Abstract
A simple alfalfa model was developed as an alternative infection model for virulence studies of the Burkholderia cepacia complex. Symptoms of disease were observed in wounded alfalfa seedlings within 7 days following inoculation of 101 to 105 CFU of most strains of the B. cepacia complex. Strains from seven genomovars of the B. cepacia complex were tested for virulence in the alfalfa model, and the degree of virulence was generally similar in strains belonging to the same genomovar. Strains of Burkholderia multivorans and some strains of Burkholderia stabilis did not cause symptoms of disease in alfalfa seedlings. Representative strains were also tested for virulence using the rat agar bead model. Most of the strains tested were able to establish chronic lung infections; B. stabilis strains were the exception. Most of the strains that were virulent in the alfalfa infection model were also virulent in the lung infection model. The B. cepacia genomovar III mutants K56pvdA::tp and K56-H15 were significantly less virulent in the alfalfa infection model than their parent strain. Therefore, this alfalfa infection model may be a useful tool for assessing virulence of strains of the B. cepacia complex and identifying new virulence-associated genes.
Burkholderia cepacia is a gram-negative bacillus commonly found in soil, vegetation, and water (10, 13). B. cepacia was originally described as a phytopathogen causing soft rot in onions by Burkholder in 1950 (2). Individual strains of B. cepacia may have the ability to protect plants from disease, cause plant disease, degrade environmental pollutants, or cause nosocomial infections (23). B. cepacia has emerged as an important opportunistic pathogen in immunocompromised patients including those with cystic fibrosis (CF) and chronic granulomatous disease (8, 10). Furthermore, patient-to-patient transmission of virulent strains (16) and multiple antibiotic resistance of B. cepacia (31) are significant concerns within the CF research community. Pulmonary infection with B. cepacia can lead to three different outcomes: long-term asymptomatic colonization, chronic colonization with a progressive pulmonary decline, and a rapid pulmonary decline with necrotizing pneumonia, fever, and occasional septicemia often referred to as cepacia syndrome (23).
Recently, B. cepacia has been classified into nine genotypically distinct but phenotypically similar species (genomovars) forming the B. cepacia complex, all of which may infect CF patients. The nine species of the B. cepacia complex are as follows: B. cepacia genomovar I, Burkholderia multivorans (formerly genomovar II), B. cepacia genomovar III, Burkholderia stabilis (formerly genomovar IV), Burkholderia vietnamiensis (formerly genomovar V) (41), B. cepacia genomovar VI (6), Burkholderia ambifaria (formerly genomovar VII) (7), Burkholderia anthina (40), and Burkholderia pyrrocinia (8). In Canada, approximately 80% of the B. cepacia complex strains that colonize CF patients belong to genomovar III, which has generally been associated with a high rate of mortality and morbidity (36). In the United States, approximately 50% of CF isolates belong to genomovar III, and 38% belong to B. multivorans (15).
B. cepacia secretes extracellular virulence factors in vitro, including hemolysin, proteases, lipase, pili, and siderophores (10). The roles of only a few of these virulence factors have been examined in animal infection models (28, 32-24). Studying virulence in in vivo animal models is often complex, time-consuming, and expensive. Because of these limitations, alternative host models have been developed for some important human pathogens. For example, a multihost pathogenesis system has been used to identify virulence-associated genes in Pseudomonas aeruginosa. Alternative host models used include Arabidopsis thaliana (24-26), Caenorhabditis elegans (38, 39), Drosophila melanogaster (9), and Galleria mellonella (14). By using this multihost pathogenesis system, some virulence-associated genes, such as gacA, lasR, ptsP, and phzB, have been shown to be important both in nonmammalian hosts and in the mouse burn infection model (18). Recently, a new, simple, and inexpensive plant model has been developed in alfalfa for P. aeruginosa (30).
Although appropriate animal models are currently used for studying B. cepacia complex chronic respiratory infections (5, 27, 32, 33), alternative infection models for preliminary analysis of virulence of the B. cepacia complex have not yet been described. The objectives of this study were to determine whether the B. cepacia complex was able to cause infection in alfalfa and to determine whether an alfalfa model could be used to differentiate genomovars of the B. cepacia complex. We also compared virulence in the alfalfa model and the well-established rat agar bead chronic infection model (3).
