/ml), and 10 mM HEPES.
Intracellular survival of L. major in macrophages.
BM Mφ were infected with L. major overnight at a 10:1 ratio and then incubated with IFN-γ (10 ng/ml; 100 IU/ml) and/or recombinant FasL (100 ng/ml) for 48 h. BM Mφ were then washed three times, lysed, and incubated for 4 days with L. major growth medium (M199 plus 10% fetal calf serum) at 26°C. [3H]thymidine was added for the last 16 h. The parasites were harvested, and the incorporation of radioactivity was measured.
Immunohistology.
Footpads or lymph nodes were frozen in optimal cutting temperature compound (Sakura Finetek, Zoeterwoude, The Netherlands), and 7-μm-thick frozen sections were prepared, fixed in methanol (5 min), and rehydrated in phosphate-buffered saline. Nonspecific binding sites were blocked in phosphate-buffered saline-1% bovine serum albumin for 30 min. The sections were then incubated with rabbit anti-mouse iNOS (Calbiochem, Schwalbach, Germany) for 60 min, followed by incubation with biotinylated donkey anti-rabbit antibody (Amersham, Freiburg, Germany) for 40 min, and revealed by streptavidin AP (Boehringer Mannheim, Rotkreuz, Switzerland). Counterstaining with hematoxylin-eosin was then performed.
Statistical analysis.
The t test for unpaired data was used in statistical analysis.
RESULTS
gld TNF−/− mice do not control the development of their lesions following infection with L. major.
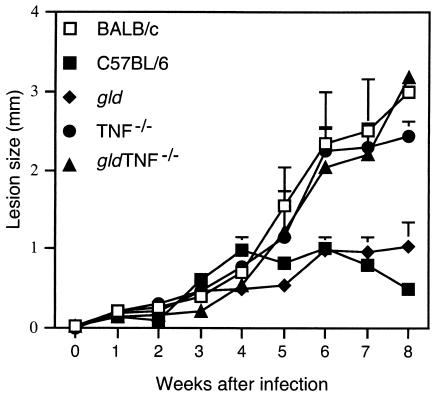
In order to study the respective roles of FasL and TNF in resistance to infections with L. major, gld TNF−/− mice on a C57BL/6 background were injected subcutaneously with 3 × 106 stationary-phase L. major promastigotes. The course of infection was compared to that of mice deficient for TNF only (TNF−/−) or FasL only (gld), both also on a C57BL/6 genetic background resistant to infection with L. major. C57BL/6 and susceptible BALB/c mice were used as controls (Fig. 1). The C57BL/6 mice developed small lesions that healed 8 weeks after infection. In contrast, the TNF−/− and gld TNF−/− mice developed lesions similar in size to those of susceptible BALB/c mice through 8 weeks of infection, after which the lesions did not increase any further in size and swelling spread to the leg and toes up to 3 months after parasite inoculation (data not shown). In 80% of the TNF−/− mice, lesion necrosis was observed 7 to 8 weeks after parasite inoculation (data not shown).
FIG. 1.
gld TNF−/− mice infected with L. major fail to control their lesions. gld TNF−/−, TNF−/−, gld, and control C57BL/6 and BALB/c mice (six mice per group) were infected subcutaneously with 3 × 106 L. major promastigotes. Increase in footpad thickness was monitored by weekly measurements. The sizes of the lesions were determined by subtracting the values obtained from the uninfected footpad from those of the infected one. The error bars represent the standard deviations of the mean amounts of footpad increase.
Mice of the gld strain developed slowly progressive lesions that were significantly smaller than those of BALB/c, TNF−/−, and gld TNF−/− mice (Fig. 1) and which stabilized at ∼1- to 1.2-mm diameter with no further increase (observed in three independent experiments up to 3 months after infection; data not shown).
Mice deficient in both FasL and TNF fail to control parasite replication within their lesions.
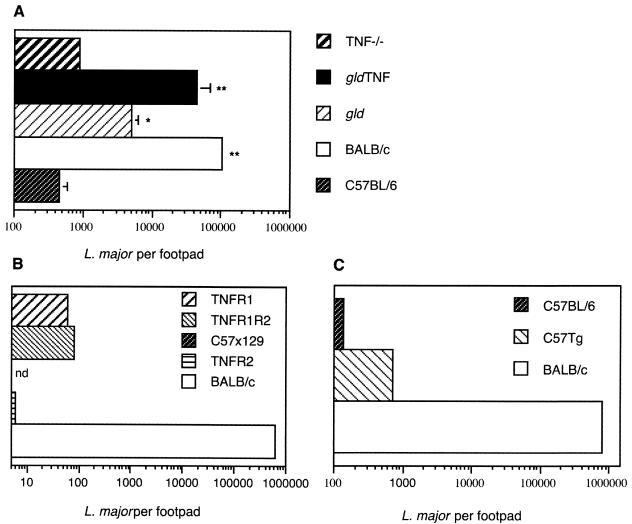
The control of parasite growth in the lesions of gld TNF−/− mice was compared to that occurring in TNF−/− and gld mice (Fig. 2). Six weeks after infection, as expected, C57BL/6 mice efficiently controlled parasite replication within the site of injection, with an average of 4 × 102 residual parasites per footpad. TNF−/− mice also controlled the number of parasites within their lesions, but slightly less efficiently, with an average of 9 × 102 parasites/footpad (Fig. 2A). The number of parasites measured in gld mice was five to six times higher than in TNF−/− mice. An even greater number of parasites was detected in the lesions of gld TNF−/− mice, with values 10 and 50 times higher than those observed in gld and TNF−/− mice, respectively (Fig. 2A). The number of parasites in lesions of gld TNF−/− mice, however, remained 2 to 5.5 times lower than those measured in the lesions of highly susceptible BALB/c mice. Significant differences in intralesional parasite numbers in gld TNF−/−, gld, and TNF−/− mice were observed in four different experiments, with parasite counts performed 4, 6, and 8 weeks after infection (Fig. 2 and data not shown). Thus, mice deficient in both TNF and FasL failed to control parasite replication at the site of parasite inoculation, while mice defective in TNF alone controlled parasite replication almost as well as resistant C57BL/6 mice, with no statistically significant difference. Mice defective for FasL controlled parasite replication within their lesions less efficiently than TNF−/− mice but better than gld TNF−/− mice. In addition, 6 weeks after infection, parasite spreading was observed in gld and gld TNF−/− mice, as revealed by the detection of parasites in the para-aortic lymph nodes, while no parasites were detected in the para-aotric lymph nodes of TNF−/− mice. Thus, among mice on the C57BL/6-resistant genetic background, the absence of both TNF and FasL resulted in the poorest control of parasite growth and dissemination.
