Abstract
A preestablished infection with the parasitic helminth, Schistosoma mansoni, significantly reduced the incidence and delayed the onset of experimental autoimmune encephalomyelitis (EAE) in C57BL/6J mice immunized with myelin oligodendrocyte glycoprotein (MOG)35-55 peptide. The altered disease progression was not solely due to the induction of a strong Th2 response, since intraperitoneal injection of schistosome eggs did not affect disease development. MOG-specific gamma interferon (IFN-γ), nitric oxide, and tumor necrosis factor alpha production by splenocytes was significantly reduced in schistosome-infected mice compared to uninfected mice. However, similar levels of interleukin-10 (IL-10) were produced in an antigen-specific manner, suggesting that the induction of antigen-specific responses was not inhibited. Analysis of in vivo cytokine production by real-time PCR indicated that IL-12p40, but not IFN-γ, transcript levels were dramatically reduced in the spinal cords of schistosome-infected, MOG-immunized mice. Furthermore, analysis of the cellular composition of the spinal cords and brains revealed that a preestablished infection with S. mansoni decreased central nervous system (CNS) inflammation, particularly of macrophages and CD4 T cells. These results suggest that schistosomiasis may negatively regulate the onset of EAE by downregulating the production of proinflammatory cytokines and altering CNS inflammation.
Multiple sclerosis (MS) is an immune-mediated disease characterized by neurodegeneration, leading to severe impairment of mobility, vision, and coordination, which ultimately results in paralysis (18). Although the cause of MS is unknown, it is clear that both genetic and environmental factors are involved in disease initiation (18). More than 2 million people are currently affected worldwide, with the highest prevalence occurring in areas farthest from the equator, suggesting that factors in areas of high latitude promote disease initiation or that factors in the equatorial regions inhibit disease initiation (18, 20, 28).
Schistosomiasis is a chronic disease caused by infection with the helminth schistosoma (5). After percutaneous infection and worm maturation, the female Schistosoma mansoni worms produce eggs within the inferior mesenteric veins (5). These eggs pass through the gut wall to exit the host through the feces or are swept into the liver and trapped in the sinusoids, where they induce granulomatous lesions (5). These lesions can cause portal hypertension, hepatic fibrosis, and portal shunting that, in severe cases, can result in death (2, 7). Two hundred million people are infected, primarily in equatorial areas: South America, Africa, Southeastern Asia, and the Philippines (7). Effective chemotherapy does exist to treat schistosome infection, but unfortunately reinfection rates are very high (7).
The inverse correlation of the prevalence of these two diseases also manifests itself immunologically, since MS and schistosomiasis invoke diametrically opposite immune responses. The form of MS that has been most extensively studied with the mouse model, experimental autoimmune encephalomyelitis (EAE), is a proinflammatory, CD4 T-cell-mediated disease induced by immunization with myelin proteins and peptides (19, 40). Induction is dependent on the type 1 cytokine interleukin-12 (IL-12) that appears to be central in macrophage activation and nitric oxide (NO) production (22). However, a recent study indicates that IL-23 and not IL-12, both of which share the same p40 subunit, may be crucial to the induction of EAE (4, 10). Although gamma interferon (IFN-γ) does not appear to be essential for disease induction, it may play an important role in disease pathogenesis (17). Other proinflammatory cytokines, tumor necrosis factor (TNF-α) and TNF-β, have also been shown to be important in disease relapse during the chronic stage (17, 37). In contrast, cytokines associated with an anti-inflammatory T helper 2 (Th2)/type 2 response appear to play a beneficial role in autoimmune diseases, and this finding suggests that predisposition toward a Th2 response can prevent or decrease the severity of EAE (34, 39).
Schistosomiasis is a well-characterized Th2 response-dominated disease (15). Shortly after the beginning of egg deposition, a strong egg-specific Th2 response develops characterized by high levels of IL-4, IL-5, and IL-13 (8, 15). Due to the chronicity and high global prevalence of schistosome infection, recent studies have begun to investigate the effect of preestablished schistosome infection on the progression of other diseases (infectious and noninfectious) (3, 9, 11, 21, 26, 38). These studies have shown that infection with S. mansoni can alter the progression of infection and noninfectious diseases (e.g., diabetes, leishmaniasis, toxoplasmosis, and atopy) and that this effect can be achieved in peripheral sites (3, 9, 11, 21, 26, 38). We investigate here whether schistosome infection can alter the initiation and progression of an immune-mediated disease of the central nervous system (CNS), EAE, and we analyze how this parasitic infection can alter the development of immune responses and disease pathology.
MATERIALS AND METHODS
Mice, parasites, and experimental infections and immunizations.
C57BL/6J were bred at the Wellington School of Medicine animal facility and utilized at 4 to 8 weeks of age. For live infection, mice were exposed percutaneously to ca. 70 S. mansoni cercariae (NIMR Puerto Rican strain) as previously described (33). Infection was assessed postmortem by quantification of egg burden in the livers of infected mice. Uninfected mice were excluded from the study. The infection success rate was 96%. All experimental procedures used in the present study were approved by the Wellington School of Medicine and Victoria University of Wellington Animal Ethics Committees.
Mice were immunized as previously described (30). Briefly, mice were injected subcutaneously in the rear flanks with 50 μg of myelin oligodendrocyte glycoprotein (MOG)35-55 peptide (Mimotopes, Clayton, Australia)/mouse in complete Freund adjuvant (CFA; 1 mg of Mycobacterium tuberculosis H37Ra/mouse; Difco, Detroit, Mich.) and given two intraperitoneal injections of pertussis toxin (200 ng/mouse; List Biologicals, Campbell, Calif.) at day 0 and day 2. Clinical scores were daily assigned and assessed as follows: 0, unaffected; 1, loss of tail tonicity; 2, flaccid tail; 3, flaccid tail and affected hind leg or legs; 4, paralyzed hind legs (both); and 5, moribund. At autopsy, tissues were fixed in zinc salts, paraffin-embedded, sectioned, and stained with hematoxylin and eosin (H&E) for histological examination. Sections were scored blindly and were assigned arbitrary scores of infiltration into the meningeal of the spinal cord as follows: 0, no mononuclear cell infiltration; 1, mild; 2, moderate; and 3, severe.
Splenocyte isolation and in vitro culture.