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The strains of the B. cepacia complex used in this study are described in Table 1. Most of the strains are from the experimental panel of the B. cepacia complex (19). All strains in this study were stored in 10% skim milk (BBL, Sparks, Md.) at −70°C. The strains were streaked from frozen stocks onto Luria-Bertani (LB) (Invitrogen, Burlington, Ontario, Canada) agar plates and incubated at 37°C for 24 to 48 h. For plant inoculation, cultures grown in LB medium overnight were diluted (1:25) with 12-ml portions of fresh LB medium in 125-ml Erlenmeyer flasks and incubated at 37°C with shaking to an approximate optical density at 600 nm of 2.0. For animal experiments, cultures were grown in dialyzed and chelated Trypticase soy broth (22) medium overnight.
TABLE 1.
Comparison of the virulence of strains of the B. cepacia complex in an alfalfa infection model
| Strain | Source and locationa | % Seedlings with symptomsb | No. of CFU (107) recovered/seedlingc | Reference |
|---|---|---|---|---|
| B. cepacia genomovar I | ||||
| ATCC 25416 | Onion, US | 73 ± 14 | 5.5 ± 3.6 | 19 |
| ATCC 17759 | Soil, Trinidad | 73 ± 13 | 15.9 ± 14 | 19 |
| Cep509 | CF, Australia | 85 ± 18 | 8.9 ± 8.1 | 19 |
| B. multivorans | ||||
| ATCC 17616 | Soil, US | 0 | 1.1 ± 0.6 | 19 |
| C5393 | CF, Canada | 0 | 1.3 ± 0.2 | 19 |
| C1576 | CF-e, UK | 0 | 1.3 ± 0.8 | 19 |
| LMG 13010 | CF, Belgium | 0 | 0.5 ± 0.4 | 19 |
| CF-A1-1 | CF-e, UK | 0 | 0.8 ± 0.6 | 19 |
| JTC | CGD, US | 0 | 1.0 ± 0.7 | 19 |
| C1962 | Clinical, UK | 0 | 1.2 ± 0.8 | 19 |
| B. cepacia genomovar III | ||||
| K56-2 | CF-e, Canada | 100 ± 0 | 8.9 ± 3.6 | 19 |
| ATCC 17765 | UTI, UK | 32 ± 13 | 8.1 ± 6.6 | 19 |
| PC715j | CF, Canada | 80 ± 18 | 8.8 ± 3.5 | 21 |
| J2315 | CF-e, UK | 65 ± 10 | 3.9 ± 5.1 | 19 |
| BC7 | CF-e, Canada | 65 ± 13 | 1.8 ± 2.6 | 19 |
| Cep511 | CF-e, Australia | 98 ± 3 | 12 ± 12 | 19 |
| PC184 | CF, US | 22 ± 13 | 3.4 ± 2.5 | 19 |
| J415 | CF, UK | 53 ± 3 | 4.0 ± 4.3 | 19 |
| B. stabilis | ||||
| LMG 14086 | Respirator, UK | 0 | 0.7 ± 0.4 | 19 |
| LMG 18888 | Clinical, Belgium | 0 | 0.7 ± 0.5 | 19 |
| LMG 14294 | CF, Belgium | 13 ± 12 | 0.9 ± 0.5 | 19 |
| C7322 | CF, Canada | 10 ± 17 | 0.3 ± 0.2 | 19 |
| B. vietnamiensis | ||||
| PC259 | CF, US | 7 ± 6 | 0.8 ± 0.7 | 19 |
| LMG 16232 | CF, Sweden | 33 ± 10 | 12 ± 12 | 19 |
| FC441 | CGD, Canada | 68 ± 8 | 23 ± 10 | 19 |
| LMG 10929 | Rice, Vietnam | 42 ± 16 | 10 ± 6 | 19 |
| B. cepacia genomovar VI | ||||
| LO6 | CF | 53 ± 24 | 13 ± 11 | 6 |
| LMG 18943 | CF, US | 25 ± 0 | 1.7 ± 1.1 | 6 |
| B. ambifaria | ||||
| ATCC 53266 | Corn roots, biocontrol, US | 63 ± 20 | 4.7 ± 1.9 | 7 |
| AMMD | Pea rhizosphere, biocontrol, US | 65 ± 20 | 7.2 ± 2.5 | 7 |
| Cep996 | CF, Australia | 53 ± 16 | 4.3 ± 3.3 | 7 |
Abbreviations: CF, infection of a CF patient; CF-e, strain that spread epidemically among patients with CF; CGD, infection of a patient with chronic granulomatous disease; UTI, patient with a urinary tract infection; US, United States; UK, United Kingdom.