FIG. 2.
Following infection with L. major, gld TNF−/− mice do not control parasite replication at the site of parasite injection. (A) Parasite loads in lesions of gld TNF−/−, TNF−/−, gld, and C57BL/6 mice 6 weeks after infection with 3 × 10 6 L. major promastigotes, determined by limiting-dilution analysis as described in Materials and Methods. The means and standard deviations of four individual footpads per group are shown. The data are representative of three experiments. **, P < 0.001 versus C57BL/6; *, P < 0.05 versus C57BL/6. (B) Parasite loads in lesions of TNFR1- (p55), TNFR2- (p75), and TNFR1R2-deficient mice on a C57BL/6 × 129 genetic background compared to the parasite loads in C57BL/6 × 129 and BALB/c mice. (C) Parasite numbers in C57BL/6 mice transgenic for soluble hTNFR1 6 weeks after infection compared to parasite numbers measured in C57BL/6 and BALB/c mice. The data in panels B and C are the means of a pool of three footpads per group and are representative of two experiments. nd, not detectable.
In order to confirm the small role of TNF in parasite control within lesions of mice infected with L. major that was observed in TNF−/− mice, the parasite load was measured 6 weeks after infection in mice deficient in TNF signaling due either to the absence of one (p55 or p75) or both (TNFRp55−/− and TNFRp55p75−/− mice) TNFRs (Fig. 2B) or to the presence of high levels of soluble hTNFRp55 protein (>25 μg/ml of serum) in C57BL/6 transgenic mice (Fig. 2C). Six weeks after infection with L. major, these mice developed large lesions (data not shown), but all controlled the parasite numbers within their lesions quite efficiently (between 50 to 1,000 parasites per lesion). Thus, the lack of a functional TNF pathway only minimally affects the control of parasite replication following infection with L. major. These results are in agreement with those of previous studies involving mice deficient in TNFRp55 and TNFRp55p75 (15, 24, 36), but they contrast with a report involving TNF−/− mice (38).
gld TNF−/− mice develop a Th1 response with elevated levels of IFN-γ in their draining lymph node cells.
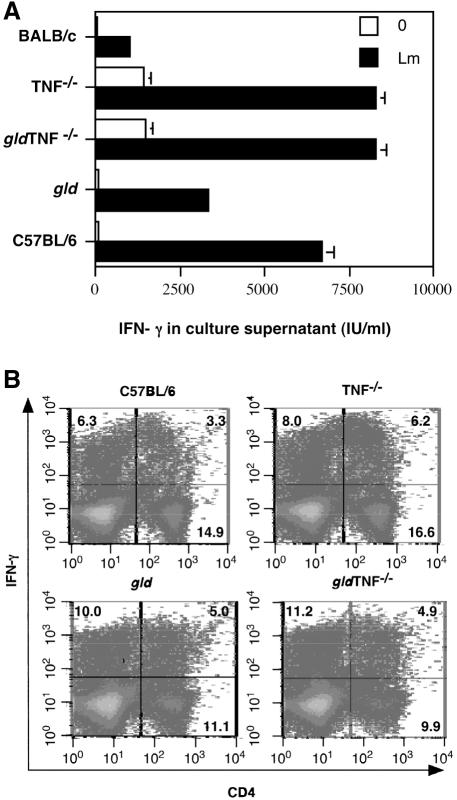
As control of parasite replication is associated with the production of IFN-γ by CD4+ Th1 cells, the type of immune response developing in the draining lymph nodes of gld TNF−/− mice following infection with L. major was compared to those in gld and TNF−/− mice. BALB/c and C57BL/6 mice were used as controls. Six weeks after infection, draining lymph node cells from mice of each group were prepared, and the levels of IFN-γ and bioactive IL-4 were analyzed in the culture supernatants of lymph node cells restimulated in vitro for 72 h with L. major (Fig. 3A). Following restimulation, the levels of IFN-γ in culture supernatants were highest in TNF−/−, gldTNF−/−, and C57BL/6 mice (seven to eight times those of BALB/c supernatants). The levels in gld mice were slightly lower, though still three times higher than the values measured in BALB/c supernatants (Fig. 4A). It is noteworthy that the levels of IFN-γ in unstimulated lymph node cells from TNF−/− and gld TNF−/− mice were already high (Fig. 3A).
