Abstract
Cryptococcus neoformans is an encapsulated, environmental fungus that can cause life-threatening meningitis. Pathogenicity of C. neoformans for macrophages and vertebrate hosts may be a mechanism selected in evolution for protection against environmental predators. In this study, we investigated whether Dictyostelium discoideum could serve as an alternate host for C. neoformans. D. discoideum has a defined genetic system which provides significant advantages for the study of fungus-amoeba interactions. Our results show that D. discoideum is susceptible to infection with C. neoformans and that the interactions are similar to those described previously for this fungus with macrophages and Acanthamoeba castellanii. Acapsular C. neoformans cells did not replicate when coincubated with D. discoideum. However, incubation of acapsular C. neoformans with D. discoideum mutants defective in myosin VII synthesis resulted in infection, validating the concept that avirulent organisms can be virulent in impaired hosts even at the unicellular level. Phagocytosis of C. neoformans by D. discoideum could be inhibited with capsule-specific antibodies and various sugars. Passage of an encapsulated C. neoformans strain through D. discoideum cultures increased virulence and was accompanied by larger capsules and faster time to melanization. These results add to the evidence implicating soil ameboid predators as important factors for the maintenance of C. neoformans virulence in the environment and suggest that D. discoideum promises to be an extremely useful system for studying the interaction of C. neoformans with phagocytic cells.
Cryptococcus neoformans is an encapsulated fungus found predominately in soils contaminated with pigeon excreta, and human infection is widely assumed to result from inhalation of desiccated yeast particles or basidiospores (23). C. neoformans is macrophage tropic in mammalian infection, but the fungus also infects environmental microorganisms, such as the amoeba Acanthamoeba castellanii and the nematode Caenorhabditis elegans (46, 52). This pathogenic fungus has developed elaborate mechanisms to evade macrophage, nematode, and amoeba phagocytosis and killing, including a unique intracellular pathogenic strategy that causes the release of polysaccharide capsule into cytoplasmic vesicles (24, 46, 52). C. neoformans infections in immunocompetent hosts often result in latency and persistence, with the yeast residing inside macrophages (27, 30). The reactivation of these sequestered fungal cells can result in active disease in immunocompromised individuals, which usually presents clinically as a life-threatening meningitis (20, 27, 42). C. neoformans' infection of A. castellanii and macrophages show striking similarities; therefore, it was postulated that the pathogenicity of C. neoformans for macrophages was the result of evolutionary selection as a mechanism for protection against phagocytic environmental predators (52). This hypothesis was further supported by a study using C. elegans, which determined that fungal virulence factors played an important role in nematode killing (46).
Virulence is a microbial characteristic that is expressed and maintained in a susceptible host (12). C. neoformans can occasionally lose virulence during in vitro culture conditions (25). However, virulence can also be restored by passage of an avirulent C. neoformans strain through mice (47). Since C. neoformans is an environmental fungus and does not depend on an animal host for replication, it is remarkable that soil isolates are virulent for many animal species, and virulence remains relatively stable in the laboratory. C. neoformans laboratory isolates consistently retain traits associated with virulence, including the formation of a polysaccharide capsule, the ability to make melanin pigments, and the capacity to express certain enzymes, such as phospholipase, urease, and superoxide dismutase (9, 15, 18, 19, 41). Previous studies with Legionella pneumophila demonstrated an increase in virulence of the bacterium after infection of amoebae (7). This precedent together with the observations made with A. castellanii and C. elegans suggest that C. neoformans virulence for mammals is maintained in the environment by selection resulting from interactions with other soil organisms (46, 52).
Dictyostelium discoideum, a free-living soil amoeba, has been exploited to study professional phagocytic processes and is a model system organism (22, 49, 51). D. discoideum is a haploid, genetically malleable amoeba and therefore is ideal for studying host-pathogen interactions. In addition, the endolysosomal and phagosomal pathways of D. discoideum have been studied extensively, and many mutants are available (10, 38). Here we explore the interaction of C. neoformans with D. discoideum and show that the latter is a suitable host for the fungus. Furthermore, infection of D. discoideum by the fungus can affect subsequent fungal virulence for mice.
MATERIALS AND METHODS
Organisms and culture conditions.
Stock cultures of D. discoideum strains were maintained at −80°C. For experimental work, D. discoideum cultures were grown axenically in HL5 medium supplemented with 100 μg of penicillin and streptomycin (GibcoBRL, Carlsbad, Calif.)/ml at 22°C with shaking at 150 rpm for no more than 10 passages (53). Alternatively, D. discoideum was grown as plaques on lawns of Klebsiella aerogenes plated on SM/5 agar (53). D. discoideum AX-4 was a gift from J. E. Segall (Albert Einstein College of Medicine, Bronx, N.Y.) and was used for most experiments unless otherwise noted. D. discoideum HTD17-1, a myosin VII null mutant, and G1-21, a nonhomologous recombinant control for HTD17-1, were gifts from M. Titus (University of Minnesota, Minneapolis, Minn.) (58). These strains were maintained in HL5 medium supplemented with 10 μg of Blasticidin S (ICN)/ml. rtoA D. discoideum, a gene disruption mutant with defects in endosomal fusion and pH regulation as well as exocytosis, and DH1 wild-type cells were a gift from R. Gomer (Rice University, Houston, Tex.) and were maintained in HL5 medium as described previously (31, 63). The DH1 cell cultures were supplemented with uracil (31, 63).
C. neoformans serotype D strains 24067 and 3501 were obtained from the American Type Culture Collection (Rockville, Md.). Strain F7 is a stable pseudohyphal mutant of 24067 (26); strain Cap67, an acapsular mutant of 3501, was obtained from E. Jacobson (Richmond, Va.) (11). The C. neoformans serotype A strain H99 was obtained from J. Perfect (Duke University Medical College, Durham, N.C.). Yeast cultures were started from frozen stock maintained at −80°C and grown in Sabouraud dextrose (SAB) broth for 48 h at 30°C with shaking at 150 rpm, collected by centrifugation, and washed three times with phosphate-buffered saline (PBS) (0.137 M NaCl, 0.003 M sodium phosphate [pH 7.4]).
Cytotoxicity assays.