Values are means ± standard deviations of three assays (20 seedlings/assay). The starting inoculum was between 1 × 105 and 3 × 105 CFU for all experiments. Any seedlings with visible symptoms including yellow leaves, stunted root, and brown necrotic regions were considered positive for symptoms of disease.
Values are means ± standard deviations of two assays (three seedlings/assay).
Alfalfa infection assay by strains of the B. cepacia complex.
Alfalfa seeds (variety 57Q77) were provided by Pioneer Hi-Bred International, Inc. (Johnston, Iowa). Alfalfa seeds were prepared as previously described (30). To disinfect and accelerate germination, they were immersed in concentrated sulfuric acid (approximately 10 ml for 300 seeds) for 20 min and then washed with 500 ml of distilled water (dH2O) four times. The seeds were covered with 60 ml of sterile dH2O in a 125-ml Erlenmeyer flask and incubated at 32°C with shaking for 6 to 8 h to encourage uniform imbibition and germination (11). The seeds were rinsed twice with 60 ml of sterile dH2O and incubated overnight in 60 ml of sterile dH2O at 32°C with shaking. The following day, the seedlings (10 per plate) were placed with sterile forceps onto the surface of water agar (deionized water solubilized with 1% Difco Bacto Agar and 1% Difco Noble agar).
Within 1 h, one leaf of each seedling was wounded by piercing the leaf with a 20-gauge needle. Immediately after the leaves were wounded, the seedlings were surface inoculated with 10-μl aliquots of diluted bacterial cells. The cultures used for the inoculum were serially diluted in 0.85% NaCl, and aliquots were plated onto LB agar plates for quantitation. Controls included seedlings wounded and inoculated with 10 μl of 0.85% NaCl and untreated seedlings. The petri plates containing seedlings were sealed with parafilm in order to maintain a high level of humidity and incubated in a 37°C warm room under a desk lamp with an average of 8 to 12 h of artificial light per day at an intensity of 640 lx. The seedlings were visually monitored for disease symptoms at 7 days postinfection (p.i.). Symptoms, including yellow leaves, stunted roots, and brown necrotic regions on the seedlings, were considered disease symptoms. At least 20 seedlings were used per bacterial inoculum.
Recovery of bacteria from infected alfalfa seedlings.
For each strain, six seedlings (three seedlings/assay) were homogenized in a Kontes tissue grinder in 1 ml of 0.85% NaCl. The resulting suspension was serially diluted in 0.85% NaCl and plated onto LB agar to determine the number of CFU per seedling. Seedlings with disease symptoms were randomly selected for bacterial quantitation except for seedlings inoculated with strains that did not cause symptoms of disease.
Animal studies.
Groups of nine male Sprague-Dawley rats (150 to 175 g) (Charles River Canada, Inc.) were given tracheostomies under anesthesia and inoculated with approximately 104 CFU of the appropriate strain embedded in agar beads as previously described (3). On days 7 and 21 p.i., the lungs from three animals from each group were removed aseptically and homogenized (Polytron homogenizer; Brinkman Instruments, Westbury, N.Y.) in 3 ml of phosphate-buffered saline (PBS) (10 mM sodium phosphate, 150 mM NaCl [pH 7.5]). The homogenates were serially diluted in PBS and plated on Trypticase soy agar and Burkholderia cepacia isolation agar (12). On day 7 p.i., the lungs of three additional animals from each group were removed en bloc, fixed in 10% formalin, and examined for qualitative and quantitative pathological changes. Infiltration of the lung with inflammatory cells and exudate was measured using a point counting method as previously described (35) with the following modifications. The lung sections were scanned using an Epson 1650 scanner, and areas of inflammation were digitized with Scion Image software and reported as the percentage of lung inflammation.
Statistical analyses.
Analysis of variance (ANOVA) and linear regression were performed with INSTAT software (GraphPad Software, San Diego, Calif.). A P value of <0.05 was considered statistically significant.
RESULTS
Ability of B. cepacia strain K56-2 to infect wounded alfalfa.
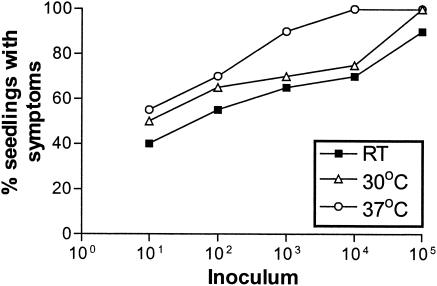
To determine whether B. cepacia strain K56-2 could cause infections in alfalfa, wounded plant seedlings were inoculated with doses of bacteria ranging from 101 to 105 CFU and were examined for symptoms. Seedlings infected with strain K56-2 had symptoms of disease that were clearly visible 7 days p.i. (Fig. 1B). At higher inoculations, 90% of the infected seedlings displayed symptoms of disease, including yellowing of the leaves and root necrosis when incubated at room temperature. Infection with as few as 101 CFU resulted in 40% of seedlings displaying symptoms (Fig. 2).
