Abstract
The variable efficacy of bacillus Calmette-Guérin (Mycobacterium bovis BCG) in protecting humans and cattle against tuberculosis has prompted a search for a more effective vaccination regimen. A prime-boost strategy was investigated in cattle naturally sensitized to environmental mycobacteria by using a combination of three DNA vaccines coding for Hsp 65, Hsp 70, and Apa for priming, followed by a boost with BCG prior to experimental challenge with virulent M. bovis. Controls were vaccinated with DNA or BCG alone or were not vaccinated. The immune responses were monitored throughout the study, and protection was assessed based on reductions in the numbers of lesions and viable mycobacteria in lymph node samples. Vaccination with BCG alone or with a DNA prime-BCG boost regimen induced high levels of antigen-specific gamma interferon (IFN-γ) in whole-blood cultures. In the prime-boost group there were fewer animals with severe lung lesions, fewer lymph nodes with lesions per animal, a smaller proportion of animals with lesions, lower mean lung and lymph node lesion scores, and less M. bovis isolated from retropharyngeal and thoracic lymph nodes compared to the results obtained for the nonvaccinated animals. The prime-boost regimen induced significant enhancement of protection in six parameters, compared with significant enhancement of protection in only two parameters for BCG alone. In addition, following challenge, in vitro IFN-γ responses against ESAT-6 and CFP-10, as well as bovine tuberculin-induced skin test and in vitro IFN-γ responses, were identified as immunological markers that predicted protection. The use of the prime-boost strategy suggested that a combination of vaccines may be better than a single vaccine for protection against tuberculosis.
Human tuberculosis is a major health problem worldwide and is responsible for an estimated 1.9 million deaths annually (12). It is predominantly caused by Mycobacterium tuberculosis. Bovine tuberculosis, caused by Mycobacterium bovis, is a major cause of economic loss in countries where it is endemic, and in some countries it may be a significant zoonotic disease problem (11). M. tuberculosis and M. bovis are closely related genetically, and vaccine development programs for both cattle tuberculosis and human tuberculosis promise to be mutually beneficial (16). Although effective chemotherapy for human tuberculosis is available, the treatment is lengthy and relatively expensive, so that widespread control of the disease is often difficult to achieve in developing countries. A better option for disease control is effective prophylactic vaccination against tuberculosis. Bovine tuberculosis in farmed animals has been eradicated in many countries by a test and slaughter program. Vaccination of cattle is an option for the control of bovine tuberculosis in countries that have wildlife reservoirs of M. bovis infection and in developing countries where a test and slaughter program is not economically viable. Bacillus Calmette-Guérin (BCG), an attenuated strain of M. bovis, has been widely used for vaccination against human tuberculosis despite controversy over its protective efficacy (14). One of the most convincing explanations for the variability in the protective efficacy of BCG in human trials is that environmental mycobacterial infections prevent or mask BCG-induced protective immunity (1, 14). Evidence from a recent cattle trial suggested that sensitization of calves to environmental mycobacteria adversely affected the subsequent protective efficacy of BCG (5), and similar conclusions have been drawn by using a mouse model of tuberculosis (3). It is therefore important to develop vaccination strategies which are effective when animals have been presensitized to environmental mycobacteria.
Thus, although BCG can stimulate gamma interferon (IFN-γ) responses and long-term memory, it may not have the capacity in some situations to stimulate a fully protective immune response. A number of promising immunogenic proteins are being evaluated as alternatives to BCG (2, 22), and recombinant proteins, such as those of the Ag85 complex and ESAT-6 (4), have given limited protection. One of the problems associated with using subunit vaccines is the lack of an appropriate adjuvant to enhance the cellular immune responses necessary for protection against tuberculosis. DNA vaccines expressing mycobacterial proteins that are key targets of mycobacterial protective immunity provide both immunogenic and adjuvant components. Protection against tuberculosis has been conferred by DNA vaccines (8, 13, 15, 17), but it has been difficult to find a vaccine that performs better than BCG by using small animal models.
Recent studies have shown that DNA vaccines are effective for priming immune responses. A prime-boost strategy with DNA vaccines and attenuated viral vectors expressing similar antigens has been successful in stimulating a protective cell-mediated immune response against viral infection (21). Strategies involving priming with one type of vaccine and boosting with another type have had some degree of success against experimental infections of M. tuberculosis in mice (26). Priming with a DNA vaccine expressing ESAT-6 and MPT63 and boosting with recombinant modified vaccinia virus also expressing these proteins gave protection as good as that provided by BCG (19). Priming with an Ag85B DNA vaccine and boosting with the BCG-Tokyo strain gave better protection than BCG-Tokyo alone gave (13), while boosting with BCG-Pasteur did not give better protection than BCG-Pasteur alone gave (24). Prime-boost vaccination strategies may be useful for enhancing protection against M. tuberculosis infections in humans and M. bovis infections in cattle, particularly in situations where BCG is not functioning optimally. In this study the efficacy of a prime-boost strategy in which DNA encoding Hsp 65, Hsp 70, and Apa was used to prime and BCG was used to boost was evaluated for protection against an experimental challenge with M. bovis in cattle naturally presensitized to environmental mycobacteria.
MATERIALS AND METHODS
Animals.
Forty-eight female calves (Friesian or Friesian cross) that were 5 to 6 months old were obtained from a tuberculosis-free accredited herd from an area of New Zealand free of bovine tuberculosis. When the calves first arrived at the isolation unit, 2 weeks prior to vaccination, the majority responded to purified protein derivative from Mycobacterium avium (avian PPD) in the IFN-γ test. The calves were placed in four groups by using a randomized stratified sampling system so that all groups contained animals with a similar distribution of responses to avian PPD in the IFN-γ test. During the trial, the calves were kept in a high-security isolation unit, where they grazed on pasture. All procedures performed on the calves had the approval of the Wallaceville Animal Research Centre Animal Ethics Committee (Upper Hutt, New Zealand).