FIG. 3.
Detection of IFN-γ and IL-4 in lymph node cells of gld TNF−/− mice 6 weeks after infection with L. major. Cells were isolated from popliteal lymph nodes of gld TNF−/− mice 6 weeks after infection with 3 × 106 stationary-phase L. major promastigotes. As controls, cells were taken from the popliteal lymph nodes of similarly infected C57BL/6, gld, TNF−/−, and BALB/c mice. (A) Cells (5 × 105) from mice in each group (six mice per group) were pooled and stimulated in vitro with (Lm) or without (0) 106 UV-irradiated L. major promastigotes for 72 h. IFN-γ production was evaluated in the supernatant as described in Materials and Methods. The data are representative of three different experiments; means and standard deviations of triplicate measurements are given. (B) Frequencies of IFN-γ+ CD4+ popliteal lymph node T cells 6 weeks after infection with L. major. gld TNF−/−, TNF−/−, gld, and C57BL/6 mice were infected subcutaneously with 3 × 106 stationary-phase L. major promastigotes. After 6 weeks, the draining popliteal lymph nodes from each group of mice were removed and pooled, and cell suspensions were prepared. Intracellular staining of CD4+ T cells was performed as described in Materials and Methods. The data are representative of three independent experiments.
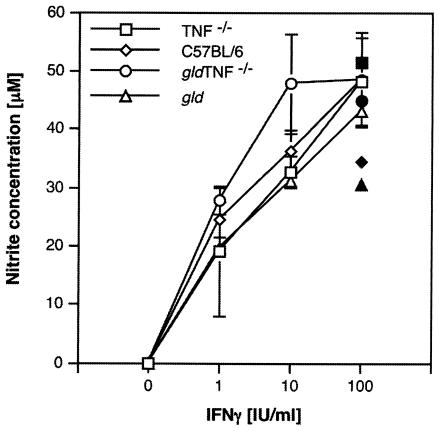
FIG. 4.
Nitric oxide production in BM Mφ. Nitric oxide production by BM Mφ from gld TNF−/− mice, as detected using the Griess reagent, was compared to that in C57BL/6, gld, and TNF−/− BM Mφ 48 h after being cultured with nonactivating doses of LPS (2 ng/ml) and increasing concentrations of IFN-γ (open symbols). Each point represents the mean and standard deviation of two different experiments with four samples per time point. Nitric oxide production was also measured after culture of the macrophages with 100 U of IFN-γ/ml and 20 ng of rTNF/ml (solid symbols).
No IL-4 was detected in culture supernatants of gld TNF−/−, TNF−/−, gld, and C57BL/6 mice 15, 30, and 60 days after infection with L. major (data not shown). As expected, elevated levels of IL-4 (3,800 pg/ml) were observed in the culture supernatants of lymph nodes of susceptible BALB/c mice.
To estimate the number of CD4+ IFN-γ-secreting cells present 6 weeks after infection, draining lymph nodes of L. major-infected mice were isolated, stimulated in vitro by phorbol myristate acetate-ionomycin for 4 h, and stained for surface CD4 expression. The IFN-γ-producing cells were detected by intracellular staining (Fig. 3B). The percentages of IFN-γ-producing cells among CD4+ T cells in the draining lymph nodes of gld, gld TNF−/−, and TNF−/− mice were comparable (5.0, 4.9, and 6.2%, respectively), while that obtained for C57BL/6 mice was lower (3.3%) (Fig. 3B). The mean fluorescence intensity in CD4+ T cells isolated from mice deprived of FasL (gld TNF−/− and gld mice) was slightly (1.5 to 2.5 times) lower than that measured in cells from C57BL/6 and TNF−/− mice, suggesting that the average output of IFN-γ per cell in cells deficient in FasL may be lower than that of C57BL/6 mice but could be compensated for by the higher percentage of cells producing IFN-γ in these mice.
A significant proportion of IFN-γ was also measured in CD4-negative cells, including predominantly CD8+ T cells (70 to 80%), and in a small percentage of NK and B cells (data not shown).
The percentages of IFN-γ+ CD4+ T cells in the draining lymph nodes of uninfected mice were 0.4 to 0.5% in C57BL/6 and gld mice and 0.9 to 1.0% in gld TNF−/− and TNF−/− mice (data not shown). Thus, CD4+ Th1 cells were differentiating in the draining lymph nodes of gld TNF−/− mice, as well as in those of TNF−/−, gld, and C57BL/6 mice, and higher levels of IFN-γ were observed in the absence of TNF.
Semiquantitative reverse transcription-PCR analysis of lymph node cell mRNA confirmed that TNF−/− and gld TNF−/− mice synthesized four to five times more IFN-γ mRNA than C57BL/6 and gld mice (data not shown). Taken together, these results demonstrate that lymph node cells of TNF−/− and gld TNF−/− mice produced IFN-γ at levels higher than that of resistant C57BL/6 mice.
Expression of NO in gld TNF−/− macrophages.