FIG. 1.
Effect of temperature on visual symptoms of pathology on alfalfa. Photographs of wounded alfalfa seedlings after inoculation with 105 CFU of B. cepacia K56-2 are shown. Seedlings were incubated at 30°C (A) and 37°C (B). In each panel, the seedling on the left is the negative control (inoculated with saline), and the seedling on the right was inoculated with K56-2.
FIG. 2.
Effects of temperature and inoculum on alfalfa infections. Groups of 20 seedlings were inoculated with doses of B. cepacia K56-2 ranging from 101 to 105 CFU and incubated at three different temperatures: room temperature (RT), 30°C, and 37°C. The results shown are the percentages of seedlings with disease symptoms visible on day 7 p.i.
Effects of incubation temperature and inoculum dose on disease symptoms.
To determine whether the incubation temperature of the seedlings affected disease symptoms, plant seedlings were infected with 101 to 105 CFU of B. cepacia strain K56-2 and incubated at room temperature, 30°C, and 37°C and examined for symptoms 7 days p.i. Both the inoculum dose and incubation temperature affected the percentage of seedlings developing symptoms of disease (Fig. 2). Incubation of the seedlings at 37°C resulted in a greater percentage of seedlings with disease at most inoculum doses (Fig. 2). These infected seedlings were smaller, with smaller yellow leaves, and stunted roots than those incubated at 30°C. There was also evidence of brown necrotic regions on the seedlings (Fig. 1). In subsequent experiments, seedlings were inoculated with 105 CFU and incubated at 37°C.
Ability of strains and genomovars of the B. cepacia complex to cause disease symptoms in alfalfa.
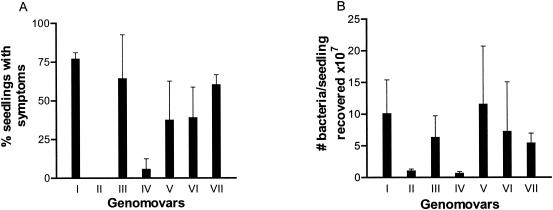
Thirty-one strains from seven different genomovars of the B. cepacia complex were tested for their ability to cause disease in alfalfa. The percentages of seedlings with disease symptoms visible on day 7 p.i. for the strains are shown in Table 1. Although strain variability was noted within genomovars, the ability to cause disease symptoms in alfalfa generally was similar in the strains of a genomovar. B. cepacia genomovars I and III appeared to be the most virulent in the alfalfa infection model, whereas B. multivorans (genomovar II) and some strains of B. stabilis (genomovar IV) were avirulent (Table 1 and Fig. 3A). As shown in Fig. 3A, the mean percentage of seedlings with symptoms for all strains in B. cepacia genomovar I was 77% compared to 0% for B. multivorans (P < 0.001 by ANOVA) and 8% for all B. stabilis strains (P < 0.001 by ANOVA). B. cepacia genomovar III strains caused symptoms in 64% of seedlings and were also significantly more virulent than B. multivorans and B. stabilis (P < 0.001 by ANOVA). B. ambifaria (genomovar VII), which caused symptoms in 60% of alfalfa sprouts, was also more virulent than B. multivorans (P < 0.01 by ANOVA) and B. stabilis (P < 0.05 by ANOVA). B. vietnamiensis and B. cepacia genomovar VI were able to cause disease symptoms in alfalfa.
FIG. 3.
Comparison of the ability of B. cepacia complex genomovars to cause infections in alfalfa. Seedlings were inoculated with 105 CFU of the strains shown in Table 1 and incubated at 37°C for 7 days. Data from Table 1 for the percentage of seedlings with symptoms of disease for each genomovar (A) and for the number of bacteria recovered (107) from individual homogenized alfalfa sprouts for each genomovar (B) are summarized. The means ± standard deviations (error bars) of the values in Table 1 are shown.
Interestingly, strains of B. multivorans and B. stabilis, with the exception of LMG 14294 and C7322, did not cause disease in the alfalfa model. There was no difference between seedlings infected with the other strains of these two genomovars and the negative-control seedlings.