DNA vaccines.
Plasmids pCMV4.65 and pCMV4.70, encoding mycobacterial antigens Hsp 65 and Hsp 70, respectively, were constructed as described previously (18). Plasmid pCMV4.apa was produced by PCR amplification of the coding sequence for the mycobacterial antigen Apa (Rv 1860), including the N-terminal signal peptide, from M. tuberculosis H37Rv genomic DNA with primers 5′-ATTGGATCCGCCATGCATCAGGTGGAC-3′ (forward primer) and 5′-TATGCGGCCGCCTCAGGCCGGTAAG-3′ (reverse primer). The amplified product was cloned into the BamHI and NotI sites of pCMV4 to produce pCMV4.apa. pCMV4 was based on plasmid pCDNA3.1 (Invitrogen, Carlsbad, Calif.), with addition of intron A from the human cytomegalovirus immediate-early gene cloned into the HindIII site (9). Protein expression from pCMV4.apa was confirmed by transfection into CV1 cells and Western blotting with an Apa-specific monoclonal antibody. The DNA plasmids were prepared for vaccination by using a QIAGEN-tip 10000 plasmid extraction kit and endotoxin-free buffers (QIAGEN Ltd., Dorking, United Kingdom), adjusted to a final concentration of 1 mg/ml in phosphate-buffered saline (PBS), and stored at −20°C prior to injection.
Bacteria.
M. bovis BCG Pasteur 1173P2 was used as the vaccine strain. The M. bovis challenge strain, 83/6235, was originally isolated from a tuberculous lesion of a brushtail possum (Trichosurus vulpecula) and has been used in previous cattle studies (6, 7). For cattle inoculation, the vaccine and challenge strains were grown to the mid-log phase in Tween-albumin broth, and the number of bacteria was estimated by the degree of turbidity. Dilutions for inoculating the cattle were made in Tween-albumin broth, and the number of CFU inoculated was determined as described previously (6).
Vaccination of cattle and challenge with M. bovis.
The cattle were divided into four groups, each containing 12 animals. One group of animals was not vaccinated (nonvaccinated group). The trial began at zero time with DNA vaccination of two of the groups (referred to below as the onset of vaccination). One group was vaccinated twice, at zero time and 3 weeks, with DNA (DNA group); another group was vaccinated twice, at zero time and 3 weeks, with DNA and then boosted with 1 × 106 CFU of BCG at 6 weeks (DNA/BCG group); and the final group received 1 × 106 CFU of BCG at 6 weeks (BCG group). For the initial DNA vaccination each animal received 1 mg of each plasmid in 1 ml, 0.1 mg intradermally and 0.9 mg intramuscularly. The second DNA vaccination was given in the same way, but only a total of 0.5 mg was inoculated. BCG was given subcutaneously. Thirteen weeks (week 13) after the trial commenced, the animals were challenged intratracheally with 1.5 × 103 CFU of M. bovis strain 83/6235 as previously described (6).
Tuberculin skin test.
The animals were inoculated intradermally with a 0.1-ml volume containing 0.1 mg of purified protein derivative from M. bovis (bovine PPD) (AgriQuality, Upper Hutt, New Zealand) in the midlateral region of the neck approximately 15 cm below the ear. The cattle were tested before challenge at 10 weeks after the onset of vaccination, when one-half the animals in each group were tested, and at 28 weeks (15 weeks after challenge), when all of the animals were tested. The test results were determined from skin thickness measurements taken at the time of inoculation and 72 h later.
Cytokine assays.
IFN-γ and interleukin-2 (IL-2) levels were measured in supernatants from whole-blood cultures stimulated with mycobacterial antigens. Heparinized blood was dispersed in three 1.5-ml aliquots, and 100-μl portions of PBS, preservative-free avian PPD (300 μg/ml; CSL Ltd., Parkville, Victoria, Australia), bovine PPD (300 μg/ml; CSL Ltd.), or a cocktail of 10 synthetic peptides derived from ESAT-6 and CFP-10 (final concentration, 4 μg/ml) (27) were added to the blood in separate wells. The blood cultures were incubated for 24 h at 37°C in a humidified atmosphere consisting of 5% CO2 in air, after which plasma supernatants were harvested from each well and stored at −20°C before they were assayed. The plasma supernatants were assayed for bovine IFN-γ by using a sandwich enzyme-linked immunosorbent assay described by Rothel et al. (23) and supplied by CSL Ltd. as a BOVIGAM EIA kit. Recombinant cattle IFN-γ (31) was used to generate standard curves, and the results were expressed in nanograms per milliliter.
The biological activity of IL-2 in plasma supernatants was determined by using the IL-2 bioassay (6). Briefly, triplicate wells containing 104 concanavalin A (Sigma Chemical Co., St. Louis, Mo.)-stimulated 4-day lymphoblasts were incubated in 200 μl of RPMI 1640 (Gibco, Grand Island, N.Y.) supplemented with 5% heat-inactivated fetal calf serum (Gibco), 2 mM l-glutamine, 50 μM 2-mercaptoethanol, 2.9 mM sodium bicarbonate, 100 μg of sodium pyruvate per ml, 1 mM nonessential amino acids (Sigma), 100 U of penicillin per ml, and 100 μg of streptomycin per ml. A 1:10 dilution of supernatant from whole-blood cultures was added to the cultures, and the cultures were incubated for 24 h before 0.25 μCi of [3H]thymidine was added to each well. The cultures were harvested 18 h later with an automatic cell harvester, and [3H]thymidine incorporation was determined with a liquid β-scintillation counter (Micro Beta, Wallac, Finland). The results were expressed as a stimulation index (SI), determined as follows: SI = (mean counts per minute for bovine PPD or avian PPD sample)/(mean counts per minute for PBS sample). Addition of a monoclonal antibody against bovine IL-2 to the concanavalin A-stimulated lymphoblasts immediately before addition of the plasma supernatants has been shown to block IL-2 bioactivity.