IFN-γ has been shown to activate iNOS (NOS2), leading to the production of NO, which is critical for the resolution of the infection (8, 37). BM Mφ from gld mice have been reported to secrete in vitro normal amounts of NO upon stimulation with IFN-γ plus nonactivating doses of LPS (6), while resident peritoneal macrophages from TNFRp55−/− and TNFRp55p75−/− mice failed to produce NO and to control parasite replication upon stimulation with IFN-γ in vitro (24). To estimate whether gld TNF−/− macrophages, lacking both TNF and FasL, had any defect in macrophage activation, BM Mφ isolated from gld TNF−/− mice were incubated with a nonactivating dose (2 ng/ml) of LPS and increasing concentrations of IFN-γ (which failed to activate macrophages at these concentrations when used alone). Forty-eight hours later, the amounts of NO secreted in culture supernatants were compared to those observed in similarly treated culture supernatants of macrophages from TNF−/−, gld, and C57BL/6 mice (Fig. 4). In several experiments, no significant differences in nitrite production were measured in cultures of macrophages from gld TNF−/−, TNF−/−, and gld mice compared with C57BL/6 macrophages. Following treatment with 20 ng of rTNF/ml, BM Mφ from gld mice also produced levels of NO comparable to those produced by C57BL/6 control mice, while BM Mφ from gld TNF−/− and TNF−/− mice produced higher levels of nitrites (Fig. 4). Thus, the LPS- plus IFN-γ-induced production of NO by macrophages from gldTNF−/− mice does not appear to depend on endogenous TNF.
Expression of iNOS and NO in lesions of gld TNF−/− mice.
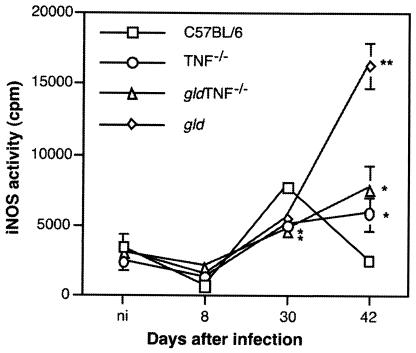
To correlate these results with the situation observed in vivo, the iNOS activities in the footpads of gld TNF−/−, TNF−/−, gld, and C57BL/6 mice were measured 0, 8, 30, and 42 days after infection with L. major (Fig. 5). Thirty days after infection, maximal iNOS activity was detected in the footpads of C57BL/6 mice. Elevated activity was also observed in TNF−/−, gldTNF−/−, and gld mice, but at lower levels (P < 0.02). Forty-two days after infection, iNOS activity dropped to basal levels in C57BL/6 mice, as expected, while it increased in TNF−/− and gld TNF−/− mice and even more so in gld mice (P < 0.001 versus C57BL/6 mice).
FIG. 5.
iNOS activities in infected footpads of gld TNF−/− mice. At the indicated times after infection with L. major, iNOS activities were measured in the footpads of gld TNF−/−, TNF−/−, and gld mice as described in Materials and Methods and compared to that of C57BL/6 mice. The data are expressed as counts per minute per milligram of footpad tissue; n = 4 to 10 footpads/time point. *, P < 0.02 versus values from C57BL/6 mice; **, P < 0.001 versus values from C57BL/6 mice.
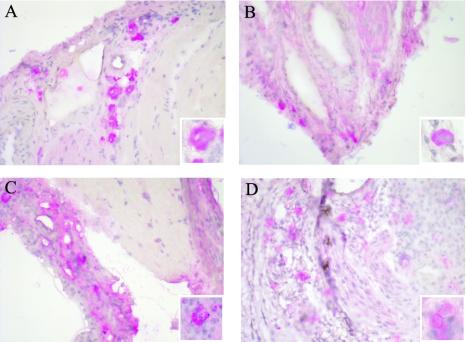
To determine putative alterations in iNOS localization in lesions of mice deficient in both TNF and FasL, histology was performed on lesions of gld TNF−/− mice 30 days after infection with L. major, and the results were compared to those for gld, TNF−/−, and C57BL/6 mice. The lesions of gld TNF−/− mice showed strong and diffuse coloration for iNOS (Fig. 6C). Similar diffuse coloration was also observed in mice deficient in TNF only (Fig. 6B), as reported previously (12). In contrast, iNOS was well localized in the lesions of gld and C57BL/6 mice (Fig. 6A and D).
FIG. 6.
Histological determination of iNOS in footpads of gld TNF−/− mice. Six weeks after infection with 3 × 106 L. major promastigotes, the expression of iNOS in the footpad lesions of C57BL/6 (A), TNF−/− (B), gld TNF−/− (C), and gld (D) mice was analyzed. Sections were stained with anti-iNOS antibody and with hematoxylin-eosin as described in Materials and Methods (magnification, ×40). Within each panel, a macrophage positive for iNOS is shown (magnification, ×100).
Thus, the production of NO in vitro following stimulation with IFN-γ and nonstimulating doses of LPS and TNF does not appear to be deficient in BM Mφ from gld TNF−/− mice. However, within the lesions of L. major-infected gld TNF−/− and TNF−/− mice 30 days after infection, iNOS appeared not only within macrophages but also diffused within the surrounding tissue.
FasL is required, together with IFN-γ, to decrease intracellular survival of L. major within macrophages.