Ability of strains of the B. cepacia complex to grow on wounded alfalfa seedlings.
To determine whether the difference in the ability to cause disease in alfalfa in strains of the different genomovars was due to differences in the ability of the strains to grow on the alfalfa seedlings, the number of bacteria present on infected seedlings was determined on day 7 p.i. (Table 1). For all strains tested, the number of bacteria recovered was at least 10-fold higher than the inoculum, indicating that all strains were able to grow on alfalfa. B. cepacia complex was able to survive on the water agar medium without seedlings but was not able to grow (data not shown). Strains of B. multivorans and B. stabilis grew poorly, generally increasing by only 10- to 100-fold, whereas strains of the other genomovars increased by 100- to 1,000-fold (Table 1 and Fig. 3B). The reduced growth of B. stabilis and B. multivorans could account for their inability to cause disease symptoms, since there was a correlation between the number of bacteria recovered and the percentage of seedlings with symptoms in all of the strains examined (r = 0.666 by linear regression; P < 0.0001).
Comparison of B. cepacia complex strain virulence in the alfalfa infection model versus the rat agar bead infection model.
Rats were infected with agar beads containing selected B. cepacia complex strains based on differences in their virulence properties in alfalfa. Quantitative bacteriology was performed on days 7 and 21 p.i. on lungs removed from infected animals, and quantitative histopathological analysis was performed on day 7 p.i. (Table 2). With the exception of B. stabilis, all the strains tested were able to establish a chronic infection (Table 2). Quantitative bacteriological analysis demonstrated that strains of B. cepacia genomovar I, B. multivorans, B. cepacia genomovar III, and B. vietnamiensis persisted in the lungs for at least 21 days. Genomovar III strains K56-2 and Pc715j have been previously shown to persist for at least 28 days (32, 34) and therefore were not tested in this study. B. stabilis strain LMG 14086 cleared within 21 days and strain LMG 14294 cleared by 7 days p.i., suggesting that these strains are not able to establish persistent infections in the agar bead infection model.
TABLE 2.
Comparison of the virulence of strains of the B. cepacia complex in a chronic respiratory infection model
| Strain | Virulence (CFU/ml/lung)a
|
% Pathology on day 7 p.i.a | |
|---|---|---|---|
| Day 7 p.i. | Day 21 p.i. | ||
| B. cepacia genomovar I | |||
| Cep509 | 8.1 × 105 ± 6.4 × 105 | 1.2 × 105 ± 1.4 × 105b | 43.0 ± 2.0 |
| B. multivorans | |||
| C5393 | 1.3 × 106 ± 1.4 × 106 | 3.7 × 105 ± 1.5 × 105 | 13.3 ± 5.9 |
| C1576 | 1.2 × 105 ± 1.7 × 105 | 4.5 × 104 ± 4.6 × 104 | 9.0 ± 4.6 |
| B. cepacia genomovar III | |||
| K56-2 | 1.5 × 106 ± 2.4 × 106e | ND | 40.5 ± 4.2e |
| PC715j | 3.5 × 105 ± 3.4 × 105f | ND | 22.8 ± 10.8f |
| J2315 | 4.6 × 103 ± 7.8 × 103 | 5.0 × 102c | 38.0 ± 21.0 |
| Cep511 | 2.7 × 105 ± 2.2 × 105 | 3.3 × 103 ± 5.1 × 103 | 12.7 ± 1.53 |
| J415 | 3.1 × 105 ± 5.2 × 105 | 6.5 × 103 ± 1.2 × 104 | 13.0 ± 1.73 |
| B. stabilis | |||
| LMG 14086 | 3.3 × 102 ± 2.9 × 102 | 0 | 20.0 ± 1.7 |
| LMG 14294 | 0 | 0 | 16.0 ± 5.6 |
| B. vietnamiensis | |||
| PC259 | 1.2 × 104 ± 2.0 × 104 | 2.0 × 102 ± 1.7 × 102 | 19.7 ± 0.6 |
| FC441 | 8.5 × 102 ± 6.6 × 102 | 7.1 × 103 ± 9.3 × 103d | 29.0 ± 1.0 |
Values are means ± standard deviations for three animals unless noted otherwise. ND, not determine
Values are mean ± standard deviation for four animals.
Values are for one animal (one animal died on day 1 p.i., and one animal died on day 2 p.i.).
Values are mean ± standard deviation for five animals.
Data previously reported by Sokol et al. (32).
Values are means ± standard deviations for eight animals in the experiment previously described by Sokol et al. (32).