C. neoformans replication in and cytotoxicity for D. discoideum was measured by CFU and trypan blue exclusion assays, respectively. D. discoideum viability determinations were confirmed with PFU on bacterial agar plates. The fungal and amoeba cells were washed with PBS, counted with a hemocytometer, and suspended at 105 cells/ml. Cell counts were confirmed by CFU on SAB agar plates incubated at 30°C. C. neoformans cells were added at a 1:1 effector-to-target ratio to D. discoideum in 96-well tissue culture plates and incubated in PBS at 22°C. The numbers of viable yeast and amoeboid cells were determined at 0, 24, and 48 h by measuring CFU and the number of D. discoideum that excluded the trypan blue dye, respectively. For CFU analysis, D. discoideum and C. neoformans cells were removed from the wells by placing the tissue culture plate on ice for 10 min followed by gentle agitation. D. discoideum cells were lysed by pulling the contents of the wells through a 27-gauge needle five times (43). At each time interval, five tissue culture wells per amoeba or fungal strain were used to ascertain viable yeast cells, and serial dilutions were plated in duplicate onto SAB agar plates, which were incubated at 30°C for 48 h. Using a trypan blue exclusion assay, the viability of D. discoideum was assessed at 0, 24, and 48 h. A 1:10 dilution of trypan blue in PBS was added to the undisrupted tissue culture wells at each time interval. The 96-well tissue culture plates were centrifuged at 220 × g for 10 min and viewed at magnification ×200. D. discoideum cells were considered dead if they were unable to exclude the blue dye. The percentage of dead D. discoideum cells was calculated from the trypan blue cytotoxicity assay by counting the number of blue cells per 100 D. discoideum cells. Five wells per experimental condition were counted. To confirm trypan blue results, PFU were assayed. The contents from wells containing a 1:1 ratio of D. discoideum to C. neoformans were removed gently after incubation on ice for 10 min. Serial dilutions were plated onto SM/5 agar with K. aerogenes, and the plates were incubated at 22°C. Plaques were visible and counted within 4 days of plating.
Phagocytosis index.
D. discoideum cells were collected by centrifugation at 600 × g, washed with PBS, and suspended in HL5 media. Washed C. neoformans cells from strains H99, 24067, 3501, F7, and Cap67 were added to the D. discoideum cells at an effector-to-target ratio of 1:1, as determined by hemocytometer counts, and incubated at 22°C for 2 h. The medium was aspirated, and the cells were fixed with ice-cold methanol for 30 min at 4°C. Cells were not washed prior to fixing to avoid removal of loosely adhered D. discoideum cells. Wells were washed with 2× PBS and stained with a 1:10 dilution of Giemsa for 45 min. The plates were viewed at magnification ×200, and five wells per experimental condition were used to determine the phagocytic index, defined as the number of amoebae with at least one internalized fungal cell (8).
Transmission electron microscopy.
Fungal and AX-4 amoeba cells were washed as described above and incubated at a 1:1 ratio in HL5 media for 2.5 h with gentle rocking. The cells were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate at room temperature overnight, and the samples were prepared according to published methods and viewed with a model 102 electron microscope (Siemens, Berlin, Germany) (59).
Phagocytosis inhibition assays.
Carbohydrate phagocytosis inhibition assays were used to determine what receptors might be involved in the phagocytosis of C. neoformans by D. discoideum AX-4. Concentrations ranging from 1 to 1,000 mM of d-mannose, d-galactose, d-xylose, and mannitol (Sigma, St. Louis, Mo.) were added to D. discoideum in 96-well plates and incubated for 1 h prior to adding C. neoformans H99 as described above. Five wells per condition were counted.
To assess the effect of antibody specific for the C. neoformans capsule, the immunoglobulin G1 (IgG1) monoclonal antibody (MAb) 18B7 and C. neoformans 24067 were added to D. discoideum in 96-well plates prepared as mentioned above for phagocytosis assays. Concentrations of MAb 18B7 were varied between 1, 10, and 100 μg/ml. The IgG1 MAb 3665 was used as an irrelevant isotype-matched control (48). Phagocytic index was determined by averaging results from five wells.
Murine studies.
C. neoformans strain 24067 cells were passaged through D. discoideum AX-4 by coincubating C. neoformans and D. discoideum cells, and virulence was determined by murine survival. Four preparations were evaluated for virulence in mice: (i) D. discoideum cells with C. neoformans cells, (ii) killed D. discoideum cells with C. neoformans cells, (iii) D. discoideum alone, and (iv) C. neoformans alone. All cells were washed, counted, and suspended in HL5 medium in 175-ml tissue culture flasks (Corning, Corning, N.Y.). Live fungal cells were added at a 1:10 ratio to D. discoideum. D. discoideum cells were killed by sonication, which was confirmed by trypan blue exclusion. All flasks were incubated at 22°C for 72 h. As determined by light microscopy, there were no whole D. discoideum cells present in the culture that contained both C. neoformans and D. discoideum cells at 72 h. Cells were removed from the tissue culture flasks and washed four times with PBS and suspended in PBS. A total of 107 C. neoformans cells or D. discoideum cells, prepared as noted above, were injected into the peritoneum of A/Jcr female mice, 7 to 9 weeks of age, from the National Cancer Institute (Bethesda, Md.). The survival time was recorded.
Phenotypic analysis of passaged C. neoformans cells.
To ascertain if there were phenotypic differences between the passaged and nonpassaged C. neoformans cells, we determined the growth rate, time to melanization, and capsule size for both groups. Immediately after coincubation with D. discoideum, the growth rate of C. neoformans was determined in SAB broth at either 30 or 37°C for 5 days by counting cells and CFU as a function of time. Cells were also streaked onto agar plates containing minimal media with 1 mM L-Dopa, incubated in the dark, and checked daily for pigmentation change, indicative of melanization (59, 62). The experiment was simultaneously performed with nonpassaged C. neoformans cells. To measure capsule size differences in C. neoformans, fungal cells were grown at 37 and 30°C for 24 h, and capsule size was measured using India ink exclusion. Micrographs were taken at ×400, and the capsule area was measured using Scion image software (National Institutes of Health, Bethesda, Md.). The capsule and cell body size of 20 cells per condition were counted, and capsule size was determined as the total organism area minus the area of the cell body in pixels.
Statistical analysis.
All experiments were performed at least two independent times. Student t test analysis and cytotoxicity graphs were compiled in Excel 2000 (Microsoft; Redmont, Wash.). Survival analysis was performed using log rank analysis (SPSS, Chicago, Ill.). (This work is from a thesis to be submitted by J.N.S. in partial fulfillment of the requirements for a Ph.D. in the Sue Golding Graduate Division of Medical Sciences, Albert Einstein College of Medicine, Bronx, N.Y.)
RESULTS
Growth of C. neoformans with D. discoideum.