Spleens were harvested, and single cell suspensions were prepared by using sterile 70-μm (pore-size) cell strainers (Falcon, Franklin Lakes, N.J.) as previously described (6). Splenocytes were resuspended at 5 × 106 cells/ml in complete T-cell medium containing Iscove Dulbecco modified Eagle medium, 5% fetal calf serum, 100 U of penicillin/ml plus 100 μg of streptomycin/ml, 10 mM HEPES, l-glutamine, and 5 × 10−5 M 2-mercaptoethanol (all from Gibco-BRL, Auckland, New Zealand). Cells (106) were cultured in 96-well flat-bottom plates (Falcon), with MOG35-55, M. tuberculosis antigen (Mtb), concanavalin A (Sigma, St. Louis, Mo.), or medium alone at 37°C and 5% CO2. Culture supernatants were harvested at 48 h for cytokine analysis.
Cytokine ELISAs.
Sandwich enzyme-linked immunosorbent assays (ELISAs) were used to measure IL-4, IL-5, IL-10, IFN-γ, and TNF-α as previously described (30). NO was measured in culture supernatants by using the Greiss reaction as described previously (14).
Spinal cord cell isolation.
Spinal cords were isolated from mice by perfusion of the spinal column with phosphate-buffered saline. The spinal cord was then minced and incubated in collagenase (Gibco-BRL) for 30 min at 37°C. The cells were then passed through a 70-μm-pore-size cell strainer to remove debris and washed with 1% fetal cald serum-0.01% NaN3 in phosphate-buffered saline (fluorescence-activated cell sorting [FACS] buffer). The cells were then centrifuged through a 37% Percoll gradient (Sigma), resuspended in FACS buffer, and stained for flow cytometry by using standard techniques.
Flow cytometry.
The expression of surface markers was quantified by flow cytometry with fluorescein isothiocyanate-, phycoerythrin-, PerCP, or cychrome C-conjugated antibodies. Rat anti-mouse CD4, CD8, B220, Gr-1, Mac-1, CD45, and isotype control antibodies were purchased from Pharmingen. Rat anti-F4/80 antibody was purchased from Serotec (Oxford, United Kingdom). Samples were analyzed by using a FACSCaliber flow cytometer and CELLQuest software (Becton Dickinson, Franklin Lakes, N.J.).
Real-time PCR.
RNA was isolated from tissues by using Trizol (Invitrogen, Auckland, New Zealand) according to the manufacturer's instructions and was reverse transcribed as previously described (35). Real-time PCR amplifications were performed in an ABI Prism 7700 sequence detector (Applied Biosystems [ABI], Scoresby, Australia) by using SYBR Green 1 (ABI) according to the manufacturer's instructions. Each PCR amplification was performed in duplicate wells, and 18S rRNA was used as a control to calculate the level of cytokine RNA. The primers used in these studies were as follows: IL-10 (44), IL-12 (31), IFN-γ forward (5′-CCTCCTGCGGCCTAGCTC-3′), IFN-γ reverse (5′-GTAACAGCCAGAAACAGCCATG-3′), and 18S (36).
Immunohistochemistry.
Zinc-fixed tissue sections were deparaffinized, rehydrated, and treated with 3% H2O2 to quench endogenous peroxidase activity. For F4/80 and B220 staining, tissues were blocked in 2% normal goat serum (Vector Laboratories, Burlingame, Calif.) and then incubated in biotinylated rat anti-F4/80 (Serotec) or B220 (Pharmingen) antibody at a 1:100 dilution for 30 min at room temperature. For CD3 staining, tissues were blocked in 2% normal horse serum (Sigma) and incubated in goat anti-mouse CD3 (Santa Cruz Biotechnology, Santa Cruz, Calif.) pAb at a 1:100 dilution for 30 min at room temperature, followed by the addition of a biotinylated donkey anti-goat immunoglobulin G antibody (Jackson Immunochemicals, West Grove, Pa.). Sections were developed by using an ABC kit (Novocastra Laboratories, Newcastle upon Tyne, United Kingdom) and diaminobenzidene (Sigma) according to the manufacturer's instructions.
Statistical analysis
Data were analyzed by using an unpaired or paired Student t test or two-way analysis of variance as indicated.
RESULTS
Infection with live S. mansoni alters the initiation and progression of EAE.
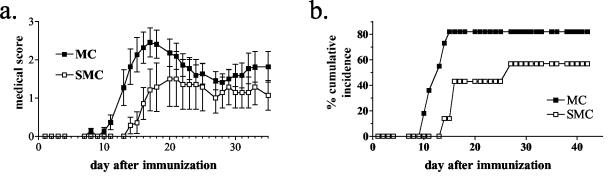
Schistosomiasis has been shown to modify the disease course of several infectious (Leishmania major and Trichuris muris) and noninfectious (type 1 diabetes) diseases (9, 11, 21). To determine whether the presence of this Th2-inducing parasite would alter the course of a disease, such as EAE, that is localized to the CNS, C57BL/6J mice were infected with the parasitic worm S. mansoni 6 weeks before the induction of EAE by MOG immunization. The 6-week period allowed the worms to begin egg laying, which induces strong egg-specific Th2 responses in the mammalian host (15). Mice that had a preestablished schistosome infection (SMC mice) had a significantly decreased incidence and delay to the onset of disease compared to uninfected, immunized mice (MC mice) (Fig. 1; P < 0.03, MC versus SMC incidence; paired Student t test from three experiments). Of the mice that did develop disease, there was no difference in the peak score between the SMC and the MC mice (3.13 ± 0.28 versus 3.50 ± 0.43 [infected versus uninfected]). In addition, no difference was seen in the recovery from acute disease between the two groups, suggesting that schistosomiasis appears to modify disease primarily at the induction phase.
FIG. 1.
Live S. mansoni infection decreases the incidence and delays the onset of EAE. C57BL/6J mice were infected with 70 cercariae percutaneously 6 weeks prior to immunization with MOG35-55 in CFA and scored daily as described in Materials and Methods. Shown are the means and the standard errors of the mean (SEM) of clinical scores from 7 to 11 individual mice. Shown are the results from one of three similar experiments. P ≤ 0.03 for the onset of disease (MC versus SMC mice).
Schistosome infection results in reduced antigen-specific production of IFN-γ, TNF-α, and NO but not in reduced production of IL-10.