Most strains of B. cepacia genomovars I and III and B. vietnamiensis tested were virulent in this animal model, as determined by the percentage of the lungs infiltrated with inflammatory exudates. B. cepacia strains Cep509, K56-2, and J2315 were the most virulent strains tested in this animal model (Table 2). Two rats infected with strain J2315 died within 48 h p.i. Generally, infections with strains of B. multivorans and B. stabilis resulted in less lung pathology than infections with strains belonging to the other genomovars tested (Table 2). A correlation between virulence in the alfalfa infection model and virulence in the rat agar bead model was demonstrated, as measured by changes in lung histopathology (r = 0.5913 by linear regression; P < 0.05). Genomovar III strain Cep511 was an exception, since it was one of the less virulent strains in terms of lung histopathology yet caused disease symptoms in 98% of the alfalfa seedlings.
Ability of B. cepacia complex strain K56-2 mutants to cause disease in the alfalfa model.
To determine whether the alfalfa model might be useful for assessing the virulence of B. cepacia complex mutants, the ability of selected mutants of strain K56-2 to cause disease symptoms in this model was examined. As shown in Table 3, K56pvdA::tp, which contains a deletion in a biosynthetic gene for production of the siderophore ornibactin (33), was significantly less virulent than K56-2. This mutant was able to grow on the wounded alfalfa but caused symptoms of disease in fewer plants. Interestingly, K56orbA::tp, which contains a mutation in the ornibactin receptor gene (32) was as virulent as K56-2 in this model. K56-2-9, which has a mutation in an extracellular zinc metalloprotease gene (8a), was also virulent in this model. K56-H15, a Tn5-OT182 insertion mutant, did not cause symptoms of disease in the alfalfa model and also did not grow as well as the parent strain on the wounded seedlings. Results with these mutant strains suggest that the alfalfa model may also be a useful tool in assessing the virulence properties of genetic mutants.
TABLE 3.
Virulence of B. cepacia K56-2 mutants in the alfalfa model
| Strain | Gene mutated | % Seedlings with symptomsa | No. of CFU (107) recovered/seedlingb | Reference |
|---|---|---|---|---|
| K56-2 (wild type) | 100 | 16 ± 6.4c | 19 | |
| K56pvdA::tp | Ornibactin biosynthesis | 30 ± 5d | 1.5 | 33 |
| K56orbA::tp | Ornibactin receptor | 98 ± 3 | 8.3 | 32 |
| K56-2-9 | Zinc metalloprotease | 100 | 6.5 | 8a |
| K56-H15 | Tn5-OT182 insertion | 3 ± 6d | 0.29 ± 0.2c,e | Unpublished data |
Values are means ± standard deviations of three assays (20 seedlings/assay). The starting inoculum was between 1 × 105 and 3 × 105 CFU. Any seedlings with visible symptoms including yellow leaves, stunted root, and brown necrotic regions were considered positive for symptoms of disease.
Values are means of one assay (two seedlings/assay) unless noted otherwise.
Values are means ± standard deviations of two assays (two seedlings/assay).
Significantly different from the value for K56-2 (P < 0.001 by ANOVA).
Significantly different from the value for K56-2 (P < 0.05 by ANOVA).
DISCUSSION
It has been known for decades that B. cepacia is a natural phytopathogen (2) and an opportunistic pathogen for CF patients (8, 10). Although several animal infection models have been used to study the virulence of strains of the B. cepacia complex (4, 5, 28, 32-24), this study describes a simple plant infection model that may have applications as an alternative model.
Silo-Suh et al. have used the wounded alfalfa model for P. aeruginosa. P. aeruginosa caused chlorosis symptoms, water-soaked lesions, and complete maceration of tissue at room temperature (30). The symptoms observed with B. cepacia complex infections were slightly different than those observed in P. aeruginosa infections. B. cepacia caused necrosis, evident by brown areas on the seedling, lack of root hair, stunting of root growth, and chlorosis, which is a yellowing of leaf tissue due to a lack of chlorophyll. P. aeruginosa infections appeared to remain localized to the leaves and resulted in a greater maceration of tissue. B. cepacia complex strain K56-2 exhibited similar symptoms of disease, whether or not the leaves were wounded prior to infection (data not shown). B. cepacia complex strain K56-2 and other B. cepacia complex strains were determined to be more virulent in seedlings incubated at 37°C than at room temperature. Previously, it was shown that the sour skin disease on onions caused by B. cepacia was most damaging at temperatures above 30°C (23). These studies suggest that there may be a difference in B. cepacia complex virulence factor expression between 37°C and lower temperatures.