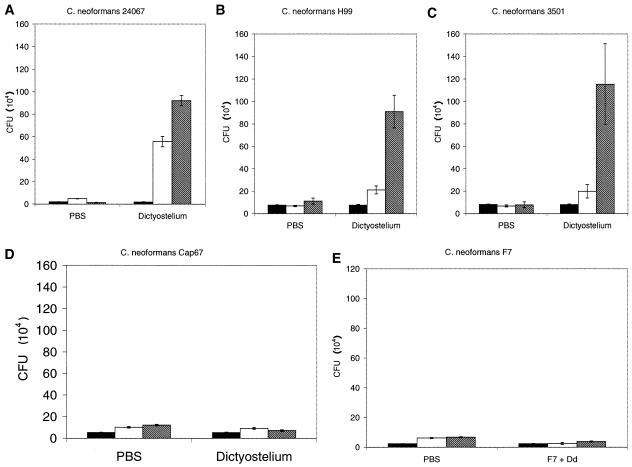
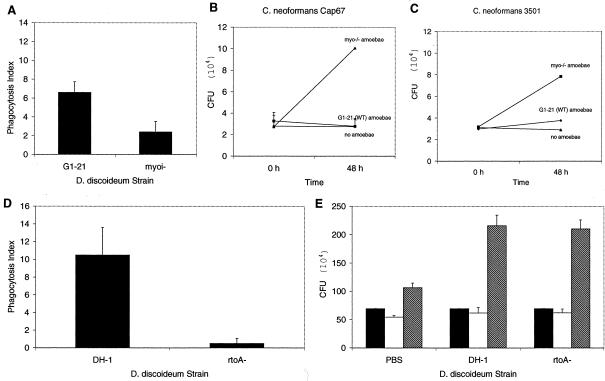
D. discoideum AX-4 was infected with C. neoformans, and fungal growth was determined by counting CFU. Wild-type C. neoformans strains were able to grow in the presence of D. discoideum, as evidenced by an increase in the number of CFU with time (Fig. 1A, B, and C). Increases in CFU were evident for both serotype A (H99) and serotype D (24067 and 3501) strains. For both strain H99 and strain 3501, there was more growth between 24 and 48 h; however, strain 24067 had the greatest growth increase in the first 24 h. The number of CFU increased between 12-fold for H99 and almost 50-fold for 24067. The CFU increases at 48 h were significant compared to CFU of C. neoformans alone for all three wild-type strains (P ≤ 0.001). In addition, results with Cap67, an avirulent acapsular mutant of 3501, indicate that the capsule is required for C. neoformans growth in D. discoideum (Fig. 1D). There was no change in the number of CFU of Cap67 when exposed to D. discoideum. At 48 h there was no difference in CFU between C. neoformans Cap67 grown in PBS or D. discoideum conditions (P = 0.445). Similar results were demonstrated for F7, a hypovirulent, pseudohyphal variant of 24067 (Fig. 1E).
FIG. 1.
Fungal CFU after incubation of C. neoformans with or without D. discoideum. Solid bars denote CFU at 0 h, open bars denote CFU at 24 h, and hatched bars denote CFU at 48 h. The error brackets represent one standard deviation. The differences between the CFU of C. neoformans strains 24067, H99, and 3501 incubated with D. discoideum and the corresponding fungal cells in PBS at 48 h were significant (P ≤ 0.001). Numbers of CFU for C. neoformans Cap67 were similar in PBS and D. discoideum for each time interval. Numbers of CFU for C. neoformans strains 24067 (serotype A) (A), H99 (serotype D) (B), 3501 (serotype A) (C), Cap67 (acapsular variant of 3501) (D), and F7 (pseudohyphal variant of 24067) (E) are shown. Each experiment was repeated with similar results.
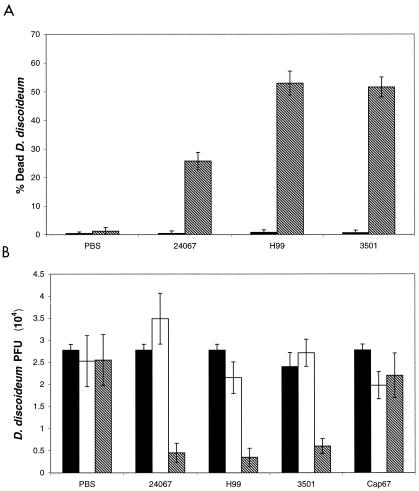
Changes in D. discoideum viability were assessed using both a trypan blue exclusion assay and PFU enumeration. The two methods produced similar results. Trypan blue exclusion results showed that C. neoformans killed D. discoideum (Fig. 2A). The greatest killing of D. discoideum occurred with C. neoformans strains H99 and 3501, with 53 and 52%, respectively, at 24 h. Incubation of D. discoideum with C. neoformans 24067, H99, and 3501 resulted in a decrease in D. discoideum viability at 48 h as indicated by the number of PFU (Fig. 2B). This decrease was significant when compared to D. discoideum in PBS only (P ≤ 0.001). In contrast, the acapsular mutant, C. neoformans Cap67, caused no significant decrease in PFU numbers from that for D. discoideum in PBS only (P = 0.397). The results from the trypan blue exclusion and PFU assay parallel each other, indicating that wild-type C. neoformans strains were lethal to D. discoideum.
FIG. 2.
Viability of D. discoideum after incubation with C. neoformans. Solid bars represent viability at 0 h, open bars represent viability at 24 h, and hatched bars represent viability at 48 h. Error brackets denote one standard deviation. (A) Percentages of amoebae that are trypan blue positive. At 48 h, P values were ≤0.001 for comparisons of amoebae incubated with any of the C. neoformans strains with D. discoideum to amoebae alone. (B) Numbers of PFU representing the total numbers of viable D. discoideum cells. At 48 h a significant decrease in PFU of D. discoideum cells was measured after incubation C. neoformans strains 24067, H99, and 3501 (P ≤ 0.001). The number of PFU of D. discoideum incubated with C. neoformans Cap67 was unchanged throughout the assay. The experiment was done twice with similar results.
Phagocytosis of C. neoformans by D. discoideum.
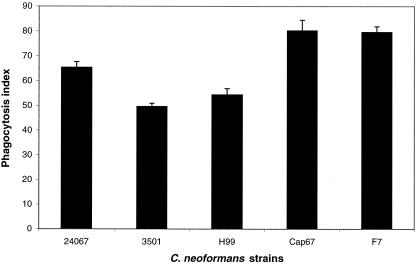
Phagocytosis assays were used to confirm that D. discoideum cells ingested C. neoformans cells. The phagocytic index showed that up to 80% of wild-type AX-4 D. discoideum cells phagocytosed at least one C. neoformans fungal cell (Fig. 3). The majority of D. discoideum cells internalized one fungal cell, though two and three C. neoformans cells were found internalized in occasional amoeboid cells. The hypovirulent C. neoformans strains F7 and Cap67 were phagocytosed in significantly higher numbers than virulent parental strains.
FIG. 3.
Phagocytosis of C. neoformans strains 24067, 3501, H99, F7, and Cap67 by D. discoideum. Bars represent the numbers of phagocytic events by D. discoideum, and error brackets denote 1 standard deviation. The phagocytosis index was determined by counting the total number of internalized fungal cells per 100 amoebae. For each experimental condition the number of repetitions was five. This experiment was repeated on different days and yielded similar results.
Intracellular interaction of C. neoformans with D. discoideum.
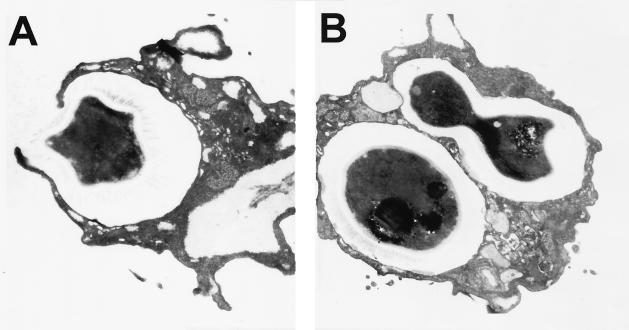
Transmission electron microscopy was used to investigate the interactions of C. neoformans and D. discoideum. Figure 4A depicts D. discoideum phagocytosing a C. neoformans strain 3501 cell. D. discoideum pseudopods can be seen surrounding the encapsulated yeast as the fungal cell is engulfed. Intracellular replication by C. neoformans cells was suggested by the observation of budding fungal cells inside D. discoideum (Fig. 4B). Ingested fungal cells were contained in a membrane-enclosed vacuole inside D. discoideum. Some D. discoideum cells appeared to have participated in at least two phagocytic events, as suggested by the presence of separate phagocytic vacuoles containing C. neoformans cells. The micrographs shown are representative for all wild-type C. neoformans strains tested and confirm that the C. neoformans cells are ingested by D. discoideum.