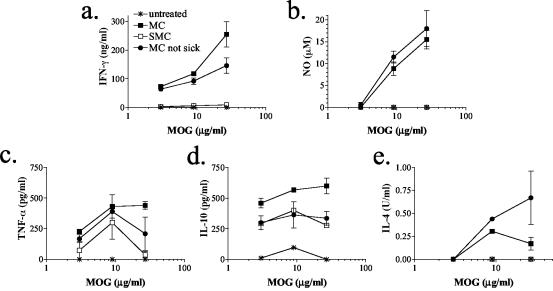
To understand whether the modulation of disease course was due to altered antigen-specific responses in the SMC mice, splenocytes were isolated and stimulated in vitro with antigen. Splenocytes from SMC and MC mice, even those that did not show overt disease, responded to MOG stimulation, indicating that both groups induced a MOG-specific T-cell response (Fig. 2). However, the production of IFN-γ, NO, and to a lesser extent TNF-α was reduced in SMC sick mice compared to MC sick mice (Fig. 2a to c). Similarly, low levels of these cytokines were seen in SMC mice that were not sick (data not shown). It is interesting that at earlier time points (i.e., 2 to 3 weeks compared to 6 weeks after immunization), similar levels of NO were produced by splenocytes from both SMC sick mice and MC sick mice despite the reduction in other proinflammatory mediators such as IFN-γ and TNF-α (data not shown). In addition, the levels of these proinflammatory mediators produced by splenocytes from SMC sick mice were even below the levels produced by the MC mice that did not develop any signs of disease (Fig. 2a to c). Interestingly, there is a reduction in the CD4 T-cell compartment in SMC mice compared to MC mice (13% versus 21%, respectively) (Table 1). However, a reduction in the CD4 T-cell compartment cannot solely explain the reduced levels of inflammatory mediators, since the level of depression seen in IFN-γ production is almost 40-fold.
FIG. 2.
Schistosome infection results in reduced MOG-specific IFN-γ (a), NO (b), and TNF-α (c) production but not in reduced MOG-specific IL-10 production (d). (e) Low levels of MOG-specific IL-4 were detected in MC culture supernatants but not in SMC culture supernatants. At 6 weeks after MOG immunization, splenocytes were isolated from S. mansoni-infected or uninfected C57BL/6J mice, pooled, and stimulated with MOG in vitro. Then, 48-h culture supernatants were assayed for IFN-γ, NO, IL-10, and TNF-α. Shown are the means and SEM of duplicate or triplicate wells from one of two similar experiments.
TABLE 1.
Cellular subsets in the spleen 6 weeks after MOG immunization
| Mouse groupa | % Cells (SD)
|
|||||
|---|---|---|---|---|---|---|
| CD4+ | CD8+ | B220+ | Mac-1+ | F4/80+ | Gr-1+ | |
| Normal | 26 (0.5) | 15 (2) | 43 (4) | 11 | 9 (0.5) | 6 |
| MC, not sick | 22 (1.5) | 14 (5) | 50 (1.5) | 16 | 16 (0.5) | 11 |
| MC, sick | 21 (1) | 13 (4) | 49 (3) | 14 | 13 (0.5) | 9 |
| SMC, sick | 13 (2.5) | 8 (1) | 30 (3) | 15 | 20 (2.5) | 6 |
Values for SMC mice that were not sick were not determined.
Although IFN-γ, NO, and TNF-α production were significantly reduced, the level of MOG-specific IL-10 produced by splenocytes from SMC mice was similar to that of MC mice that did not develop overt disease (Fig. 2d). IL-10 levels in both these groups were less than those seen after MOG stimulation of splenocytes from MC sick mice (Fig. 2d). No MOG-specific IL-4 was detected in the supernatants from SMC splenocytes, and only low levels were detected in culture supernatants from MC mice (Fig. 2e). These results indicate that, although MOG-specific proinflammatory mediator production is significantly reduced by schistosome infection, MOG-specific responses do develop, as evidenced by the production of IL-10. In addition, the lack of antigen-specific IL-4 indicates that the MOG-specific Th response has not undergone immune switching from Th1 to Th2 by schistosome infection.
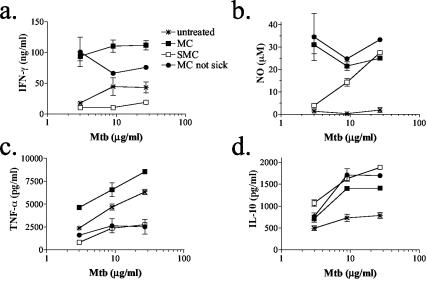
Analysis of Mtb-specific responses revealed a similar impaired production of IFN-γ, NO, and TNF-α by splenocytes from SMC mice (Fig. 3a to c). Furthermore, IL-10 production was unaffected by schistosome infection compared to MC mice, thus supporting the conclusion that antigen-specific responses do develop in the SMC mice despite the significant depression in type 1 responses (Fig. 3d). Finally, the lack of IL-4 production by splenocytes in response to either MOG or Mtb underscores that these mice do not switch the type of response development from Th1 to Th2 by schistosome infection (data not shown). Instead, MOG or Mtb stimulation of splenocytes from SMC mice promotes IL-10 production primarily and may signify a switch to a Th3 or regulatory phenotype.
FIG. 3.
Schistosome infection results in reduced Mtb-specific IFN-γ (a), NO (b), and TNF-α (c) production but not in reduced IL-10-specific production (d). At 6 weeks after MOG immunization, splenocytes were isolated from S. mansoni-infected or uninfected C57BL/6J mice and stimulated with Mtb in vitro. Then, 48-h culture supernatants were assayed for IFN-γ, NO, IL-10, and TNF-α. Shown are the means and SEM of duplicate or triplicate wells from one of two similar experiments.
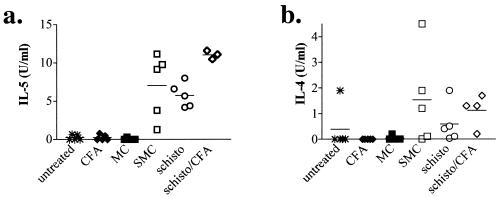
To determine whether the strong Th1 inducing immunizations (i.e., CFA) would alter the induction of Th2 responses by schistosome infection, plasma was collected and assayed for IL-5 and IL-4. Figure 4a shows that only mice infected with S. mansoni had detectable levels of IL-5 in the plasma, and similar results were found for IL-4 (Fig. 4b). However, no significant difference was seen between MOG-immunized, infected mice (Fig. 4, SMC) and infected mice that were not immunized or immunized with CFA alone (Fig. 4). Moreover, immunization with CFA alone appeared to magnify Th2 responses in schistosome-infected mice compared to infected but unimmunized mice (Fig. 4). These results indicate that CFA immunization per se did not diminish Th2 responses in vivo and that a strong systemic Th2 cytokine environment was present during EAE.