IFN-γ producing cells.
The ELISPOT assay was used to quantify the numbers of IFN-γ-producing cells in all animals 10 weeks after the onset of vaccination. The method followed was the method used to quantify mouse IFN-γ-producing cells, with minor modifications (24). Briefly, MAIP S45 plates (Millipore, Bedford, Mass.) were coated overnight with mouse anti-bovine IFN-γ capture antibody (BioSource Europe S.A.) at a concentration of 2 μg/ml. They were then washed with medium (RPMI 1640 containing 25 mM HEPES [Gibco]), blocked with fetal calf serum, and rewashed with medium supplemented with 50 μM 2-mercaptoethanol, 0.1 mM nonessential amino acids (100×; Gibco), 100 U of penicillin (Sigma) per ml, 100 mg of streptomycin (Gibco) per ml, 2 mM l-glutamine, and 5% CPSR-1 (Sigma) (complete medium). Peripheral blood mononuclear cells were purified from heparinized whole blood by density centrifugation with Lymphoprep 1.077 (Axis-Shield, Oslo, Norway). They were washed in PBS and then in complete medium and plated in complete medium at a concentration of 2 × 105 cells per well together with avian PPD or bovine PPD (CSL Ltd.) at a concentration of 60 μg/ml, Hsp 65 or Hsp 70 (Lionex Ltd., Braunschweig, Germany) at a concentration of 10 μg/ml, concanavalin A at a concentration of 5 μg/ml, or PBS. After 24 h the plates were washed with deionized H2O and PBS containing 0.05% Tween 20. A polyclonal rabbit anti-bovine IFN-γ-detecting antibody was added, and the plates were incubated for 1 h at room temperature, washed with PBS containing 0.05% Tween 20, and incubated for 1 h at room temperature with anti-rabbit immunoglobulin G-alkaline phosphatase conjugate (Sigma). The plates were washed again, and spots were developed in the dark at room temperature with 5-bromo-4-chloro-3-indolylphosphate (BCIP)-nitroblue tetrzolium substrate (Sigma). The plates were washed with copious amounts of tap water and air dried, and the spots were counted with an automated AID ELISPOT reader (Autoimmun Diagnostika GmbH, Stassberg, Germany).
Necropsy procedure and bacterial isolation.
All cattle were subjected to extensive postmortem examination 16 weeks after challenge. The lungs were initially palpated for detection of any nodules and then sliced with a knife (10-mm-thick slices) to aid in visualization of any lesions. A lung lesion severity score was determined by using the following scale: 0, no lesions; 1, 1 to 9 lesions; 2, 10 to 29 lesions; 3, 30 to 99 lesions; 4, 100 to 199 lesions; 5, ≥200 lesions. Twenty-five lymph nodes from each animal were removed and inspected after they were finely sliced as described in the detailed necropsy procedure reported by Corner et al. (10). A lesion severity score was determined for the most severely affected lymph node from each animal by using the following scale: 0, no lesions; 1, 1 to 5 small lesions (diameter, 1 to 4 mm); 2, 5 to 19 small lesions; 3, ≥20 small lesions; 4, medium-size lesion (diameter, 5 to 9 mm); 5, large lesion (diameter, ≥10 mm). Samples from the left and right bronchial, anterior and posterior mediastinal, and left and right retropharyngeal lymph nodes were collected from all animals for histopathological examination and bacterial culture. Additional samples were collected from any lesions observed in other lymph nodes or organs. Sections were stained by using the hematoxylin-eosin and Ziehl-Neelsen methods and were examined microscopically. Bacterial culture from the lungs was carried out only for lung samples containing a tuberculous lesion as M. bovis is rarely found in bovine lung tissue not associated with lesions, particularly at the stage of experimental infection used in this study (de Lisle, unpublished observation).
Statistical analyses.
Results of the IL-2 and IFN-γ assays were log10 transformed to achieve homogeneity of variance and were analyzed by analysis of variance by using Fisher's individual error rate. Comparisons of skin test responses, numbers of lymph nodes with lesions, and lesion severity scores were also made by using analysis of variance and Fisher's individual error rate. A comparison of the proportions of animals with macroscopic tuberculous lesions in the different vaccine groups was carried out by using Fisher's exact test.
RESULTS
Responses to avian PPD prior to vaccination.
In order to test for preexposure to environmental mycobacteria and to assign calves to one of the four vaccine groups, they were bled, and whole-blood cultures were stimulated with avian PPD 2 weeks prior to the onset of vaccination. The results are shown in Table 1 together with the responses to bovine PPD at that time for comparison. The mean IFN-γ response to avian PPD was more than twice the mean response to bovine PPD, suggesting that the herd had been exposed to environmental mycobacteria. Eight of the 12 animals in the nonvaccinated group, 8 of the 12 animals in the DNA group, 9 of the 12 animals in the DNA/BCG group, and 9 of the 12 animals in the BCG group had IFN-γ responses to avian PPD of ≥0.5 ng per ml.
TABLE 1.
Prevaccination IFN-γ responses to avian PPD
| Group | IFN-γ response 2 weeks prior to the onset of vaccination (ng of IFN-γ/ml)a
|
|
|---|---|---|
| Avian PPD | Bovine PPD | |
| Nonvaccinated | 1.10 ± 0.25 | 0.40 ± 0.12 |
| DNA | 1.09 ± 0.24 | 0.45 ± 0.16 |
| DNA/BCG | 1.14 ± 0.26 | 0.47 ± 0.11 |
| BCG | 1.12 ± 0.24 | 0.48 ± 0.10 |
Amount of IFN-γ in whole-blood cultures from 12 calves per group. The values are means ± standard errors of the means.