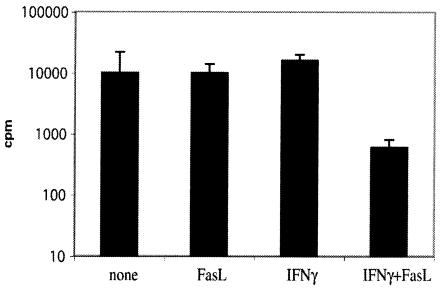
The results presented in this report show that in lesions of TNF−/− and gld TNF−/− mice, high levels of iNOS were detectable; however, only TNF−/− mice that had a functional Fas/FasL pathway were able to control parasite growth within the lesions almost completely. Upregulation of Fas expression by IFN-γ on macrophages infected with L. major was previously reported and correlated with sensitivity to FasL-mediated apoptosis of BM Mφ infected with L. major (6). To determine if FasL would also exercise a direct effect on parasite viability within macrophages, L. major-infected BM Mφ were exposed for 48 h to rFasL in the presence or absence of IFN-γ. Incubation of macrophages with a mixture of FasL and IFN-γ led to considerable reduction in parasite numbers (Fig. 7), correlating with stimulation of NO production by these cells (data not shown), whereas treatment with either substance alone failed to affect the survival of L. major or to activate NO release. In addition, BM Mφ stimulated with similar doses of either IFN-γ or FasL alone, in the absence of a nonactivating dose of LPS, failed to produce detectable levels of NO, while costimulation with IFN-γ and FasL resulted in the production of NO (data not shown).
FIG. 7.
Leishmanicidal activity requires both FasL and IFN-γ. BM Mφ were infected with stationary-phase L. major promastigotes for 16 h at a ratio of 10:1. The cells were then incubated in the presence of medium, rFasL (100 ng/ml), IFN-γ (10 ng/ml), or both IFN-γ and FasL; 48 h later, the macrophages were lysed and the released parasites were cultured in vitro for 4 days at 26°C. [3H]thymidine was added in the last 16 h of culture, and proliferation was measured. The error bars indicate standard deviations (mean of two experiments).
Of note, macrophages incubated with a mixture of rTNF (0.1 to 40 ng/ml) and FasL (1,200 ng/ml), combined or not with nonactivating doses of LPS, did not increase their output of NO, nor did they exercise leishmanicidal activity (data not shown). Thus, the Fas/FasL pathway appears to be involved in both apoptosis of the macrophages hosting L. major (6) and, together with IFN-γ, killing of the intracellular parasites. Deficiencies in both of these mechanisms may result in the phenotype observed in gld and gld TNF−/− mice following infection with L. major.
DISCUSSION
In the present study, we have showed that after infection with L. major, mice deficient in both TNF and FasL (gld TNF−/− mice) did not heal their lesions and failed to control parasite replication despite the development of a strong Th1 response, with levels of IFN-γ higher than those measured in resistant C57BL/6 mice. Mice deficient in TNF alone also failed to heal their lesions but almost completely controlled replication of L. major within the site of parasite inoculation. Such control of parasite replication was also observed in mice deficient in TNF signaling due to a lack of TNFRp55 or of both TNFRp55 and TNFRp75 (references 24 and 36 and this report), as well as in C57BL/6 transgenic mice overexpressing soluble hTNFR1. Thus, injection of L. major into mice deficient in TNF signaling due to three different defects resulted in infections in which parasite replication at the site of infection was quite well controlled. This indicates that TNF is only minimally involved in the control of parasite replication, at least in mice exhibiting a functional Fas/FasL pathway. Only in mice deficient in the pathway was TNF observed to play a more important role, since gld TNF−/− mice consistently had 10 to 50 times more parasites in their lesions than gld mice (Fig. 2A). Interestingly, these experiments demonstrate a lack of correlation between lesion size and parasite load. Indeed, mice deficient in the TNF pathway exhibited large lesions with minimal parasite load, while on the contrary, gld mice developed lesions that were significantly smaller than those of TNF−/− or BALB/c mice while carrying a high number of intralesional parasites. Of note, the number of parasites measured in the lesions of gld TNF−/− mice on the C57BL/6 resistant genetic background always remained 10 times lower than those observed in highly susceptible BALB/c mice, presumably due to the numerous genetic factors involved in resistance to infection with L. major (2).
It was recently reported that TNF−/− mice and susceptible BALB/c mice did not control parasite dissemination and succumbed to infection 6 weeks after inoculation with L. major (38). However, the number of parasites within the lesions was not investigated in that study. With the strain of L. major used in our study, neither TNF−/− nor BALB/c mice succumbed to infection up to 12 weeks after parasite inoculation, suggesting that the differences observed may be due to the use of different parasite strains. In addition, although both studies made use of TNF−/− mice on the same C57BL/6 background, the embryonic stem cells used to produce such mice were different, i.e., they were of 129 origin in the present report and of C57BL/6 origin in the study of Wilhelm et al. (38). Although both strains are resistant to infection with L. major, differences due to the genetic background of the embryonic stem cells may also account for the dissimilarities observed.