FIG. 4.
Transmission electron micrographs of C. neoformans strain 3501 cells interacting with D. discoideum. (A) C. neoformans is being engulfed by the pseudopods of a D. discoideum cell 2 h postinfection. (B) Two individual phagocytic events by one D. discoideum cell 2 h postinfection. In one event the C. neoformans cells are in membrane-bound vacuoles. The second event shows a budding C. neoformans cell in a vacuole. (Magnifications: ×24,000 [A] and ×18,000 [B]). The micrographs shown are representative of what was observed under the microscope.
D. discoideum mutants affect growth of C. neoformans.
The genetics of D. discoideum are well defined, and an assortment of known null mutants is available. Previously characterized amoeba mutants defective in phagocytoses and exocytosis were analyzed to explore the usefulness of D. discoideum mutants to study the interaction of amoeboid cells with C. neoformans.
The myoi D. discoideum mutant is a null mutant for myosin VII (58). D. discoideum myosin VII is important in cell adhesion and particle adhesion during phagocytosis (58). Phagocytosis of the hypovirulent C. neoformans strain Cap67 by the myoi null D. discoideum mutant was only 49% of the G1-21 nonhomologous control D. discoideum phagocytic index (P = 0.042) (Fig. 5A). Also, the D. discoideum myoi null mutant was more permissive to growth of C. neoformans strain Cap67 (Fig. 5B). The number of CFU of Cap67 increased dramatically compared to the absence of growth for Cap67 cells grown with D. discoideum G1-21 control cells (P = 0.002). When evaluating the effect of the myoi mutant on the wild-type C. neoformans strain 3501, a less dramatic result was seen; however, the myoi null D. discoideum cells were still more permissive to growth of 3501 (P = 0.041) (Fig. 5C).
FIG. 5.
Interaction of C. neoformans strains with D. discoideum myoi and rtoA mutants. (A) Phagocytosis of C. neoformans Cap67 cells by D. discoideum myoi or wild-type cells. Wild-type D. discoideum cells phagocytosed significantly more fungal cells than myoi null cells (P = 0.042). Solid bars represent the numbers of phagocytic events by D. discoideum, and error brackets denote one standard deviation. The phagocytosis index was determined by counting the number of D. discoideum cells with at least one internalized C. neoformans cell. For each experimental condition the number of repetitions was five. (B) Number of CFU of Cap67 after incubation with myoi null D. discoideum cells (▪), wild-type D. discoideum cells (♦), and alone (▴). Error bars denote one standard deviation. (C) Number of CFU of 3501 after incubation with myoi null D. discoideum cells (▪), wild-type D. discoideum cells (♦), and alone (▴). Error bars denote one standard deviation. (D) Phagocytosis of C. neoformans H99 cells by D. discoideum rtoA or wild-type cells. Wild-type D. discoideum cells phagocytosed significantly more fungal cells than rtoA null cells (P ≤ 0.001). Solid bars represent the numbers of phagocytic events by D. discoideum, and error brackets denote one standard deviation. For each experimental condition the number of repetitions was five. (E) C. neoformans H99 cell counts after incubation with either wild-type or rtoA D. discoideum cells. Bars represent numbers of CFU at different times; solid bars denote CFU at 0 h, open bars denote CFU at 24 h, and hatched bars denote CFU at 48 h. The error brackets represent one standard deviation. There are no significant differences between C. neoformans growth with wild-type and rtoA D. discoideum cells. These experiments were repeated on different days and yielded similar results.
A second mutant was used to evaluate the contribution of the amoeboid cell exocytosis phenotype to the interaction of C. neoformans and D. discoideum. Through homologous recombination, rtoA mutants were created which had normal endocytosis but reduced and poor exocytosis (5, 63). rtoA mutants are defective in endosomal vesicle fusion and the regulation of both endosomal and cytosolic pH (5, 63). Surprisingly, phagocytosis assays revealed that the rtoA D. discoideum cells phagocytosed fewer fungal cells than parental D. discoideum DH1 controls for C. neoformans (Fig. 5E) (P ≤ 0.001). This reduced phagocytosis, however, did not translate to reduced growth as determined by numbers of CFU (Fig. 5). The rtoA mutant supported growth as well as DH-1 control cells (P = 0.665).
Phagocytosis inhibition studies.
To understand the interaction between D. discoideum and C. neoformans, we evaluated the efficiency of phagocytosis using several potential inhibitors. Four sugars were investigated for their ability to inhibit phagocytosis: d-mannose, d-xylose, d-galactose, and mannitol. At sugar concentrations of 0.5 M or higher, there was a marked decrease in phagocytosis of C. neoformans by D. discoideum strain AX-4 with each compound (data not shown). Concentration of 1 M d-mannose inhibited C. neoformans phagocytosis by 90% (Fig. 6). Trypan blue exclusion assays ascertained that 1 M mannose was not cytotoxic to the D. discoideum cells (data not shown). In fact, D. discoideum cells with added sugars had a slightly higher percentage of live cells than did those incubated in PBS alone.
FIG. 6.
Inhibition of D. discoideum phagocytosis of C. neoformans 24067 cells by 1 M mannose and MAb 18B7. Solid bars represent the number of phagocytic events by D. discoideum, and error brackets denote one standard deviation. The phagocytosis index was determined by counting the number of D. discoideum cells with at least one internalized C. neoformans cell. For each experimental condition the number of repetitions was five.
The C. neoformans capsule is primarily composed of glucuronoxylomannan, and antibodies against this polysaccharide are opsonic. The IgG1 MAb 18B7 binds glucuronoxylomannan and enhances phagocytosis of C. neoformans cells by macrophages (45). Since it has been shown that the C. neoformans capsule is essential for fungal virulence and capsule-specific antibodies enhance phagocytosis by macrophages, we investigated the effect of 18B7 binding to yeast cells on D. discoideum phagocytosis (13), (36). Binding of 18B7 to the capsule of C. neoformans inhibited phagocytosis by D. discoideum (Fig. 5). In fact, 18B7 inhibited phagocytosis in a dose-dependent manner, since 1 μg of antibody/ml had no inhibitory affect while both 10 and 100 μg/ml of 18B7 significantly inhibited phagocytosis of C. neoformans by D. discoideum (P = 0.005 and P ≤ 0.001, respectively) (data not shown). In contrast, an isotype-matched control antibody that does not bind to the capsule had no effect on phagocytosis (P = 0.749).
Incubation of C. neoformans with D. discoideum increases virulence in mice.