T-cell responses after vaccination and challenge.
In order to determine whether there was any association between IFN-γ or IL-2 responses and protection against experimental infection with M. bovis, IFN-γ and IL-2 cytokine responses were measured at regular intervals during the vaccine trial. Differences between the vaccine groups were observed in the IFN-γ (Fig. 1A) and IL-2 responses (Fig. 1B) to bovine PPD. The BCG and DNA/BCG groups exhibited significantly higher mean peripheral blood IFN-γ responses 8 to 10 weeks after the onset of vaccination than the DNA and nonvaccinated groups exhibited (P < 0.01). DNA vaccination was nevertheless effective at stimulating an immune response, since compared to nonvaccinated animals, there was a significant increase in the frequency of bovine PPD-specific IFN-γ-secreting cells, as measured by the ELISPOT assay, prior to challenge (week 10) (P < 0.05). There was also a small but not significant increase in IFN-γ-producing cells specific for Hsp 65 and Hsp 70 (Table 2). There was minimal correlation between the mean numbers of IFN-γ-producing cells for bovine PPD for the different groups and the mean levels of soluble IFN-γ released from bovine PPD-stimulated whole-blood cultures.
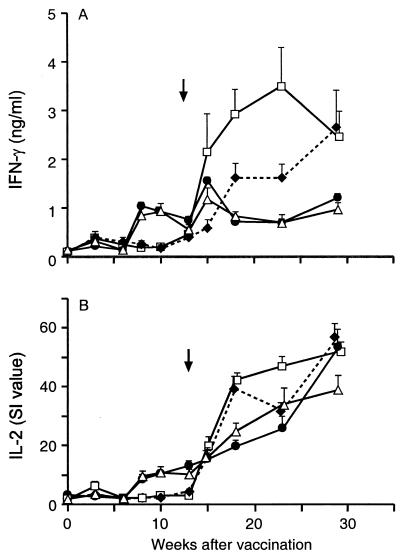
FIG. 1.
IFN-γ and IL-2 responses after vaccination and challenge. Plasma from whole-blood cultures of nonvaccinated animals (⧫) or animals vaccinated with DNA alone (□), with DNA and BCG (▵), or with BCG alone (•) were stimulated in vitro with bovine PPD and assayed for IFN-γ (A) or IL-2 (B). The error bars indicate standard errors of the means. The arrow indicates the time of challenge.
TABLE 2.
Numbers of IFN-γ-producing cells 10 weeks after the onset of vaccination (10 weeks after the initial DNA vaccination and 4 weeks after BCG vaccination)
| Group | Log10 no. of IFN-γ spot-forming cells/106 PBMCa
|
|||
|---|---|---|---|---|
| Avian PPD | Bovine PPD | Hsp 65 | Hsp 70 | |
| Nonvaccinated | 1.98 ± 0.15 | 1.62 ± 0.21 | 1.65 ± 0.15 | 1.47 ± 0.19 |
| DNA | 2.15 ± 0.16 | 2.06 ± 0.12b | 1.76 ± 0.17 | 1.94 ± 0.16 |
| DNA/BCG | 2.05 ± 0.13 | 1.97 ± 0.14 | 1.32 ± 0.19 | 1.36 ± 0.21 |
| BCG | 1.91 ± 0.15 | 1.69 ± 0.12 | 1.27 ± 0.08 | 1.25 ± 0.09 |
Log10 number of IFN-γ spot-forming cells per 106 peripheral blood mononuclear cells (PBMC). The values are means ± standard errors of the means.
The value is significantly different from the value for the nonvaccinated group (P < 0.05).
At week 15, 2 weeks after challenge, all the vaccinated groups exhibited significantly higher bovine PPD-induced IFN-γ responses than the nonvaccinated group exhibited (P < 0.01). The BCG group had a slightly higher IFN-γ response at this time than the DNA/BCG group, but the difference was not significant (P = 0.058). By week 18 (5 weeks postchallenge) the IFN-γ response was lower in the groups which received BCG than in the other two groups (P < 0.01), and this difference was maintained until the animals were killed. The pattern of IL-2 responses was broadly similar. At 8 to 10 weeks after the onset of vaccination, the mean responses for the BCG and DNA/BCG groups were higher than the mean responses for the DNA and nonvaccinated groups (P < 0.01). At week 15 (2 weeks postchallenge) there were no significant differences between the groups, but by week 18 (5 weeks postchallenge), the DNA and nonvaccinated groups exhibited greater responses that the other two groups exhibited (P < 0.01).
Pathological and microbiological findings.
The DNA/BCG group exhibited significant reductions in five parameters associated with pathology compared to the data for the nonvaccinated group, whereas there were only two reductions for the BCG group and no reductions for the DNA group (Table 3). There were no significant differences between the DNA/BCG group and the BCG group. The proportion of animals with macroscopic tuberculous lung lesions and the proportion of animals with lymph node lesions in the DNA/BCG group were significantly less than the proportions in the nonvaccinated group (P < 0.05 and P < 0.01, respectively); the proportion in the BCG group was significantly less for lymph node lesions (P < 0.05) but not for lung lesions. The mean lymph node severity scores of the DNA/BCG and BCG groups were significantly lower than the score of the nonvaccinated group (P < 0.001 and P < 0.01, respectively), but the mean lung severity score was significantly different only for the DNA/BCG group (P < 0.01). The mean number of lymph nodes with lesions per animal was significantly lower than the value for the nonvaccinated group only for the DNA/BCG group (P < 0.05). There were significantly fewer animals with severe tuberculous pneumonia (>25 lung lesions) in the DNA/BCG group than in the nonvaccinated group (P < 0.05), while the differences were not significant for the other two vaccinated groups (data not shown). The lymph node lesions in animals belonging to the different groups varied from 1 to 25 mm in diameter and had yellow calcified caseous centers, while the lung lesions consisted of a series of small nodules that were predominantly 2 to 5 mm in diameter and had yellow caseous centers. All tuberculous lesions were restricted to the thoracic cavity except in five animals (two animals that were not vaccinated, one animal that was vaccinated with DNA, and two animals that were vaccinated with DNA and BCG) in which lesions were also found in the submandibular, parotid, or retropharyngeal lymph nodes.