FIG. 4.
MOG immunization does not alter systemic Th2 cytokine levels during schistosomiasis. Sera from uninfected and S. mansoni-infected mice were collected 2 weeks after immunization with CFA with or without MOG. IL-5 (a) and IL-4 (b) levels were determined by ELISA. Shown are the group means (bars) and values for individual mice (three to five per group) from one of three similar experiments.
Production of IL-12 is significantly reduced in spinal cords in schistosome-infected, MOG-immunized mice.
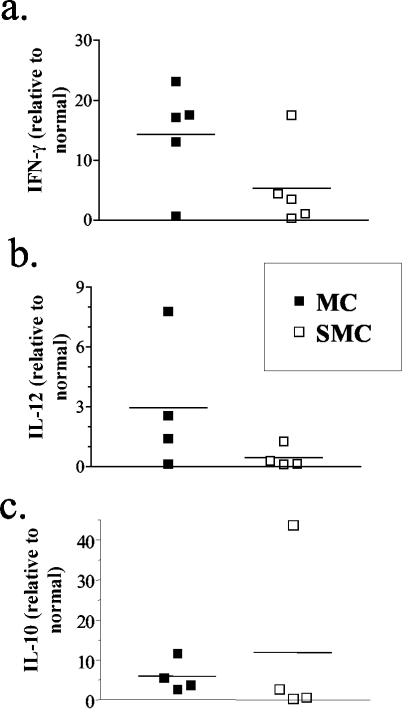
Analysis of the antigen-specific T-cell responses that developed after MOG immunization of infected or uninfected mice revealed a decreased production of proinflammatory mediators by splenocytes from SMC mice. To investigate whether a similar decrease in cytokine production was evident at the site of inflammation, mRNA was isolated from sick MC and SMC mice and analyzed by real-time PCR. In contrast to the splenocyte responses, IFN-γ mRNA was not as strongly downregulated in the spinal cords of SMC mice compared to MC mice, although the decrease was significant (Fig. 5a). IL-10 mRNA levels were highly variable but did not show any significant differences between the groups (Fig. 5c). However, IL-12p40 mRNA levels were consistently decreased in the spinal cords of SMC mice compared to MC mice (Fig. 5b). Taken together, these results support the idea that schistosome infection reduces the level of proinflammatory mediator production by splenocytes and in the CNS during EAE.
FIG. 5.
Transcript levels of IL-12p40 (b) and IFN-γ (a) but not of IL-10 (c) are reduced in spinal cords during EAE by schistosome infection. Spinal cords were isolated from uninfected and infected mice at 2 weeks after immunization, and mRNA was isolated. After cDNA production, IFN-γ, IL-12p40, and IL-10 transcript levels were determined by real-time PCR. Values were calculated as the fold increase versus untreated, normal controls. Shown are group means (bars) and values for individual mice (four to five per group) from one of two experiments. P ≤ 0.05 (MC versus SMC mice) for IFN-γ (as determined by one-tailed Student t test).
Infiltration of the CNS by inflammatory cells is altered by schistosome infection.
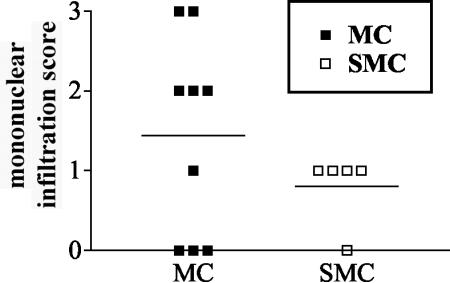
To determine whether the decreased proinflammatory mediator production could be correlated to a decreased presence of infiltrating inflammatory cells, sections from spinal cord and brain tissue were compared at 2 and 6 weeks after MOG immunization by standard H&E staining. At 2 weeks postimmunization the numbers of inflammatory cells and lesions were similar in all sick mice, whereas no lesions were visible in mice that did not develop disease supporting a correlation between CNS inflammation and disease (data not shown). However, the numbers and sizes of the inflammatory lesions were significantly reduced in the sick SMC mice compared to sick MC mice by 6 weeks after immunization (Fig. 6), suggesting that preinfection with S. mansoni may promote the resolution of lesions in the CNS.
FIG. 6.
Inflammation in the spinal cord is reduced in SMC mice compared to MC mice by 6 weeks after MOG immunization. H&E-stained sections of spinal cord were examined and graded for meningeal infiltration as follows: 0, no mononuclear cell infiltration; 1, mild; 2, moderate; and 3, severe. Shown are the results from five to nine mice per group from one of three experiments.
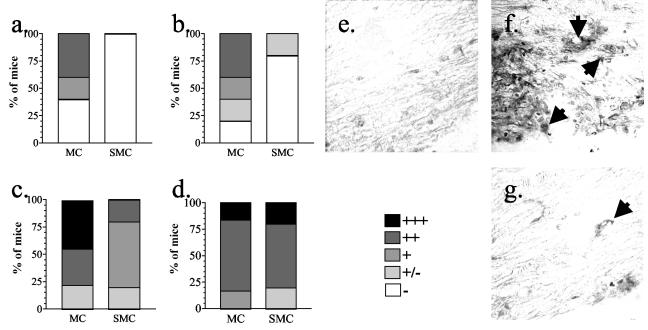
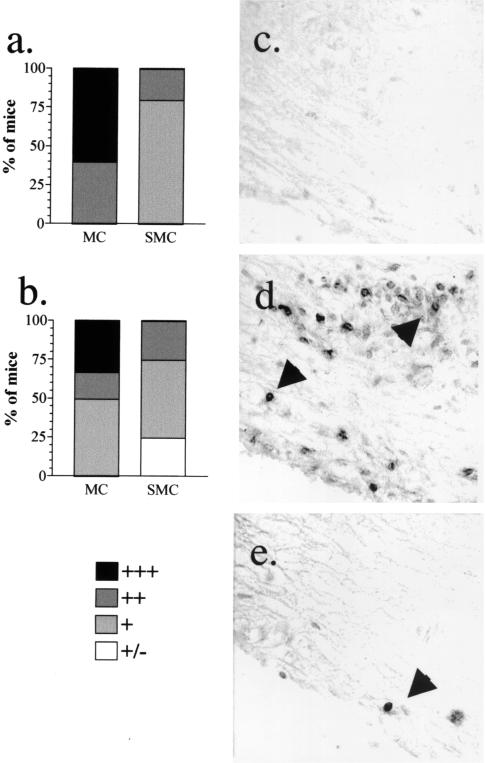
The infiltration of specific subsets of leukocytes was examined to determine whether differences existed in the types of leukocytes that formed the inflammatory lesions in sick SMC and MC mice. Although lesions from SMC and MC mice were similar in size and number at 2 weeks after immunization, dramatic differences were found in the composition of those lesions. No F4/80+ macrophages were detected in the spinal cords from sick SMC mice, whereas high levels were found in spinal cords from sick MC mice (Fig. 7a). A similar difference was found in the number of macrophages in the brains of sick SMC and MC mice (Fig. 7b). Analysis of cells isolated from the the spinal cords of sick mice confirmed this reduction in infiltrating macrophages (Mac-1+ CD45high cells), although similar percentages of infiltrating CD4+ T cells were found in sick SMC and MC mice (Table 2). In contrast, the MC mice that had not yet developed EAE had significantly more CD4+ T cells infiltrating into the spinal cords than SMC mice without disease (Table 2). B220+ cells were at similar to slightly lower levels in the spinal cords of sick SMC mice compared to MC mice (Fig. 8a). However, in the brain, more B220+ cells were found in the sick SMC mice (Fig. 8c). In normal mice, F4/80+ cells, B220+ cells, and CD4+ T cells were rarely found (Fig. 7 and 8 and data not shown). The decrease in macrophage infiltration with a similar level of lymphocyte infiltration suggests that schistosome infection alters the activation or migration of macrophages at or prior to the peak of disease.