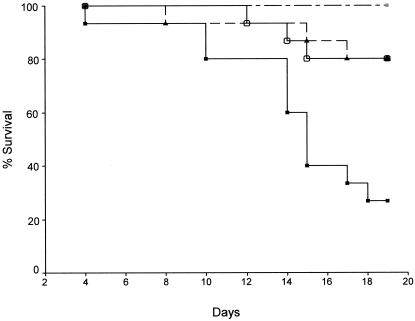
Mice infected with C. neoformans grown with live D. discoideum AX-4 had significantly shorter survival times than mice infected with C. neoformans grown alone or with killed D. discoideum (P ≤ 0.001) (Fig. 7). Mice infected with C. neoformans grown with killed D. discoideum lived as long as mice infected with C. neoformans grown in medium only. No deaths occurred for mice infected with D. discoideum alone.
FIG. 7.
Survival of A/J mice infected with either 107 C. neoformans cells grown with live D. discoideum (−▪−) (n = 15), 107 C. neoformans cells grown with killed D. discoideum (−○−) (n = 15), 107 C. neoformans cells (-▴-) (n = 15), or 107 live D. discoideum cells (-×-) (n = 5). The graph shows that C. neoformans 24067 primed by growth with live D. discoideum is more lethal than C. neoformans grown either alone or with killed D. discoideum (P ≤ 0.005 for both). Also, D. discoideum alone was not pathogenic. There were no significant differences in the survival of mice infected with C. neoformans alone and C. neoformans grown with killed D. discoideum (P = 0.974). This experiment was performed twice with similar results.
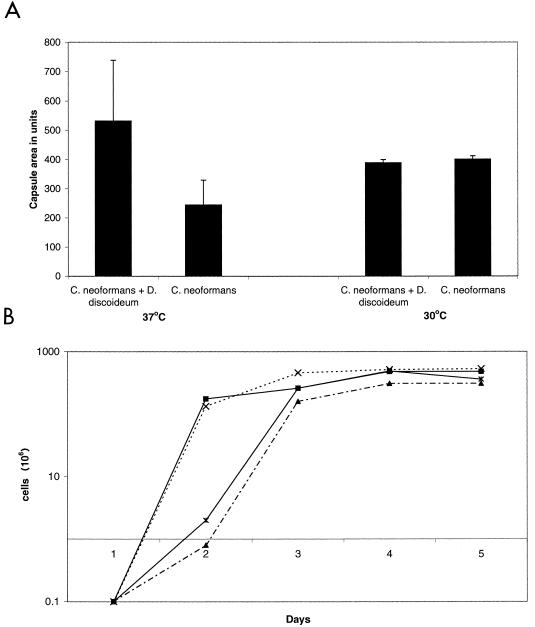
We analyzed D. discoideum-passaged and nonpassaged C. neoformans cells for growth rate, time to melanization, and capsule size to assess phenotypic differences that may be associated with alterations in virulence. The capsule size of fungal cells passaged with D. discoideum was larger than that of cells grown in medium at 37°C (P ≤ 0.001) (Fig. 8A). The average capsule size of the D. discoideum-passaged C. neoformans cells grown at 37°C was more than twice the size of the capsule from C. neoformans grown alone. However, cells grown at 30°C had no noticeable capsule size difference. There were no differences in the growth rates between C. neoformans cells passaged with or without D. discoideum (Fig. 8B). C. neoformans passaged through D. discoideum melanized more rapidly than the nonpassaged control at both 30 and 37°C. The difference in melanization became noticeable at day 4, with pronounced differences by day 5.
FIG. 8.
Phenotypic differences between D. discoideum passaged or unpassaged C. neoformans cells. (A) Capsule area of C. neoformans cells grown at 37 or 30°C after passage. Bars represent capsule area, and error brackets denote one standard deviation. At 37°C there is a difference between D. discoideum passaged C. neoformans cells and unpassaged cells (P ≤ 0.001). (B) Growth curves at 37 and 30°C of passaged or unpassaged C. neoformans cells. Growth curves are similar for passaged C. neoformans grown at 37°C (×) compared to unpassaged C. neoformans at 37°C (▪) and for passaged C. neoformans at 30°C (*) compared to unpassaged C. neoformans at 30°C (▴). This experiment was repeated with similar results.
DISCUSSION
A. castellanii was the first phagocytic organism to be identified as an alternative host for C. neoformans infection (52). Recent studies have extended the alternative host range for C. neoformans to the nematode C. elegans (46). Here we broaden the consortium of alternative host models for the study of C. neoformans virulence to include D. discoideum. The availability of D. discoideum as an alternative host is of particular significance because this organism can be easily maintained, and the genetics are well defined. Furthermore, D. discoideum is recognized as a model system for study of phagocytic processes and microbial pathogenesis (49). Many D. discoideum mutants are available with phenotypes ranging from defects in phagocytic processes to impaired motility. In this study we show that D. discoideum is suitable as a model host for C. neoformans infection and can affect cryptococcal virulence.
C. neoformans was readily ingested by D. discoideum, and the interaction between the fungal and amoeboid cells resulted in the death of the host cell and proliferation of the yeast. D. discoideum mutants in myosin and exocytosis were more susceptible to C. neoformans as illustrated by the fact that an acapsular mutant which is avirulent for wild-type D. discoideum replicated and killed D. discoideum mutants. Most interestingly, C. neoformans virulence was enhanced by passage through live D. discoideum culture. These results significantly extend prior findings made with A. castellanii to demonstrate that interaction of amoeboid cells with C. neoformans cells can alter fungal virulence.
Myosin VII is a member of a large family of proteins, but its specific function is not well understood. Earlier studies have associated myosin VII mutations with deafness in humans (37). Recent studies with D. discoideum suggest that it has important roles in adhesion and phagocytosis, specifically with particle adhesion and phagocytosis (56, 58). Myosin VII null cells, myoi cells, were reported to phagocytose particles at only 20% of the wild-type rate due to a decrease in particle binding (56, 58). In our experiments, the myoi mutant was less efficient in ingesting C. neoformans cells than the G1-21 wild-type D. discoideum control. However, the myoi mutant was also significantly more permissive to C. neoformans growth than wild-type D. discoideum, suggesting a role for myosin VII in amoeboid defense against live yeast. These results may seem paradoxical given that C. neoformans is a facultative intracellular parasite (24). The specific defect in the myoi null D. discoideum cells was determined to involve particle binding, cell-to-cell adhesion, and cell-to-substratum adhesion; however, these characterization assays employed either latex beads or heat-killed Saccharomyces cerevisiae in a nonadherent cell assay (58). Our live fungal cells could have exerted additional stresses on the D. discoideum cells that were not present in assays using killed yeast cells. Similar phagocytic differences were observed in experiments using L. pneumophila in which a myosin I double mutant was more permissive to bacterial growth only in adherent culture conditions (51).
rtoA is an important gene for exocytosis and phagosomal pH regulation for D. discoideum (5, 6, 63). Prior studies using both beads and nonpathogenic yeast cells in a nonadherent cell assay suggested that phagocytosis was not impaired in this mutant (5, 6). However, for C. neoformans, D. discoideum cells deficient in RtoA manifested significantly reduced phagocytosis. C. neoformans cells are encapsulated, and the interaction of amoeboid cells with this yeast may be significantly different than that with nonpathogenic yeasts. It was proposed that RtoA is required for lipid bilayer fusion through transient cell membrane association (5). Therefore, RtoA− D. discoideum mutants may be defective in phagocytosis in our adherent cell assay compared to the case in previous assays, which identified the RtoA mutational affect in a nonadherent amoeba cell assay. Hence, the interaction of C. neoformans with RtoA-deficient D. discoideum revealed an unexpected phenotype in the host cells. It is interesting that both parental control strains G1-21 and HTD-17 were less efficient at phagocytosing C. neoformans cells than the AX-4 wild-type D. discoideum strain used for all other amoeba experiments.