Following infection with L. major, mice expressing a nonfunctional FasL (gld) only partially controlled the parasite burden, as reported previously (6), much less efficiently than TNF−/− mice but more efficiently than gld TNF−/− mice. The differences observed in the control of parasite numbers at the site of parasite inoculation in gld TNF−/−, TNF−/−, and gld mice were not due to defective development of a Th1 type of immune response, as the elevated levels of IFN-γ detected in their draining lymph node cells, together with a lack of IL-4, were characteristic of a Th1 response. Interestingly, significantly higher levels of IFN-γ were measured in mice deficient in TNF.
iNOS activity was detected 30 days after infection with L. major in gld, gld TNF−/−, and TNF−/− mice but at levels lower than that measured in the lesions of C57BL/6 mice. Histology revealed that, in addition to the presence of iNOS within macrophages, diffuse expression was observed in the lesions of both TNF−/− and gld TNF−/− mice. Similar diffuse iNOS expression within the lesions of infected TNF−/− mice was reported previously and was also observed in draining lymph nodes late in infection (38). However, the diffuse aspect of iNOS cannot explain the failure to control parasite replication that was observed in the lesions of gld TNF−/− mice, since TNF−/− mice successfully controlled parasite replication despite exhibiting diffuse iNOS staining. The defective control of parasite replication observed in the lesions of gld TNF−/− mice is most likely due to the absence of a FasL pathway. The numbers of macrophages within the lesions of gld mice have been reported to be increased following infection with L. major (6), so the significant increase in iNOS activity measured 42 days after infection (Fig. 5B) may result from a higher number of activated macrophages within the lesion. Such an accumulation of macrophages was also observed by histochemical analysis in gld TNF−/− mice 42 and 60 days after infection with L. major (data not shown), confirming that the number of macrophages present within a lesion does not always correlate with the size of the lesion. In gld TNF−/− mice, the number of macrophages present in the lesions is comparable to that in gld mice but the iNOS activity measured is lower, revealing that TNF likely accounts for a great part of the iNOS activation measured late in infection.
TNF has been reported to play an essential homeostatic role in limiting the duration of the inflammatory process (23) and in the control of inflammation in several models of infection, including infections with L. major (15, 28). In this report, we show that mice lacking TNF, despite the control of parasite replication at the site of L. major infection, develop large inflammatory lesions that do not resolve during the period tested. Thus, TNF appears to be essential in the resolution of the inflammatory lesion during infection with L. major but not in the control of parasite replication.
The mechanism of action of FasL in controlling parasite replication might involve induction of macrophage apoptosis by FasL-expressing CD4+ Th1 lymphocytes. It has been shown that macrophages upregulate the surface expression of Fas in response to IFN-γ and may thereby become susceptible to apoptosis upon interaction with CD4+ Th1 cells expressing FasL (6). Macrophage apoptosis could limit the number of macrophages at the site of parasite replication, and more IFN-γ per parasitized macrophage may become available. In this report, we show that gld, TNF−/−, and gld TNF−/− mice have higher levels of iNOS activity within their lesions late in infection. While small amounts of NO have been shown to inhibit macrophage apoptosis (21, 22), elevated amounts of NO released from cytokine-activated macrophages have been reported to act to stimulate macrophage effector functions and simultaneously to induce apoptosis (1). Furthermore, high levels of NO were shown to induce soluble-FasL release in vitro, as well as upregulation of Fas expression in macrophages (25). FasL release was also reported to be triggered by phagocytosis and to induce the apoptosis of bystander monocytes (5). Thus, the high level of activity of iNOS and its resulting NO observed within the lesions of TNF−/− mice may sensitize macrophages to Fas-mediated apoptosis through interactions with CD4+ Th1 cells expressing FasL (26, 40) and/or through secretion of FasL by activated macrophages, resulting in negative feedback favoring deletion of macrophages within the lesion. In gld TNF−/− mice, as well as in gld mice, NO is unable to induce Fas/FasL-mediated apoptosis of macrophages due to deficient FasL, resulting in increased numbers of macrophages within the lesion (6), thereby allowing L. major proliferation. Thus, NO might at the same time induce microbicidal functions within the macrophages and regulate macrophage numbers at the site of parasite inoculation through interactions with FasL. We further show that in vitro, FasL is indeed required for macrophage leishmanicidal activity in the presence of NO.
Thus, we show here that early after infection, TNF does not play an important role in parasite control during infection with L. major. We further demonstrate that FasL, acting in synergy with IFN-γ and iNOS, appears to be essential in the control of parasite replication within the lesions, acting on both the apoptosis of macrophages and their microbicidal function.
Acknowledgments
We thank Jürg Tschopp for providing gld TNF−/− mice and rFasL; H. Bluethmann, Hoffmann la Roche, Basel, Switzerland, for providing TNFRp55, TNFRp55p75, and B6 × 129 mice; W. Held for providing NK1.1 MAb; and H. Himmelrich for stimulating discussions. We also thank G. Badic and E. Sauberli for histology and R. Cornaz and Y. Hauyon for technical assistance.
This work was supported by grants from the Swiss National Foundation for Scientific Research (no. 3200-067447.01 to F.T.-C, 3200-06206.00 to I.G., and 31-56796.99 to J.L.).