FIG. 7.
Infiltration of F4/80+ macrophages into the spinal cord and brain is reduced in SMC mice compared to MC mice. Paraffin-embedded spinal cord (a, c, and e to g) or brain (b and d) sections from MC, SMC, or untreated control mice were stained with anti-F4/80 monoclonal antibody. The number of F4/80+ cells was qualitatively assessed in spinal cords (a and c) and brains (b and d) from tissues isolated 2 weeks (a and b) or 6 weeks (c and d) after immunization. More F4/80+ cells are found in the spinal cord of an MC (f) mouse compared to an SMC mouse (g) at 6 weeks after immunization. (e) Few F4/80+ cells were detected in untreated control mice. No staining was found when an isotype control antibody was used (data not shown). Shown are the results from five to nine mice per group from one of three experiments.
TABLE 2.
Flow cytometry analysis of spinal cord cells 6 weeks after MOG immunization
| Mouse groupa | % Cells (expected cell type)
|
||||
|---|---|---|---|---|---|
| CD4+ CD45+ (CD4+ T cells) | CD8+ CD45+ (CD8+ T cells) | Mac-1+ Gr-1+ (Neutrophils) | CD45high Mac-1+ (Macrophages) | CD45int Mac-1+ (Microglia) | |
| Normal | 2 | 1 | 1 | 0.9 | 16 |
| MC, not sick | 2.4 | 1.4 | 2.4 | 2.8 | 45 |
| MC, sick | 13.3 | 4 | 4.1 | 15 | 37 |
| SMC, sick | 6 | 2.5 | 2.1 | 5 | 41 |
Values for SMC mice that were not sick were not determined.
FIG. 8.
Infiltration of T cells is reduced in SMC mice compared to MC mice at 6 weeks postimmunization. Paraffin-embedded spinal cord (a and c to e) or brain (b) sections from MC, SMC, or untreated control mice were stained with anti-CD3 monoclonal antibody. The numbers of CD3+ cells were qualitatively assessed in spinal cords (a) and brains (b) from tissues isolated 6 weeks after immunization. More CD3+ cells were found in the spinal cord of an MC mouse (d) than in the spinal cord of a SMC mouse (e) at 6 weeks after immunization. Few CD3+ cells were detected in untreated control mice (c). No staining was found when an isotype control antibody was used (data not shown). Shown are the results from four to seven mice per group from one of three experiments.
At 6 weeks postimmunization, the composition of inflammatory infiltrates was again examined. At this time point, the peak of disease had passed, and mice remained only moderately sick, with an average disease score of 1.5. In contrast to the 2-week time point, the spinal cord lesions from SMC mice had significantly fewer T cells, F4/80+ macrophages, and B220+ cells than did the spinal cord lesions from MC mice (Fig. 7 and 8 and data not shown). A dramatic decrease in T cells and macrophages was also found when spinal cord cells were isolated and analyzed by flow cytometry (Table 2). This reduction correlated with the general reduction in inflammation seen in H&E-stained tissue sections (data not shown). However, in the brain the reduction was less evident, and similar or slightly lower levels of macrophages and B cells were found in SMC mice compared to MC mice (Fig. 7 and 8). Taken together, these results indicate that schistosome infection alters macrophage inflammation throughout the course of the disease, and this alteration may be responsible for the altered course of disease in SMC mice. Furthermore, the dramatic decrease in spinal cord inflammation at 6 weeks after immunization in SMC mice may point toward an accelerated resolution of the inflammatory lesions in these mice.
DISCUSSION
The mutual exclusion of the global incidence of the immune-mediated disease MS and the parasitic disease schistosomiasis suggests that chronic parasitic infection may exert a negative effect on the induction of MS (20). Whereas many potential confounding factors exist, the studies presented here indicate that there is a direct negative effect on the induction and progression of autoreactive T cells by a preestablished S. mansoni infection by using the EAE mouse model of MS. In addition to the decreased incidence and delayed induction of disease, SMC mice had significantly reduced IFN-γ, TNF-α, and IL-12 responses while maintaining similar IL-10 levels. These results indicate that, although the Th1 responses are reduced, development of antigen-specific responses is not prevented. In SMC mice that did develop disease, inflammatory lesions were significantly reduced by 6 weeks postimmunization. Moreover, the composition of macrophages and T cells in the lesions was dramatically altered. Infiltrating macrophages were absent from inflammatory lesions of SMC mice, and although CD4 T cells were at similar levels during the peak of disease, they were significantly reduced at 6 weeks postimmunization. These results suggest that schistosomiasis may be affecting the course of EAE by targeting the macrophage compartment during the initiation phase and by downregulating Th1 responses without inducing a Th2 switch.