C. neoformans infection follows the inhalation of yeast cells into the lungs. This initial infection is generally contained by lung granulomas involving macrophages (1, 14, 28, 29). However, if the host becomes immunocompromised, asymptomatic infections can reactivate and cause cryptococcosis (20). Patients with underlying phagocyte deficiencies are well known to be more susceptible to disease resulting from C. neoformans infection (4, 40, 55). In parallel with the human experience, where immune deficiencies can enhance susceptibility, the myoi and rtoA D. discoideum mutants were also more susceptible to C. neoformans infection. Therefore, these experiments validate the concept that host defects increase susceptibility to pathogenic fungi for unicellular host-pathogen interactions and suggest that the interaction of C. neoformans with mutant D. discoideum is analogous to that observed for some immunocompromised states in mammals. Furthermore, identification of susceptibility genes in D. discoideum suggests that homologous genes in mammals have a similar function with regard to fungal pathogenesis.
Phagocytosis is mediated through cell surface receptor binding particles, which initiate a signal transduction cascade resulting in actin polymerization and particle internalization into a phagosome (32). This process of receptor-mediated phagocytosis is similar in Dictyostelium and macrophages (2, 3). To identify the mechanism by which D. discoideum interacted with C. neoformans for phagocytosis, we investigated the effect of specific sugars and antibodies on the efficiency of phagocytosis. The monoclonal antibody 18B7, specific for the C. neoformans polysaccharide capsule, inhibits C. neoformans phagocytosis by D. discoideum. Fc-like receptors are unlikely to be involved in particle internalization processes of D. discoideum, since these are vertebrate receptors that mediated their activity through tyrosine kinase activity, a process not required for D. discoideum phagocytosis (50). Furthermore, there is no reason to suppose that D. discoideum would have a receptor for ingesting antibody-coated C. neoformans, since immunoglobulins are products of multicellular animals. However, surface-expressed glycoproteins have been implicated as D. discoideum phagocytic receptors, and antibodies designed against oligosaccharides of these D. discoideum glycoproteins inhibited phagocytosis and cross-reacted with a range of membrane proteins (16, 17). The mechanism by which antibody binding to the capsule reduces D. discoideum phagocytosis is most likely due to blocking sites in the polysaccharide that are recognized by amoeba phagocytic receptors. Alternatively, antibody binding could cause a conformational change of the C. neoformans capsule, masking the C. neoformans residues that are recognized by D. discoideum phagocytic receptors (54).
Phagocytosis was also inhibited by the four carbohydrates tested, with d-mannose having the greatest effect, suggesting that carbohydrate receptors on the surface of D. discoideum may be involved in recognition and ingestion of yeast cells. These results would be more suggestive of a specific effect if one of the sugars did not affect phagocytosis of C. neoformans by D. discoideum. However, the interaction of D. discoideum with C. neoformans through a mannose-like receptor would imply that it is similar to the interaction of C. neoformans with macrophages and dendritic cells, where the mannose receptor is used to bind cryptococcal cells through the cryptococcal mannoprotein (39).
Passaging C. neoformans through D. discoideum cultures significantly increased virulence for mice, as evidenced by shorter survival times for mouse groups infected with these passaged cells. To our knowledge this is the first demonstration that the interaction of the fungus with a nonmammalian host can modify C. neoformans virulence. To investigate the mechanism for increased virulence, we analyzed three phenotypes that are associated with virulence: capsule size, melanin formation, and growth rate. The capsule is essential in virulence and has antiphagocytic as well as cytotoxic properties, while melanization protects against oxygen and nitrogen free radicals and microbicidal peptides (24, 33-36, 60, 61). Passaged C. neoformans cells expressed larger capsules and melanized faster than nonpassaged cells. Given recent studies linking capsule size to virulence (21) and the high likelihood that more-rapid melanization could have enhanced survival of yeast cells in mice, these observations suggest an explanation for the enhanced C. neoformans virulence through microevolution. Although the molecular and genetic mechanisms responsible for this phenomenon are not understood, it is clear that C. neoformans is capable of rapid phenotypic change. The ability of an organism to undergo microevolution is believed to be a vital characteristic for survival, allowing a pathogen to change its virulence and specificity (44). The passage experiments provide a powerful example of the capacity of this organism to undergo rapid change when confronted with certain selective pressures. In this regard, our findings suggest that the D. discoideum system could provide an excellent model for studying the effects of microevolution of C. neoformans virulence.
The capsule size increase was noted only in passaged cells grown at 37°C, which may be linked to the cAMP-PKA signal transduction pathway in C. neoformans, which has been identified as a regulatory pathway for capsule production, melanin formation, mating, and virulence (57). Similar changes in regulation could be responsible for the decrease in time to melanization observed for the passaged C. neoformans cells. Interestingly, passaging C. neoformans through D. discoideum did not alter the results of the amoeba killing assay. Unpassaged and passaged cells had nearly identical abilities to kill the amoebae and to grow in the presence of amoebae. This may be due to host specificity and possibly to capsule and melanin expression being already optimal for D. discoideum pathogenicity. Nevertheless, murine survival studies with the passaged C. neoformans cells illustrate a clear change in C. neoformans virulence after D. discoideum passage.
In summary, our results indicate that (i) D. discoideum can be used to study C. neoformans phagocytosis, (ii) C. neoformans virulence is enhanced after passage through D. discoideum, and (iii) enhancement of virulence is correlated with capsule size and melanization. The results with the D. discoideum mutants suggest new roles for the myosin VII and RtoA genes, illustrating the power of this system for study of host genetic factors that are important in antifungal defense. The findings in this study are consistent with and support the hypothesis that C. neoformans virulence for mammalian hosts is maintained in the environment by predatory microorganisms in soils.
Acknowledgments
We are grateful to R. Kessin for his valuable advice and help in establishing the D. discoideum system. We are thankful to R. Gomer and M. Titus for the use of their D. discoideum mutants and to the J. Segal laboratory for their initial D. discoideum instructions and reagents.
J.N.S. is supported by National institutes of Health Training Grant T32GM 07491. J.D.N. is supported by AI01489, and A.C. is supported by National Institutes of Health Awards AI33774, AI13342, and HL59842.