TABLE 3.
Pathological and microbiological parameters of protection against experimental infection with M. bovis
| Group | No. of animals with lung lesions/ total no. | No. of animals with lymph node lesions/ total no. | Mean lung scorea | Mean lymph node scoreb | Mean no. of lymph nodes with lesions per animal | No. of M. bovis culture-positive animals/ total no. | No. of retropharyn- geal and thoracic lymph nodes M. bovis positive/ total no. | Mean log10 CFU of M. bovis for retropharyngeal and thoracic lymph nodes |
|---|---|---|---|---|---|---|---|---|
| Nonvaccinated | 9/12 | 12/12 | 2.67 ± 0.56 | 3.75 ± 0.33 | 2.42 ± 0.42 | 12/12 | 32/72 | 1.89 ± 0.43 |
| DNA | 8/12 | 12/12 | 2.92 ± 0.67 | 4.00 ± 0.33 | 2.58 ± 0.29 | 12/12 | 35/72 | 2.03 ± 0.44 |
| DNA/BCG | 3/12c | 5/12d | 0.42 ± 0.26d | 1.08 ± 0.43e | 1.08 ± 0.49c | 9/12 | 22/72 | 1.36 ± 0.34d |
| BCG | 5/12 | 7/12c | 1.33 ± 0.54 | 1.92 ± 0.56d | 1.33 ± 0.41 | 10/12 | 21/72 | 1.51 ± 0.42 |
Lung lesion scores: 0, no lesions; 1, 1 to 9 lesions; 2, 10 to 29 lesions; 3, 30 to 99 lesions; 4, 100 to 199 lesions; 5, ≥200 lesions.
Lymph node lesion score of the most severely affected node for each animal based on the following scale: 0, no lesions; 1, 1 to 5 small lesions (diameter, 1 to 4 mm); 2, 5 to 19 small lesions; 3, ≥20 small lesions; 4, medium-size lesion (diameter, 5 to 9 mm); 5, large lesion (diameter, ≥10 mm).
Significantly different from the value for the nonvaccinated group (P < 0.05).
Significantly different from the value for the nonvaccinated group (P < 0.01).
Significantly different from the value for the nonvaccinated group (P < 0.001).
Histologically, the tuberculous granulomata in the lungs and lymph nodes had a central necrotic area which was usually mineralized. The necrotic areas were surrounded by epithelioid macrophages and lymphocytes, and the lesions were walled off by fibrosis. The lymph node lesions in animals vaccinated with BCG or with DNA and BCG generally had less necrosis and mineralization, were smaller than the lesions in animals belonging to the other two groups, and appeared to have been established more recently. There were no gross or histopathological differences between the DNA and nonvaccinated groups.
When the microbiological results were compared, all animals in the nonvaccinated and DNA groups were M. bovis culture positive, whereas 10 of the 12 animals in the BCG group and 9 of the 12 animals in the DNA/BCG group were positive (Table 3). The mean number of M. bovis CFU cultured from the retropharyngeal and thoracic lymph nodes was significantly lower than the mean number for the nonvaccinated group only for the DNA/BCG group (P < 0.05). The difference between the DNA/BCG group and the BCG group was not significant.
Skin test and IFN-γ responses as indicators of protection.
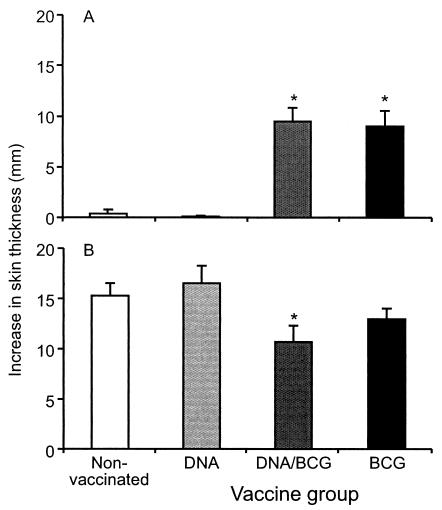
Prior to challenge, all animals that received BCG gave a skin test value of ≥4 mm in response to bovine PPD. The skin test responses to bovine PPD of the nonvaccinated animals and the animals vaccinated with DNA were negligible (≤1 mm) (Fig. 2A), although a weak skin test response to avian PPD was observed for some animals (data not shown). After challenge, all groups gave positive skin test responses to bovine PPD (Fig. 2B), but they were significantly lower in the DNA/BCG group than in the nonvaccinated controls (P < 0.05). The bovine PPD responses in BCG-vaccinated animals were also lower after challenge than the responses of nonvaccinated or DNA-vaccinated animals, although the differences were not statistically significant.
FIG. 2.
Skin test responses as indicators of protection: skin test responses to bovine PPD 10 weeks after the onset of vaccination (A) or 15 weeks after challenge (B). The values are means, and the error bars indicate standard errors of the means. An asterisk indicates that the value is significantly different from the value for the nonvaccinated group (P < 0.05).