Schistosomiasis has been shown previously to modulate the course of noninfectious (e.g., diabetes and atopy) and infectious diseases (e.g., toxoplasmosis, leishmaniasis, and T. muris infection) in humans and mice (3, 9, 11, 21, 26). Infection of nonobese diabetic mice with S. mansoni significantly inhibited the development of diabetes, demonstrating that schistosomiasis can inhibit the induction of an autoimmune disease, albeit by an undetermined mechanism (9). Using an infectious disease model, Curry et al. demonstrated that the injection of schistosome eggs into susceptible mice infected with T. muris switched Th response development from the Th1 to Th2 phenotype and thus induced Th2-dependent worm expulsion (11). Although a switch of the MOG-specific response from Th1 to Th2 can lead to reduced EAE in mice, our results suggest that no such immune deviation occurs after S. mansoni infection. Despite the dramatic decrease in IFN-γ production, no concurrent MOG-specific IL-4 production was detected in SMC mice. This conclusion supports the idea that the alteration in the course of EAE may not be solely mediated by T cells.
Recently, it has been reported that the injection of schistosome eggs and thus induction of an egg-specific Th2 response provides significant protection against the development of EAE in SJL mice (38). However, from our studies it is clear that it is not solely the presence of a strong Th2 response that is mediating protection in our system. Despite the differences in the models (egg injection versus live infection) used, the results from Sewell et al. are complementary to ours in that a decrease in gross CNS infiltration, a decreased number of IL-12-producing CD11b+ cells in the brain, and an increase in Th2 responses to T-cell mitogens was found in egg-injected proteolipid protein, (PLP)-immunized SJL mice (38). Our results extend these findings by demonstrating that, although Th1 responses are severely impaired, antigen-specific responses do develop in SMC mice, as demonstrated by the similar production of MOG-induced IL-10 in SMC and MC mice. The maintenance of IL-10 but not of IFN-γ could indicate a development or expansion of Th3 or regulatory T cells. However, this possibility requires further investigation. In addition, we show that macrophage and T-cell infiltration are consistently impaired in SMC mice. Together, these results suggest that an alteration in macrophage responses, as well as in T-cell responses, by schistosomiasis can regulate the course of EAE.
Macrophages have been shown to be essential to the development of clinical EAE, since depletion or deactivation of macrophages inhibits disease development in an adoptive transfer model (27, 43). Because macrophage depletion blocked the invasion of self-reactive T cells, these studies underscore an important role for macrophages in mediating the initial infiltration of inflammatory cells into the brain even in the presence of activated T cells (43). In the present study, we noted an attenuation of EAE by schistosomiasis and decreased CNS inflammation despite the development of MOG-specific T-cell responses. These data suggest that an alteration in macrophage activation by schistosome infection may be contributing to the modulation of clinical disease.
Previous work has shown that schistosomiasis can induce alternative activation of macrophages and impair type 1-associated macrophage effector functions (16, 21, 29). Macrophages isolated from schistosome-infected mice are unable to kill L. major in vitro, even in the presence of high levels of IFN-γ, and this defect is associated with reduced NO production (21). Parasite killing could be induced by in vitro neutralization of IL-10 but not IL-4, suggesting that IL-10 was responsible for suppressing IFN-γ-mediated macrophage functions during schistosomiasis (21). Other recent studies have shown that stimulation of macrophages by helminth products or Fcγ receptor ligation by antigen-antibody complexes leads to alternative activation of macrophages, as evidenced by increased IL-10 production or arginase 1 expression (13, 16, 25). These studies demonstrate that schistosomiasis alters macrophage activation and effector functions and support the idea that the essential role for macrophages in the induction of EAE may be inhibited by schistosomiasis.
Like macrophages, IL-12p40 has been shown to be important in the initiation of EAE (4, 10, 22). In addition, recent work supports a crucial role for IL-23, a heterodimer composed of IL-12p40 and p19, and not the IL-12p70 heterodimer in EAE induction (4, 10). In our studies we found a significant decrease in IL-12p40 transcripts in the spinal cords of SMC mice versus MC mice, and this reduction in IL-12p40 correlates to a decreased infiltration of macrophages into the brains and spinal cords of SMC mice. Moreover, a reduction in the number of IL-12-producing CD11b+ cells in the brains of schistosome egg-injected, PLP-immunized mice has also been reported (38). Taken together, these studies indicate that schistosomiasis leads to a reduction in IL-12p40 during EAE, although these assays, by measuring IL-12p40, cannot distinguish IL-12p70 from IL-23. Given that alternative activation is known to inhibit IL-12p40 production by LPS-stimulated macrophages (41), these results support the idea that schistosomiasis may be inhibiting EAE induction by altering macrophage activation or effector functions.
B cells have been shown to play both a protective and detrimental role in EAE (12, 24, 42). Studies have clearly shown that B cells contribute to the severity of EAE primarily through antibody-mediated demyelination (42). The critical role that antibody plays has been demonstrated by the reconstitution of disease in MOG-immunized B-cell−/− mice treated with serum from rMOG or MOG35-55 peptide-primed mice (23). Although these studies indicate that B cells contribute to immunopathogenesis of EAE by producing autoantibodies, Fillatreau et al. found that B cells can negatively regulate EAE severity through the production of IL-10 (12). Even though our studies may support a role for macrophages in regulating EAE during schistosomiasis, a protective role for B cells in mediating this effect cannot be ruled out and may be involved in the regulation of macrophage activation. Schistosomiasis activates B cells to produce high levels of anti-egg and anti-worm antibodies (7), and macrophages can be alternatively activated through Fcγ receptor ligation (13). Indeed, genetic deletion of the inhibitory Fcγ receptor IIb in mice prevents the development of EAE, suggesting a potential complementary role for both B cells and macrophages in our system (1). In addition, Palanivel et al. have shown that schistosome egg antigens can induce IL-10 production by B-1 cells in the peritoneal cavity, and IL-10 has been shown to downregulate type 1-associated macrophage effector functions during schistosomiasis (21, 32). Therefore, the possibility that B cells play a crucial role in the decreased incidence and delayed onset of EAE by schistosomiasis either directly or by altering macrophage activation merits further investigation.
The negative effect of schistosome infection on the induction and development of EAE demonstrated here indicates that, despite the presence of many confounding factors, the high prevalence of schistosomiasis in certain areas may contribute to the correspondingly low frequency of MS. Although our studies only investigated a direct interaction between these two diseases, it is possible that additional autoimmune diseases can be influenced by schistosomiasis or other chronic parasitic infections. The mechanism by which schistosomiasis can alter EAE induction is still unclear but does not appear to be through a suppression of antigen-specific T-cell responses or immune deviation from Th1 to Th2. Instead, our studies support a regulatory role for macrophages and potentially B cells in mediating CNS inflammation.