Editor: T. R. Kozel
REFERENCES
- 1.Abadi, J., and L. Pirofski. 1999. Antibodies reactive with the cryptococcal capsular polysaccharide glucuronoxylomannan are present in sera from children with and without human immunodeficiency virus infection. J. Infect. Dis. 180:915-919. [DOI] [PubMed] [Google Scholar]
- 2.Aizawa, H., Y. Fukui, and I. Yahara. 1997. Live dynamics of Dictyostelium cofilin suggests a role in remodeling actin latticework into bundles. J. Cell Sci. 110:2333-2344. [DOI] [PubMed] [Google Scholar]
- 3.Aizawa, H., M. Sameshima, and I. Yahara. 1997. A green fluorescent protein-actin fusion protein dominantly inhibits cytokinesis, cell spreading, and locomotion in Dictyostelium. Cell Struct. Funct. 22:335-345. [DOI] [PubMed] [Google Scholar]
- 4.Allen, D. M., and H. H. Chng. 1993. Disseminated Mycobacterium flavescens in a probable case of chronic granulomatous disease. J. Infect. 26:83-86. [DOI] [PubMed] [Google Scholar]
- 5.Brazill, D. T., D. R. Caprette, H. A. Myler, R. D. Hatton, R. R. Ammann, D. F. Lindsey, D. A. Brock, and R. H. Gomer. 2000. A protein containing a serine-rich domain with vesicle fusing properties mediates cell cycle-dependent cytosolic pH regulation. J. Biol. Chem. 275:19231-19240. [DOI] [PubMed] [Google Scholar]
- 6.Brazill, D. T., L. R. Meyer, R. D. Hatton, D. A. Brock, and R. H. Gomer. 2001. ABC transporters required for endocytosis and endosomal pH regulation in Dictyostelium. J. Cell Sci. 114:3923-3932. [DOI] [PubMed] [Google Scholar]
- 7.Brieland, J. K., J. C. Fantone, D. G. Remick, M. LeGendre, M. McClain, and N. C. Engleberg. 1997. The role of Legionella pneumophila-infected Hartmannella vermiformis as an infectious particle in a murine model of Legionnaire's disease. Infect. Immun. 65:5330-5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brummer, E., and D. A. Stevens. 1994. Anticryptococcal activity of macrophages: role of mouse strain, C5, contact, phagocytosis, and L-arginine. Cell Immunol. 157:1-10. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan, K. L., and J. W. Murphy. 1998. What makes Cryptococcus neoformans a pathogen? Emerg. Infect. Dis. 4:71-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buczynski, G., J. Bush, L. Zhang, J. Rodriguez-Paris, and J. Cardelli. 1997. Evidence for a recycling role for Rab7 in regulating a late step in endocytosis and in retention of lysosomal enzymes in Dictyostelium discoideum. Mol. Biol. Cell 8:1343-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulmer, G. S., M. D. Sans, and C. M. Gunn. 1967. Cryptococcus neoformans. I. Nonencapsulated mutants. J. Bacteriol. 94:1475-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casadevall, A., and L. Pirofski. 2001. Host-pathogen interactions: the attributes of virulence. J. Infect. Dis. 184:337-344. [DOI] [PubMed] [Google Scholar]
- 13.Chang, Y. C., and K. J. Kwon-Chung. 1994. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol. Cell. Biol. 14:4912-4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, L. C., D. L. Goldman, T. L. Doering, L. Pirofski, and A. Casadevall. 1999. Antibody response to Cryptococcus neoformans proteins in rodents and humans. Infect. Immun. 67:2218-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, S. C., L. C. Wright, R. T. Santangelo, M. Muller, V. R. Moran, P. W. Kuchel, and T. C. Sorrell. 1997. Identification of extracellular phospholipase B, lysophospholipase, and acyltransferase produced by Cryptococcus neoformans. Infect. Immun. 65:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chia, C. P. 1996. A 130-kDa plasma membrane glycoprotein involved in Dictyostelium phagocytosis. Exp. Cell. Res. 227:182-189. [DOI] [PubMed] [Google Scholar]
- 17.Chia, C. P., and E. J. Luna. 1989. Phagocytosis in Dictyostelium discoideum is inhibited by antibodies directed primarily against common carbohydrate epitopes of a major cell-surface plasma membrane glycoprotein. Exp. Cell Res. 181:11-26. [DOI] [PubMed] [Google Scholar]
- 18.Cox, G. M., H. C. McDade, S. C. Chen, S. C. Tucker, M. Gottfredsson, L. C. Wright, T. C. Sorrell, S. D. Leidich, A. Casadevall, M. A. Ghannoum, and J. R. Perfect. 2001. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol. Microbiol. 39:166-175. [DOI] [PubMed] [Google Scholar]
- 19.Cox, G. M., J. Mukherjee, G. T. Cole, A. Casadevall, and J. R. Perfect. 2000. Urease as a virulence factor in experimental cryptococcosis. Infect. Immun. 68:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dromer, F., O. Ronin, and B. Dupont. 1992. Isolation of Cryptococcus neoformans var. gattii from an Asian patient in France: evidence for dormant infection in healthy subjects. J. Med. Vet. Mycol. 30:395-397. [PubMed] [Google Scholar]
- 21.D'Souza, C. A., J. A. Alspaugh, C. Yue, T. Harashima, G. M. Cox, J. R. Perfect, and J. Heitman. 2001. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol. Cell. Biol. 21:3179-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eichinger, L., S. S. Lee, and M. Schleicher. 1999. Dictyostelium as model system for studies of the actin cytoskeleton by molecular genetics. Microsc. Res. Tech. 47:124-134. [DOI] [PubMed] [Google Scholar]
- 23.Ellis, D. H., and T. J. Pfeiffer. 1990. Ecology, life cycle, and infectious propagule of Cryptococcus neoformans. Lancet 336:923-925. [DOI] [PubMed] [Google Scholar]
- 24.Feldmesser, M., Y. Kress, P. Novikoff, and A. Casadevall. 2000. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 68:4225-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franzot, S. P., B. C. Fries, W. Cleare, and A. Casadevall. 1998. Genetic relationship between Cryptococcus neoformans var. neoformans strains of serotypes A and D.J. Clin. Microbiol. 36:2200-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franzot, S. P., J. Mukherjee, R. Cherniak, L. C. Chen, J. S. Hamdan, and A. Casadevall. 1998. Microevolution of a standard strain of Cryptococcus neoformans resulting in differences in virulence and other phenotypes. Infect. Immun. 66:89-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Hermoso, D., G. Janbon, and F. Dromer. 1999. Epidemiological evidence for dormant Cryptococcus neoformans infection. J. Clin. Microbiol. 37:3204-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldman, D. L., H. Khine, J. Abadi, D. J. Lindenberg, P. La, R. Niang, and A. Casadevall. 2001. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 107:E66. [DOI] [PubMed] [Google Scholar]
- 29.Goldman, D. L., H. Khine, J. Abadi, D. J. Lindenberg, L. Pirofski, R. Niang, and A. Casadevall. 2001. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 107:E66. [DOI] [PubMed] [Google Scholar]