The Arabidopsis infection model has been a successful tool to find virulence-associated genes in P. aeruginosa (18, 24-26). Although the virulence of the B. cepacia complex was not tested in the Arabidopsis model in this study, the simplicity of the alfalfa model offers several advantages over the Arabidopsis model. Studies with Arabidopsis are routinely performed using 3- to 8-week-old plants (24) and require special incubators and considerable space for plant growth. Results can be obtained from the alfalfa model within 9 days, and no special equipment is necessary. Most strains of B. cepacia complex were able to establish an infection in alfalfa, whereas only a few strains of P. aeruginosa, UCBPP-PA14 and UCBPP-PA29, were able to infect Arabidopsis leaves (25). Many strains of P. aeruginosa can also infect alfalfa (30); therefore, the alfalfa model applications are not limited to only a few strains. Although the Arabidopsis model has been very useful for assessing virulence factors in some strains of P. aeruginosa, the alfalfa model may be useful in studying the virulence of strains of several genomovars in the B. cepacia complex, with the possible exceptions of B. multivorans and B. stabilis. One advantage of the Arabidopsis model over the alfalfa model is that the Arabidopsis genome sequence is available, making it possible to investigate the effects of host genes on infection (1, 37). Because alfalfa cannot be easily manipulated genetically, it would be interesting to determine whether B. cepacia complex caused similar infections in seedlings of the related model legume Medicago truncatula (the M. truncatula genome sequence is being determined [15]).
The ability of bacteria to grow on alfalfa seedlings correlated with their ability to cause disease symptoms (Fig. 3). Less bacteria were recovered from seedlings infected with B. multivorans or B. stabilis strains than from seedlings infected with the other genomovars, and infection with these strains rarely resulted in disease symptoms. There were differences in the percentage of seedlings displaying symptoms in strains within a genomovar, suggesting that there is variability among strains of the same species.
For strains tested in both the alfalfa and rat agar bead models, there was a general correlation in virulence for the two infection models. B. stabilis was generally avirulent in both models. B. multivorans was able to persist in the lung but did not result in severe symptoms of lung pathology and also did not cause disease symptoms in alfalfa. Previously, the agar bead model has primarily been used for B. cepacia genomovar III strains, but this study shows that strains from five genomovars (genomovars I to V) can establish lung infections in this model, although B. stabilis strains appear to be cleared rapidly (Table 2). These observations correlate with those reported by LiPuma et al. (17), which demonstrate that all B. cepacia complex species (genomovars I to VII) have been found to infect CF patients. These results suggest that the agar bead model may be useful for virulence analysis in many genomovars of the B. cepacia complex.
The most virulent strains tested in either the alfalfa or rat agar bead infection model belonged to B. cepacia genomovar I or III (Fig. 3A and Table 1 and 2). Interestingly, B. cepacia genomovar III strains were also found to cause greater lung pathology than B. multivorans in a leukopenic mouse model (4). In the leukopenic mouse model, however, all strains of B. cepacia genomovar III cleared within 16 days, whereas genomovar III strains persisted in the agar bead lung infection model for at least 21 days p.i. The establishment of a persistent chronic infection by genomovar III strains in the agar bead model is more consistent with the clinical profile of patients infected with these strains. BC7, a genomovar III CF isolate, was shown to persist in the lungs of Cftr−/− mice for at least 9 days and result in lung histopathological changes (27). Genomovar III strains were also shown to be invasive in a mouse agar bead model and an A549 cell invasion assay (4). Preliminary data suggest an association between poorer clinical outcome and infection with strains of B. cepacia genomovar III versus B. multivorans (20).
Strains of B. multivorans, two strains of B. stabilis, and B. vietnamiensis PC259 were avirulent in the alfalfa model. B. stabilis strains cleared from the lungs in both the rat agar bead model and the leukopenic mouse model (4), suggesting that in contrast to genomovar III strains, a correlation exists between all three models for B. stabilis. Using well-differentiated airway epithelial cell cultures, Schwab et al. (29) demonstrated that unlike other species of the B. cepacia complex, B. stabilis does not produce biofilms. A B. stabilis strain was able to penetrate the epithelium, but the majority of bacteria were located between epithelial cells (29). In contrast, genomovar III strains formed biofilms, which subsequently invaded and destroyed epithelial cells (29). The inability to form biofilms or the reduced ability to disrupt the actin cytoskeleton may account for the reduced virulence of B. stabilis in lung infection models.