Acknowledgments
This work was supported by the National Multiple Sclerosis Society (PP0795) and the Neurological Foundation of New Zealand (0220PG). B.T.B. is the recipient of the Wellcome Trust Senior Research Fellowship in Medical Sciences, New Zealand. Schistosome life cycle stages for this work were supplied through NIH-NIAID contract N01-A1-55270.
We thank Ann Thornton, Joan Nicol, and B. Angell for technical assistance and T. Gatehouse at AgResearch, Wallaceville, New Zealand, for her supply of schistosome eggs. We also thank Brett Delahunt for histological analysis.
Editor: J. M. Mansfield
REFERENCES
- 1.Abdul-Majid, K. B., A. Stefferl, C. Bourquin, H. Lassmann, C. Linington, T. Olsson, S. Kleinau, and R. A. Harris. 2002. Fc receptors are critical for autoimmune inflammatory damage to the central nervous system in experimental autoimmune encephalomyelitis. Scand. J. Immunol. 55:70-81. [DOI] [PubMed] [Google Scholar]
- 2.Arap Siongok, T. K., A. A. Mahmoud, J. H. Ouma, K. S. Warren, A. S. Muller, A. K. Handa, and H. B. Houser. 1976. Morbidity in schistosomiasis mansoni in relation to intensity of infection: study of a community in Machakos, Kenya. Am. J. Trop. Med. Hyg. 25:273-284. [DOI] [PubMed] [Google Scholar]
- 3.Araujo, M. I., A. A. Lopes, M. Medeiros, A. A. Cruz, L. Sousa-Atta, D. Sole, and E. M. Carvalho. 2000. Inverse association between skin response to aeroallergens and Schistosoma mansoni infection. Int. Arch. Allergy Immunol. 123:145-148. [DOI] [PubMed] [Google Scholar]
- 4.Becher, B., B. G. Durell, and R. J. Noelle. 2002. Experimental autoimmune encephalomyelitis and inflammation in the absence of IL-12. J. Clin. Investig. 110:493-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, H. W., and F. A. Neva. 1975. Blood flukes of man, p. 239-252. In Basic clinical parasitology, 4th ed. Appleton-Century-Crofts, New York, N.Y.
- 6.Brunet, L. R., F. D. Finkelman, A. W. Cheever, M. A. Kopf, and E. J. Pearce. 1997. IL-4 protects against TNF-alpha-mediated cachexia and death during acute schistosomiasis. J. Immunol. 159:777-785. [PubMed] [Google Scholar]
- 7.Butterworth, A. E., A. J. Curry, D. W. Dunne, A. J. Fulford, G. Kimani, H. C. Kariuki, R. Klumpp, D. Koech, G. Mbugua, J. H. Ouma, et al. 1994. Immunity and morbidity in human schistosomiasis mansoni. Trop. Geogr. Med. 46:197-208. [PubMed] [Google Scholar]
- 8.Chiaramonte, M. G., L. R. Schopf, T. Y. Neben, A. W. Cheever, D. D. Donaldson, and T. A. Wynn. 1999. IL-13 is a key regulatory cytokine for Th2 cell-mediated pulmonary granuloma formation and IgE responses induced by Schistosoma mansoni eggs. J. Immunol. 162:920-930. [PubMed] [Google Scholar]
- 9.Cooke, A., P. Tonks, F. M. Jones, H. O'Shea, P. Hutchings, A. J. Fulford, and D. W. Dunne. 1999. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 21:169-176. [DOI] [PubMed] [Google Scholar]
- 10.Cua, D., J. Sherlock, Y. Chen, C. A. Murphy, B. Joyce, B. Seymour, L. Lucian, W. To, S. Kwan, T. Churakova, S. Zurawski, M. Wiekowski, S. A. Lira, D. Gorman, R. A. Kastelein, and J. D. Sedgwick. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammmation of the brain. Nature 421:744-748. [DOI] [PubMed]
- 11.Curry, A. J., K. J. Else, F. Jones, A. Bancroft, R. K. Grencis, and D. W. Dunne. 1995. Evidence that cytokine-mediated immune interactions induced by Schistosoma mansoni alter disease outcome in mice concurrently infected with Trichuris muris. J. Exp. Med. 181:769-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fillatreau, S., C. H. Sweenie, M. J. McGeachy, D. Gray, and S. M. Anderton. 2002. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3:944-950. [DOI] [PubMed] [Google Scholar]
- 13.Gerber, J. S., and D. M. Mosser. 2001. Stimulatory and inhibitory signals originating from the macrophage Fcγ receptors. Microbes Infect. 3:131-139. [DOI] [PubMed] [Google Scholar]
- 14.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126:131-138. [DOI] [PubMed] [Google Scholar]
- 15.Grzych, J. M., E. Pearce, A. Cheever, Z. A. Caulada, P. Caspar, S. Heiny, F. Lewis, and A. Sher. 1991. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J. Immunol. 146:1322-1327. [PubMed] [Google Scholar]
- 16.Hesse, M., M. Modolell, A. C. La Flamme, M. Schito, J. M. Fuentes, A. W. Cheever, E. J. Pearce, and T. A. Wynn. 2001. Differential regulation of NOS-2 and arginase-1 by type-1/type-2 cytokines in vivo. Granuloma pathology is shaped by the pattern of l-arginine metabolism. J. Immunol. 167:6533-6544. [DOI] [PubMed] [Google Scholar]
- 17.Hjelmstrom, P., A. E. Juedes, and N. H. Ruddle. 1998. Cytokines and antibodies in myelin oligodendrocyte glycoprotein-induced experimental allergic encephalomyelitis. Res. Immunol. 149:794-804; 847-848; 855-860. [DOI] [PubMed] [Google Scholar]
- 18.Kahana, E. 2000. Epidemiologic studies of multiple sclerosis: a review. Biomed. Pharmacother. 54:100-102. [DOI] [PubMed] [Google Scholar]
- 19.Kuchroo, V. K., R. A. Sobel, J. C. Laning, C. A. Martin, E. Greenfield, M. E. Dorf, and M. B. Lees. 1992. Experimental allergic encephalomyelitis mediated by cloned T cells specific for a synthetic peptide of myelin proteolipid protein: fine specificity and T-cell receptor V beta usage. J. Immunol. 148:3776-3782. [PubMed] [Google Scholar]
- 20.Kurtzke, J. F. 2000. Multiple sclerosis in time and space: geographic clues to cause. J. Neurovirol. 6(Suppl. 2):S134-S140. [PubMed] [Google Scholar]
- 21.La Flamme, A. C., P. Scott, and E. J. Pearce. 2002. Schistosomiasis delays lesion resolution during Leishmania major infection by impairing parasite killing by macrophages. Parasite Immunol. 24:339-345. [DOI] [PubMed] [Google Scholar]