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Albina, J. E., S. Cui, R. B. Mateo, and J. S. Reichner. 1993. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J. Immunol. 150:5080-5085. [PubMed] [Google Scholar]
- 2.Beebe, A., S. Mauze, N. J. Schork, and R. L. Coffman. 1997. Serial backcross mapping of multiple loci associated with resistance to Leishmania major in mice. Immunity 6:551-557. [DOI] [PubMed] [Google Scholar]
- 3.Bogdan, C., H. Moll, W. Solbach, and M. Rollinghoff. 1990. Tumor necrosis factor-alpha in combination with interferon-gamma, but not with interleukin 4 activates murine macrophages for elimination of Leishmania major amastigotes. Eur. J. Immunol. 20:1131-1135. [DOI] [PubMed] [Google Scholar]
- 4.Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2000. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 12:64-76. [DOI] [PubMed] [Google Scholar]
- 5.Brown, S. B., and J. Savill. 1999. Phagocytosis triggers macrophage release of Fas ligand and induces apoptosis of bystander leukocytes. J. Immunol. 162:480-485. [PubMed] [Google Scholar]
- 6.Conceiçao-Silva, F., M. Hahne, M. Schröter, J. Louis, and J. Tschopp. 1998. The resolution of lesions induced by Leishmania major in mice requires a functional Fas (APO-1, CD95) pathway of cytoxicity. Eur. J. Immunol. 28:237-245. [DOI] [PubMed] [Google Scholar]
- 7.Desbarats, J., J. E. Stone, L. Lin, Z. F. Zakeri, G. S. Davis, L. M. Pfeiffer, R. G. Titus, and M. K. Newell. 2000. Rapid early onset lymphocyte cell death in mice resistant, but not susceptible to Leishmania major infection. Apoptosis 5:189-196. [DOI] [PubMed] [Google Scholar]
- 8.Diefenbach, A., H. Schindler, N. Donhauser, E. Lorenz, T. Laskay, J. MacMicking, M. Rollinghoff, I. Gresser, and C. Bogdan. 1998. Type 1 interferon (IFNα/β) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity 8:77-87. [DOI] [PubMed] [Google Scholar]
- 9.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407-2412. [PubMed] [Google Scholar]
- 10.Favre, N., and P. Erb. 1993. Use of the CTL44 cell line, a derivate of CTL/L cells, to identify and quantify mouse interleukin-4 by bioassay. J. Immunol. Methods 164:213-220. [DOI] [PubMed] [Google Scholar]
- 11.Garcia, I., Y. Miyazaki, K. Araki, M. Araki, R. Lucas, G. E. Grau, G. Milon, Y. Belkaid, C. Montixi, W. Lesslauer, and P. Vassali. 1995. Transgenic mice expressing high levels of soluble TNF-R1 fusion protein are protected from lethal septic shock and cerebral malaria, and are highly sensitive to Listeria monocytogenes and Leishmania major infections. Eur. J. Immunol. 25:2401-2407. [DOI] [PubMed] [Google Scholar]
- 12.Green, S. J., S. Mellouk, S. L. Hoffman, M. S. Meltzer, and C. A. Nacy. 1990. Cellular mechanisms of nonspecific immunity to intracellular infection: cytokine-induced synthesis of toxic nitrogen oxides from l-arginine by macrophages and hepatocytes. Immunol. Lett. 25:15-19. [DOI] [PubMed] [Google Scholar]
- 13.Huang, F. P., D. Xu, E. O. Esfandiari, W. Sands, X. Q. Wei, and F. Y. Liew. 1998. Mice defective in Fas are highly susceptible to Leishmania major infection despite elevated IL-12 synthesis, strong Th1 responses, and enhanced nitric oxide production. J. Immunol. 160:4143-4147. [PubMed] [Google Scholar]
- 14.Ju, S. T., H. Cui, D. J. Panka, R. Ettinger, and A. Marshak-Rothstein. 1994. Participation of target Fas protein in apoptosis pathway by CD4+ Th1 and CD8+ cytotoxic T cells. Proc. Natl. Acad. Sci. USA 91:4185-4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanaly, S. T., M. Nashleanas, B. Hondowicz, and P. Scott. 1999. TNF receptor p55 is required for elimination of inflammatory cells following control of intracellular pathogens. J. Immunol. 163:3883-3889. [PubMed] [Google Scholar]
- 16.Korner, H., M. Cook, D. S. Riminton, F. A. Lemckert, R. M. Hoek, B. Ledermann, F. Kontgen, B. Fazekas de St Groth, and J. D. Sedgwick. 1997. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in organogenesis and spatial organization of lymphoid tissue. Eur. J. Immunol 27:2600-2609. [DOI] [PubMed] [Google Scholar]
- 17.Liew, F. Y., S. Millott, C. Parkinson, R. M. Palmer, and S. Moncada. 1990. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from l-arginine. J. Immunol. 144:4794-4797. [PubMed] [Google Scholar]
- 18.Louis, J. A., E. Moedder, R. Behin, and H. D. Engers. 1979. Recognition of protozoan parasite antigens by murine T lymphocytes. Eur. J. Immunol. 9:841-847. [DOI] [PubMed] [Google Scholar]
- 19.Lucas, R., F. Tacchini-Cottier, R. Guler, D. Vesin, S. Jemelin, M. L. Olleros, G. Marchal, J. L. Browning, P. Vassalli, and I. Garcia. 1999. A role for lymphotoxin beta receptor in host defense against Mycobacterium bovis BCG infection. Eur. J. Immunol. 29:4002-4010. [DOI] [PubMed] [Google Scholar]