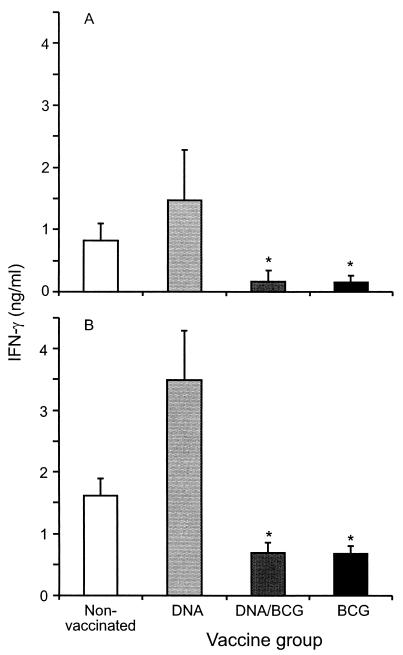
In addition, the in vitro IFN-γ responses of the vaccinated and nonvaccinated animals to a peptide cocktail containing synthetic peptides derived from ESAT-6 and CFP-10 as well as against bovine PPD were compared 10 weeks after challenge. Not only were significantly lower ESAT-6- and CFP-10-specific IFN-γ responses observed in the animals vaccinated either with BCG or with BCG and DNA than in the nonvacccinated or DNA-vaccinated animals (Fig. 3A) (P < 0.05), but there were also significantly reduced bovine PPD responses in the same groups (Fig. 3B) (P < 0.05).
FIG. 3.
IFN-γ responses to an ESAT-6-CFP-10 peptide cocktail and bovine PPD as indicators of protection. IFN-γ responses to an ESAT-6- and CFP-10-derived peptide cocktail (A) or bovine PPD (B) were determined 10 weeks after challenge. The values are means, and the error bars indicate standard errors of the means. An asterisk indicates that the value is significantly different from the values for the nonvaccinated and DNA-vaccinated groups (P < 0.05).
DISCUSSION
A prime-boost vaccination strategy based on priming with Hsp 65, Hsp 70, and Apa DNA and boosting with BCG was shown to result in significant enhancement in six pathological and microbiological parameters of protection following experimental challenge with M. bovis. In comparison, vaccination with BCG alone enhanced only two of the protection parameters. However, there were no significant differences between the DNA/BCG and BCG groups. Sensitization of the herd to environmental mycobacteria may have contributed to the low level of protection observed with BCG in this trial. However, there was no correlation between the intensity of IFN-γ responses to avian PPD in individual animals before vaccination and the intensity of IFN-γ responses to bovine PPD after vaccination with BCG or protection against challenge (data not shown). This result is not surprising as immune responses to avian PPD can fluctuate markedly when cattle are exposed to environmental mycobacteria and a response at a single time point may not be a good indicator of exposure.
The pathological and microbiological parameters of protection for the DNA/BCG group included reductions in the number of animals with lung lesions, the mean lung lesion score, the number of animals with lymph node lesions, the mean lymph node lesion score, the mean number of lymph nodes with lesions per animal, and the mean lymph node bacterial count. In contrast, the BCG vaccine alone resulted in significant reductions in only the number of animals with lymph node lesions and the mean lymph node lesion score, while the DNA vaccine alone induced no protection. Larger group sizes would be needed to demonstrate significant differences between the DNA/BCG and BCG groups, but the enhancement in six parameters of protection for the DNA/BCG group compared to the enhancement in only two of the parameters for the BCG group suggested that DNA priming and BCG boosting induced better protection in the outbred cattle than a single vaccine induced.
Recent studies have shown that high levels of cell-mediated immunity can be stimulated by consecutive use of DNA vaccines and attenuated poxvirus or modified vaccinia virus Ankara strain (MVA) vectors encoding similar heterologous antigens (21). The success of this strategy in vaccination against viral infections emphasizes the conclusion that DNA vaccines can be very effective for priming immune responses. Similar types of vaccination regimens have been used in mouse models of tuberculosis with various degrees of success. Priming with a DNA vaccine followed by boosting with MVA encoding the same proteins gave protection similar to that provided by BCG (19). Priming with a DNA vaccine and boosting with a live mycobacterial vaccine have given conflicting results in mice. In one study, priming with ESAT-6 and Ag85A DNA and boosting with either BCG Pasteur or a newly attenuated M. bovis strain enhanced immune responses but not protection against M. bovis infection (24). In contrast, priming with Ag85B and boosting with the BCG-Tokyo strain enhanced protection against M. tuberculosis in mice (13). It is possible that differences in the DNA vaccine, the type of BCG strain, or the type of challenge may explain the differences in the efficacy. The vaccine efficacy determined in mouse models of tuberculosis does not necessarily equate with that found in cattle (25). The present study confirmed these observations because Hsp 65, Hsp70, and Apa DNA vaccines have been shown to be protective in mice (15, 17), but their efficacy in cattle was apparent only when DNA vaccination was combined with a live vaccine boost.
Data from a number of studies suggest that IFN-γ is the major effector cytokine against mycobacterial infections; however, mechanisms of protective immunity are more complex, as IFN-γ is also associated with disease progression (28, 31, 32). In cattle, previous work has shown that the best association between IFN-γ responses and protection occurs when vaccination induces a strong IFN-γ response to bovine PPD at 2 to 4 weeks after vaccination, which is followed by a sharp peak of IFN-γ production at 2 weeks after challenge and then a decline as the infection is controlled (30). This type of response pattern was detected in this work in animals vaccinated with the DNA prime-BCG boost regimen or with BCG alone and to some extent reflected protection against bovine tuberculosis. These kinetics of IFN-γ responses were also associated with lower bacilliary loads postmortem. The DNA-vaccinated group, which was not protected, exhibited a strong IFN-γ response immediately after challenge, but this response continued to increase and became the highest response seen in any of the groups. A rapid IFN-γ response after challenge alone is therefore not necessarily an indication of protective immunity per se, while a continued increase in the IFN-γ response after challenge may indicate that the animal is not protected. Therefore, a rapid increase in IFN-γ responses after challenge, followed by a subsequent decrease, may be the crucial indicator of protection rather than the absolute amount of IFN-γ produced. In addition, IFN-γ-producing effector cells may be short lived and in the absence of continued strong antigenic stimuli may not develop into long-term effector memory cells (33).