- 22.Leonard, J. P., K. E. Waldburger, and S. J. Goldman. 1995. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J. Exp. Med. 181:381-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyons, J. A., M. J. Ramsbottom, and A. H. Cross. 2002. Critical role of antigen-specific antibody in experimental autoimmune encephalomyelitis induced by recombinant myelin oligodendrocyte glycoprotein. Eur. J. Immunol. 32:1905-1913. [DOI] [PubMed] [Google Scholar]
- 24.Lyons, J. A., M. San, M. P. Happ, and A. H. Cross. 1999. B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by a short encephalitogenic peptide. Eur. J. Immunol. 29:3432-3439. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald, A. S., P. Loke, and J. E. Allen. 1999. Suppressive antigen-presenting cells in helminth infection. Pathobiology. 67:265-268. [DOI] [PubMed] [Google Scholar]
- 26.Marshall, A. J., L. R. Brunet, Y. van Gessel, A. Alcaraz, S. K. Bliss, E. J. Pearce, and E. Y. Denkers. 1999. Toxoplasma gondii and Schistosoma mansoni synergize to promote hepatocyte dysfunction associated with high levels of plasma TNF-alpha and early death in C57BL/6 mice. J. Immunol. 163:2089-2097. [PubMed] [Google Scholar]
- 27.Martiney, J. A., A. J. Rajan, P. C. Charles, A. Cerami, P. C. Ulrich, S. Macphail, K. J. Tracey, and C. F. Brosnan. 1998. Prevention and treatment of experimental autoimmune encephalomyelitis by CNI-1493, a macrophage-deactivating agent. J. Immunol. 160:5588-5595. [PubMed] [Google Scholar]
- 28.Miller, D. H., S. R. Hammond, J. G. McLeod, G. Purdie, and D. C. Skegg. 1990. Multiple sclerosis in Australia and New Zealand: are the determinants genetic or environmental? J. Neurol. Neurosurg. Psychiatry 53:903-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills, C. D., K. Kincaid, J. M. Alt, M. J. Heilman, and A. M. Hill. 2000. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164:6166-6173.10843666 [Google Scholar]
- 30.Nicolson, K., S. Freland, C. Weir, B. Delahunt, R. A. Flavell, and B. T. Backstrom. 2002. Induction of experimental autoimmune encephalomyelitis in the absence of c-Jun N-terminal kinase 2. Int. Immunol. 14:849-856. [DOI] [PubMed] [Google Scholar]
- 31.Overbergh, L., D. Valckx, M. Waer, and C. Mathieu. 1999. Quantification of murine cytokine mRNAs using real-time quantitative reverse transcriptase PCR. Cytokine 11:305-312. [DOI] [PubMed] [Google Scholar]
- 32.Palanivel, V., C. Posey, A. M. Horauf, W. Solbach, W. F. Piessens, and D. A. Harn. 1996. B-cell outgrowth and ligand-specific production of IL-10 correlate with Th2 dominance in certain parasitic diseases. Exp. Parasitol. 84:168-177. [DOI] [PubMed] [Google Scholar]
- 33.Pearce, E. J., A. Cheever, S. Leonard, M. Covalesky, R. Fernandez-Botran, G. Kohler, and M. Kopf. 1996. Schistosoma mansoni in IL-4-deficient mice. Int. Immunol. 8:435-444. [DOI] [PubMed] [Google Scholar]
- 34.Racke, M. K., A. Bonomo, D. E. Scott, B. Cannella, A. Levine, C. S. Raine, E. M. Shevach, and M. Rocken. 1994. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J. Exp. Med. 180:1961-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reed, S. G., C. E. Brownell, D. M. Russo, J. S. Silva, K. H. Grabstein, and P. J. Morrissey. 1994. IL-10 mediates susceptibility to Trypanosoma cruzi infection. J. Immunol. 153:3135-3140. [PubMed] [Google Scholar]
- 36.Schmittgen, T. D., and B. A. Zakrajsek. 2000. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J. Biochem. Biophys. Methods 46:69-81. [DOI] [PubMed] [Google Scholar]
- 37.Selmaj, K., C. S. Raine, B. Cannella, and C. F. Brosnan. 1991. Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J. Clin. Investig. 87:949-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sewell, D., Z. Qing, E. Reinke, D. Elliot, J. Weinstock, M. Sandor, and Z. Fabry. 2003. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int. Immunol. 15:59-69. [DOI] [PubMed] [Google Scholar]
- 39.Shaw, M. K., J. B. Lorens, A. Dhawan, R. DalCanto, H. Y. Tse, A. B. Tran, C. Bonpane, S. L. Eswaran, S. Brocke, N. Sarvetnick, L. Steinman, G. P. Nolan, and C. G. Fathman. 1997. Local delivery of interleukin 4 by retrovirus-transduced T lymphocytes ameliorates experimental autoimmune encephalomyelitis. J. Exp. Med. 185:1711-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinman, L. 1999. Assessment of animal models for MS and demyelinating disease in the design of rational therapy. Neuron 24:511-514. [DOI] [PubMed] [Google Scholar]
- 41.Sutterwala, F. A., G. J. Noel, R. Clynes, and D. M. Mosser. 1997. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J. Exp. Med. 185:1977-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svensson, L., K. B. Abdul-Majid, J. Bauer, H. Lassmann, R. A. Harris, and R. Holmdahl. 2002. A comparative analysis of B cell-mediated myelin oligodendrocyte glycoprotein-experimental autoimmune encephalomyelitis pathogenesis in B cell-deficient mice reveals an effect on demyelination. Eur. J. Immunol. 32:1939-1946. [DOI] [PubMed] [Google Scholar]
- 43.Tran, E. H., K. Hoekstra, N. van Rooijen, C. D. Dijkstra, and T. Owens. 1998. Immune invasion of the central nervous system parenchyma and experimental allergic encephalomyelitis, but not leukocyte extravasation from blood, are prevented in macrophage-depleted mice. J. Immunol. 161:3767-3775. [PubMed] [Google Scholar]
- 44.Xia, D., A. Sanders, M. Shah, A. Bickerstaff, and C. Orosz. 2001. Real-time polymerase chain reaction analysis reveals an evolution of cytokine mRNA production in allograft acceptor mice. Transplantation 72:907-914. [DOI] [PubMed] [Google Scholar]