- 20.Lynch, D. H., M. L. Watson, M. R. Alderson, P. R. Baum, R. E. Miller, T. Tough, M. Gibson, T. Davis-Smith, C. A. Smith, K. Hunter, et al. 1994. The mouse Fas-ligand gene is mutated in gld mice and is part of a TNF family gene cluster. Immunity 1:131-136. [DOI] [PubMed] [Google Scholar]
- 21.Mannick, J. B., K. Asano, K. Izumi, E. Kieff, and J. S. Stamler. 1994. Nitric oxide produced by human B lymphocytes inhibits apoptosis and Epstein-Barr virus reactivation. Cell 79:1137-1146. [DOI] [PubMed] [Google Scholar]
- 22.Mannick, J. B., X. Q. Miao, and J. S. Stamler. 1997. Nitric oxide inhibits Fas-induced apoptosis. J. Biol. Chem. 272:24125-24128. [DOI] [PubMed] [Google Scholar]
- 23.Marino, M. W., A. Dunn, D. Grail, M. Inglese, Y. Noguchi, E. Richards, A. Jungbluth, H. Wada, M. Moore, B. Williamson, S. Basu, and L. J. Old. 1997. Characterization of tumor necrosis factor-deficient mice. Proc. Natl. Acad. Sci. USA 94:8093-8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nashleanas, M., S. Kanaly, and P. Scott. 1998. Control of Leishmania major infection in mice lacking TNF receptors. J. Immunol. 160:5506-5513. [PubMed] [Google Scholar]
- 25.Niinobu, T., K. Fukuo, O. Yasuda, M. Tsubakimoto, M. Mogi, H. Nishimaki, S. Morimoto, and T. Ogihara. 2000. Negative feedback regulation of activated macrophages via Fas-mediated apoptosis. Am. J. Physiol. Cell Physiol. 279:C504-C509. [DOI] [PubMed]
- 26.Ramsdell, F., M. Seaman, R. Miller, K. Picha, M. Kennedy, and D. Lynch. 1994. Differential ability of Th1 and Th2 T cells to express Fas ligand and to undergo activation-induced cell death. Int. Immunol. 6:1545-1553. [DOI] [PubMed] [Google Scholar]
- 27.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 28.Skerrett, S. J., T. R. Martin, E. Y. Chi, J. J. Peschon, K. M. Mohler, and C. B. Wilson. 1999. Role of the type 1 TNF receptor in lung inflammation after inhalation of endotoxin or Pseudomonas aeruginosa. Am. J. Physiol. 276:L715-L727. [DOI] [PubMed]
- 29.Slade, S. J., and J. Langhorne. 1989. Production of interferon-γ during infection of mice with Plasmodium chabaudi chabaudi. Immunobiology 179:353-365. [DOI] [PubMed] [Google Scholar]
- 30.Stalder, T., S. Hahn, and P. Erb. 1994. Fas antigen is the target molecule for CD4+ T cell mediated cytotoxicity. J. Immunol. 152:1127-1133. [PubMed] [Google Scholar]
- 31.Takahashi, T., M. Tanaka, C. I. Brannan, N. A. Jenkins, N. G. Copeland, T. Suda, and S. Nagata. 1994. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell 76:969-976. [DOI] [PubMed] [Google Scholar]
- 32.Taswell, C. 1981. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J. Immunol. 126:1614-1619. [PubMed] [Google Scholar]
- 33.Theodos, C. M., L. Povinelli, R. Molina, B. Sherry, and R. G. Titus. 1991. Role of tumor necrosis factor in macrophage leishmanicidal activity in vitro and resistance to cutaneous leishmaniasis in vivo. Infect. Immun. 59:2839-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Titus, R. G., M. Marchand, T. Boon, and J. A. Louis. 1985. A limiting dilution assay for quantifying Leishmania major in tissue of infected mice. Parasite Immunol. 7:545-555. [DOI] [PubMed] [Google Scholar]
- 35.Titus, R. G., B. Sherry, and A. Cerami. 1989. Tumor necrosis factor plays a protective role in experimental murine cutaneous leishmaniasis. J. Exp. Med. 170:2097-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vieira, L. Q., M. Goldschmidt, M. Nashleanas, K. Pfeffer, T. Mak, and P. Scott. 1996. Mice lacking the TNF receptor p55 fail to resolve lesions caused by infection with Leishmania major, but control parasite replication. J. Immunol. 157:827-835. [PubMed] [Google Scholar]
- 37.Wei, X.-Q., I. G. Charles, A. Smith, G.-J. Feng, F.-P. Huang, D. Xu, W. Muller, S. Moncada, and F. Y. Liew. 1995. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375:408-411. [DOI] [PubMed] [Google Scholar]
- 38.Wilhelm, P., U. Ritter, S. Labbow, N. Donhauser, M. Rollinghoff, C. Bogdan, and H. Korner. 2001. Rapidly fatal leishmaniasis in resistant C57BL/6 mice lacking TNF. J. Immunol. 166:4012-4019. [DOI] [PubMed] [Google Scholar]
- 39.Yagita, H., S. Hanabuchi, Y. Asano, T. Tamura, H. Nariuchi, and K. Okumura. 1995. Fas-mediated cytotoxicity—a new immunoregulatory and pathogenic function of Th1 CD4+ T cells. Immunol. Rev. 146:223-239. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, X., T. Brunner, L. Carter, R. W. Dutton, P. Rogers, L. Bradley, T. Sato, J. C. Reed, D. Green, and S. L. Swain. 1997. Unequal death in T helper cell (Th)1 and Th2 effectors: Th1 but not Th2 effectors undergo rapid Fas/FasL-mediated apoptosis. J. Exp. Med. 185:1837-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]