This cattle model of tuberculosis provides a way to monitor the efficacy of particular vaccination strategies by measuring various pathological, microbiological, and immunological parameters of protection. This study highlighted several such parameters of protection. It has been shown previously that BCG-vaccinated, protected calves have lower IFN-γ responses to ESAT-6, a parameter that also correlates with the amounts of pathological changes that occurr during infection (28). In this work, these observations were confirmed and extended by establishing that protected animals (the DNA/BCG group or the BCG group) exhibited significantly lower in vitro IFN-γ responses than unprotected animals (the nonvaccinated group or the DNA group) exhibited when they were stimulated with an ESAT-6-CFP-10 peptide cocktail or with bovine PPD after challenge. In addition, there were less intense tuberculin skin test reactions after challenge in the animals that received DNA and BCG than in the nonvaccinated animals and the animals that received DNA, demonstrating that this test can serve as a surrogate indicator of protection.
DNA vaccination does not interfere with diagnosis of tuberculosis by the tuberculin skin test (29). However, in the present study DNA vaccination alone was not effective for protecting cattle against bovine tuberculosis. If combination DNA-BCG vaccines are to complement test and slaughter policies in the control of bovine tuberculosis, then development of new diagnostic tools to differentiate vaccinated animals from infected animals (20) is essential. Differential diagnostic tests with antigens such as ESAT-6 and CFP-10, which are not expressed by BCG, can distinguish between BCG-vaccinated and M. bovis-infected cattle (27, 28), and a similar approach could be used to distinguish DNA-vaccinated cattle from infected cattle. Such reagents fulfill a prerequisite of vaccine development in that they allow continuation of test and slaughter control policies along with the use of vaccines that do not express these distinguishing antigens.
Acknowledgments
This work was supported by grants from the New Zealand Ministry of Agriculture and Forestry (Policy Management) and DEFRA UK. Jose Candido Ferraz was supported by CNPq-Brasil.
We thank Allison McCarthy, Keith Hamel, Natalie Parlane, and Gary Yates for excellent technical assistance and Lilian Morrison for the statistical analyses.
Footnotes
We dedicate this paper to Jo Colson (1948-2003), who encouraged and supported this work and made a significant contribution to tuberculosis research.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Bloom, B. R., and P. E. M. Fine. 1994. The BCG experience: implications for future vaccines against tuberculosis, p. 531-557. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. American Society for Microbiology, Washington, D.C.
- 2.Bosio, C. M., and I. M. Orme. 1998. Effective, nonsensitizing vaccination with culture filtrate proteins against Mycobacterium bovis infections in mice. Infect. Immun. 66:5048-5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt, L., J. Feino Cunha, A. Weinreich Olsen, B. Chilima, P. Hirsch, R. Appelberg, and P. Andersen. 2002. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect. Immun. 70:672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandt, L., M. Elhay, I. Rosenkrands, E, Lindblad, and P. Andersen. 2000. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect. Immun. 68:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buddle, B. M., B. J. Wards, F. E. Aldwell, D. M. Collins, and G. W. de Lisle. 2002. Influence of sensitisation to environmental mycobacteria on subsequent vaccination against bovine tuberculosis. Vaccine 20:1126-1133. [DOI] [PubMed] [Google Scholar]
- 6.Buddle, B. M., D. Keen, A. Thomson, G. Jowett, A. R. McCarthy, J. Heslop, G. W. De Lisle, J. L. Stanford, and F. E. Aldwell. 1995. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res. Vet. Sci. 59:10-16. [DOI] [PubMed] [Google Scholar]
- 7.Buddle, B. M., G. W. de Lisle, A. Pfeffer, and F. E. Aldwell. 1995. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine 13:1123-1130. [DOI] [PubMed] [Google Scholar]
- 8.Chambers, M. A., A. Williams, G. Hatch, D. Gavier-Widen, G. Hall, K. Huygen, D. Lowrie, P. D. Marsh, and R. G. Hewinson. 2002. Vaccination of guinea pigs with DNA encoding the mycobacterial antigen MPB83 influences pulmonary pathology but not hematogenous spread following aerogenic infection with Mycobacterium bovis. Infect. Immun. 70:2159-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman, B. S., R. M. Thayer, K. A. Vincent, and N. L. Haigwood. 1991. Effect of intron A from human cytomegalovirus (Towne) immediate-early gene on heterologous expression in mammalian cells. Nucleic Acids Res. 19:3979-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corner, L. A., L. Melville, K. McCubbin, K. J. Small, B. S. McCormick, P. R. Wood, and J. S. Rothel. 1990. Efficiency of inspection procedures for detection of tuberculous lesions in cattle. Aust. Vet. J. 67:389-392. [DOI] [PubMed] [Google Scholar]