- 30.Goldman, D. L., S. C. Lee, A. J. Mednick, L. Montella, and A. Casadevall. 2000. Persistent Cryptococcus neoformans pulmonary infection in the rat is associated with intracellular parasitism, decreased inducible nitric oxide synthase expression, and altered antibody responsiveness to cryptococcal polysaccharide. Infect. Immun. 68:832-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomer, R. H., I. S. Yuen, and R. A. Firtel. 1991. A secreted 80 × 10(3) Mr protein mediates sensing of cell density and the onset of development in Dictyostelium. Development 112:269-278. [DOI] [PubMed] [Google Scholar]
- 32.Greenberg, S., and S. Grinstein. 2002. Phagocytosis and innate immunity. Curr. Opin. Immunol. 14:136-145. [DOI] [PubMed] [Google Scholar]
- 33.Jacobson, E. S., and H. S. Emery. 1991. Catecholamine uptake, melanization, and oxygen toxicity in Cryptococcus neoformans. J. Bacteriol. 173:401-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobson, E. S., and S. B. Tinnell. 1993. Antioxidant function of fungal melanin. J. Bacteriol. 175:7102-7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozel, T. R., and E. C. Gotschlich. 1982. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J. Immunol. 129:1675-1680. [PubMed] [Google Scholar]
- 36.Kozel, T. R., and T. G. McGaw. 1979. Opsonization of Cryptococcus neoformans by human immunoglobulin G: role of immunoglobulin G in phagocytosis by macrophages. Infect. Immun. 25:255-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Libby, R. T., and K. P. Steel. 2000. The roles of unconventional myosins in hearing and deafness. Essays Biochem. 35:159-174. [DOI] [PubMed] [Google Scholar]
- 38.Maniak, M., R. Rauchenberger, R. Albrecht, J. Murphy, and G. Gerisch. 1995. Coronin involved in phagocytosis: dynamics of particle-induced relocalization visualized by a green fluorescent protein tag. Cell 83:915-924. [DOI] [PubMed] [Google Scholar]
- 39.Mansour, M. K., L. S. Schlesinger, and S. M. Levitz. 2002. Optimal T cell responses to Cryptococcus neoformans mannoprotein are dependent on recognition of conjugated carbohydrates by mannose receptors. J. Immunol. 168:2872-2879. [DOI] [PubMed] [Google Scholar]
- 40.Marques, S. A., A. M. Robles, A. M. Tortorano, M. A. Tuculet, R. Negroni, and R. P. Mendes. 2000. Mycoses associated with AIDS in the Third World. Med. Mycol. 38:269-279. [PubMed] [Google Scholar]
- 41.McFadden, D. C., and A. Casadevall. 2001. Capsule and melanin synthesis in Cryptococcus neoformans. Med. Mycol. 39:19-30. [PubMed] [Google Scholar]
- 42.Mitchell, T. G., and J. R. Perfect. 1995. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin. Microbiol. Rev. 8:515-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moffat, J. F., and L. S. Tompkins. 1992. A quantitative model of intracellular growth of Legionella pneumophila in Acanthamoeba castellanii. Infect. Immun. 60:296-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morschhauser, J., G. Kohler, W. Ziebuhr, G. Blum-Oehler, U. Dobrindt, and J. Hacker. 2000. Evolution of microbial pathogens. Philos. Trans. R Soc. Lond. B Biol. Sci. 355:695-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukherjee, J., A. Casadevall, and M. D. Scharff. 1993. Molecular characterization of the humoral responses to Cryptococcus neoformans infection and glucuronoxylomannan-tetanus toxoid conjugate immunization. J. Exp. Med. 177:1105-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mylonakis, E., F. M. Ausubel, J. R. Perfect, J. Heitman, and S. B. Calderwood. 2002. Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc. Natl. Acad. Sci. USA 99:15675-15680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhodes, J. C., and D. H. Howard. 1980. Isolation and characterization of arginine auxotrophs of Cryptococcus neoformans. Infect Immun. 27:910-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rothstein, T. L., and M. L. Gefter. 1983. Affinity analysis of idiotype-positive and idiotype-negative Ars-binding hybridoma proteins and Ars-immune sera. Mol. Immunol. 20:161-168. [DOI] [PubMed] [Google Scholar]
- 49.Saxe, C. L. 1999. Learning from the slime mold: Dictyostelium and human disease. Am. J. Hum. Genet. 65:25-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seastone, D. J., L. Zhang, G. Buczynski, P. Rebstein, G. Weeks, G. Spiegelman, and J. Cardelli. 1999. The small Mr Ras-like GTPase Rap1 and the phospholipase C pathway act to regulate phagocytosis in Dictyostelium discoideum. Mol. Biol. Cell 10:393-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solomon, J. M., A. Rupper, J. A. Cardelli, and R. R. Isberg. 2000. Intracellular growth of Legionella pneumophila in Dictyostelium discoideum, a system for genetic analysis of host-pathogen interactions. Infect. Immun. 68:2939-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steenbergen, J. N., H. A. Shuman, and A. Casadevall. 2001. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl. Acad. Sci. USA 98:15245-15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sussman, M. 1987. Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions. Methods Cell Biol. 28:9-29. [DOI] [PubMed] [Google Scholar]
- 54.Taborda, C. P., and A. Casadevall. 2002. CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement-independent antibody-mediated phagocytosis of Cryptococcus neoformans. Immunity 16:791-802. [DOI] [PubMed] [Google Scholar]
- 55.Temeck, B. K., D. J. Venzon, C. A. Moskaluk, and H. I. Pass. 1994. Thoracotomy for pulmonary mycoses in non-HIV-immunosuppressed patients. Ann. Thorac. Surg. 58:333-338. [DOI] [PubMed] [Google Scholar]
- 56.Titus, M. A. 1999. A class VII unconventional myosin is required for phagocytosis. Curr. Biol. 9:1297-1303. [DOI] [PubMed] [Google Scholar]
- 57.Tolkacheva, T., P. McNamara, E. Piekarz, and W. Courchesne. 1994. Cloning of a Cryptococcus neoformans gene, GPA1, encoding a G-protein alpha-subunit homolog. Infect. Immun. 62:2849-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tuxworth, R. I., I. Weber, D. Wessels, G. C. Addicks, D. R. Soll, G. Gerisch, and M. A. Titus. 2001. A role for myosin VII in dynamic cell adhesion. Curr. Biol. 11:318-329. [DOI] [PubMed] [Google Scholar]
- 59.Wang, Y., P. Aisen, and A. Casadevall. 1995. Cryptococcus neoformans melanin and virulence: mechanism of action. Infect. Immun. 63:3131-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, Y., and A. Casadevall. 1994. Decreased susceptibility of melanized Cryptococcus neoformans to UV light. Appl. Environ. Microbiol. 60:3864-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang, Y., and A. Casadevall. 1994. Susceptibility of melanized and nonmelanized Cryptococcus neoformans to nitrogen- and oxygen-derived oxidants. Infect. Immun. 62:3004-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williamson, P. R., K. Wakamatsu, and S. Ito. 1998. Melanin biosynthesis in Cryptococcus neoformans. J. Bacteriol. 180:1570-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wood, S. A., R. R. Ammann, D. A. Brock, L. Li, T. Spann, and R. H. Gomer. 1996. RtoA links initial cell type choice to the cell cycle in Dictyostelium. Development 122:3677-3685. [DOI] [PubMed] [Google Scholar]