- 11.Cosivi, O., J. M. Grange, C. J. Daborn, M. C. Raviglione, T. Fujikura, D. Cousins, R. A. Robinson, H. F. Huchzermeyer, I. de Kantor, and F. X. Meslin. 1998. Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg. Infect. Dis. 4:59-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. W.H.O. Global Surveillance and Monitoring project. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 13.Feng, C. G., U. Palendira, C. Demangel, J. M. Spratt, A. Malin, and W. Britton. 2001. Priming with DNA immunisation augments protective efficacy of Mycobacterium bovis Bacille Calmette-Guerin against tuberculosis. Infect. Immun. 69:4174-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fine, P. E. M. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 15.Garapin, A., L. Ma, P. Pescher, M. Lagranderie, and G. Marchal. 2001. Mixed immune response induced in rodents by two naked DNA genes coding for mycobacterial glycosylated proteins. Vaccine 19:2830-2841. [DOI] [PubMed] [Google Scholar]
- 16.Hewinson, R. G., H. M. Vordermeier, and B. M. Buddle. 2003. Use of the bovine model of tuberculosis for the development of improved vaccines and diagnostics. Tuberculosis 83:119-130. [DOI] [PubMed] [Google Scholar]
- 17.Lowrie, D. B., C. L. Silva, M. J. Colston, S. Ragno, and R. E. Tascon. 1997. Protection against tuberculosis by a plasmid DNA vaccine. Vaccine 15:834-838. [DOI] [PubMed] [Google Scholar]
- 18.Lowrie, D. B., R. E. Tascon, V. L. Bonato, V. M. Lima, L. H. Faccioli, E. Stavropoulos, M. J. Colston, R. G. Hewinson, K. Moelling, and C. L. Silva. 1999. Therapy of tuberculosis in mice by DNA vaccination. Nature 400:269-271. [DOI] [PubMed] [Google Scholar]
- 19.McShane, H., R. Brookes, S. Gilbert, and A. V. S. Hill. 2001. Enhanced immunogenicity of CD4+ T-cell response and protective efficacy of a DNA-modified vaccinia virus Ankara prime-boost vaccination regimen for murine tuberculosis. Infect. Immun. 69:681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollock, J. M., R. M. Girvin, K. A. Lightbody, R. A. Clements, S. D. Neill, B. M. Buddle, and P. Andersen. 2000. Assessment of defined antigens for the diagnosis of bovine tuberculosis in skin test-reactor cattle Vet. Rec. 146:659-665. [DOI] [PubMed] [Google Scholar]
- 21.Ramshaw, I. A., and A. J. Ramsay. 2000. The prime-boost strategy: exciting prospects for improved vaccination. Immunol. Today 21:160-162. [DOI] [PubMed] [Google Scholar]
- 22.Roberts, A. D., M. G. Sonnenberg, D. L. Ordway, S. K. Furney, P. J. Brennan, T. Belisle, and I. M. Orme. 1995. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. J. Immunol. 85:502-508. [PMC free article] [PubMed] [Google Scholar]
- 23.Rothel, J. S., S. L. Jones, L. A. Corner, J. C. Cox, and P. R. Wood. 1990. A sandwich enzyme immunoassay for bovine interferon-gamma and its use for the detection of tuberculosis in cattle. Aust. Vet. J. 67:134-137. [DOI] [PubMed] [Google Scholar]
- 24.Skinner, M. A., A. J. Ramsay, G. S. Buchan, D. L. Keen, C. Ranasinge, L. Slobbe, D. M. Collins, G. W. de Lisle, and B. M. Buddle. 2003. A DNA prime-live vaccine boost strategy in mice can augment IFN-γ responses to mycobacterial antigens but does not increase the protective efficacy of two attenuated strains of Mycobacterium bovis against bovine tuberculosis. Immunology 108:548-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skinner, M. A., D. N. Wedlock, and B. M. Buddle. 2001. Vaccination of animals against Mycobacterium bovis. Rev. Sci. Tech. Off. Int. Epizoot. 20:112-132. [DOI] [PubMed] [Google Scholar]
- 26.Tanghe, A., S. D'Souza, V. Rosseels, O. Denis, T. H. M. Ottenhoff, W. Dalemans, C. Wheeler, and K. Huygen. 2001. Improved immunogenicity and protective efficacy of a tuberculosis DNA vaccine encoding Ag85 by protein boosting. Infect. Immun. 69:3041-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vordermeier, H. M., A. Whelan, P. C. Cockle, L. Farrant, N. Palmer, and R. G. Hewinson. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vordermeier, H. M., M. A. Chambers, P. J. Cockle, A. O. Whelan, J. Simmons, and R. G. Hewinson. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70:3026-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vordermeier, H. M., P. J. Cockle, A. O. Whelan, S. Rhodes, M. A. Chambers, D. Clifford, K. Huygen, R. Tascon, D. Lowrie, M. J. Colston, and R. G. Hewinson. 2000. Effective DNA vaccination of cattle with the mycobacterial antigens MPB83 and MPB70 does not compromise the specificity of the comparative intradermal tuberculin skin test. Vaccine 19:1246-1255. [DOI] [PubMed] [Google Scholar]
- 30.Wedlock, D. N., B. Vesosky, M. A. Skinner, G. W. de Lisle, I. M. Orme, and B. M. Buddle. 2000. Vaccination of cattle with Mycobacterium bovis culture filtrate proteins and interleukin-2 for protection against bovine tuberculosis. Infect. Immun. 68:5809-5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wedlock, D. N., M. A. Skinner, G. W. DeLisle, and B. M. Buddle. 2002. Control of Mycobacterium bovis infections and the risk to human population. Microbes Infect. 4:471-480. [DOI] [PubMed] [Google Scholar]
- 32.Wilsher, M. L., C. Hagan, R. Prestidge, A. U. Wells, and G. Murison. 1999. Human in vitro immune responses to Mycobacterium tuberculosis. Tuber. Lung Dis. 79:371-377. [DOI] [PubMed] [Google Scholar]
- 33.Wu, C. Y., J. R. Kirman, M. J. Rotte, D. F. Davey, S. P. Perfetto, E. G. Rhee, B. L. Freidag, B. J. Hill, D. C. Douek, and R. A. Seder. 2002. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat. Immunol. 3:852-858. [DOI] [PubMed] [Google Scholar]