Although B. cepacia genomovar I strains are not isolated often from CF patients (17, 20), their virulence in both the alfalfa and agar bead models was comparable to that of genomovar III strains (Fig. 3A and Table 2). Genomovar I strains were not examined in the leukopenic mouse model or the human airway epithelial cell invasion assays (4, 29).
The B. cepacia mutants K56pvdA::tp, K56orbA::tp, and K56-2-9 were previously reported to be less virulent than K56-2 in the agar bead model, as measured by decreased persistence of bacteria in the lung and decreased lung histopathological changes (8a, 32, 33). In the alfalfa infection host model, only K56pvdA::tp and K56-H15 were less virulent than the wild-type strain (Table 3). The difference in the virulence of the K56pvdA::tp and K56orbA::tp mutants on alfalfa may be explained by their different capabilities to utilize siderophore-mediated iron uptake systems. Both ferric-salicylic acid uptake and ferric-ornibactin uptake are defective in K56pvdA::tp, whereas only ferric-ornibactin uptake is defective in K56orbA::tp (32, 33). It is also possible that ornibactin has a direct effect on virulence in addition to its role in iron acquisition. The mutation responsible for the decrease in virulence in K56-H15 is currently under investigation. It will be interesting to determine the nature of this mutation and whether this mutant also has reduced virulence in the lung infection model.
In summary, we have developed an alfalfa infection model for B. cepacia complex strains. Of 31 strains tested, 21 strains belonging to seven different genomovars caused disease symptoms in alfalfa. Strains that were virulent in the alfalfa model were generally virulent in the rat agar bead model. Therefore, this alternative model may potentially be used to assess the virulence of strains, to identify new virulence-associated genes in B. cepacia complex strains, and to identify common virulence factors in both plant and animal infections.
Acknowledgments
This study was supported in part by the Canadian Institutes of Health Research (PAS), Public Health Service grant AI-19146 (D.E.O.) from the National Institute of Allergy and Infectious Diseases, and Veterans Administration Medical Research Funds (D.E.O.).
We thank E. Mahenthiralingam for providing the majority of the strains used in this study; Pioneer Hi-Bred International, Inc., for providing the alfalfa seeds; and Brant Pohorelic for assistance with the animal experiments.
Editor: V. J. DiRita
REFERENCES
- 1.Bevan, M., K. Mayer, O. White, J. A. Eisen, D. Preuss, T. Bureau, S. L. Salzberg, and H. W. Mewes. 2001. Sequence and analysis of the Arabidopsis genome. Curr. Opin. Plant Biol. 4:105-110. [DOI] [PubMed] [Google Scholar]
- 2.Burkholder, W. H. 1950. Sour skin, a bacterial rot of onion bulbs. Phytopathology 40:115-117. [Google Scholar]
- 3.Cash, H. A., D. E. Woods, B. McCullough, W. G. Johanson, Jr., and J. A. Bass. 1979. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am. Rev. Respir. Dis. 119:453-459. [DOI] [PubMed] [Google Scholar]
- 4.Chu, K. K., D. J. Davidson, T. K. Halsey, J. W. Chung, and D. P. Speert. 2002. Differential persistence among genomovars of the Burkholderia cepacia complex in a murine model of pulmonary infection. Infect. Immun. 70:2715-2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cieri, M. V., N. Mayer-Hamblett, A. Griffith, and J. L. Burns. 2002. Correlation between an in vitro invasion assay and a murine model of Burkholderia cepacia lung infection. Infect. Immun. 70:1081-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coenye, T., J. J. LiPuma, D. Henry, B. Hoste, K. Vandemeulebroecke, M. Gillis, D. P. Speert, and P. Vandamme. 2001. Burkholderia cepacia genomovar VI, a new member of the Burkholderia cepacia complex isolated from cystic fibrosis patients. Int. J. Syst. E vol. Microbiol. 51:271-279. [DOI] [PubMed] [Google Scholar]
- 7.Coenye, T., E. Mahenthiralingam, D. Henry, J. J. LiPuma, S. Laevens, M. Gillis, D. P. Speert, and P. Vandamme. 2001. Burkholderia ambifaria sp. nov., a novel member of the Burkholderia cepacia complex including biocontrol and cystic fibrosis-related isolates. Int. J. Syst. E vol. Microbiol. 51:1481-1490. [DOI] [PubMed] [Google Scholar]
- 8.Coenye, T., P. Vandamme, J. R. Govan, and J. J. LiPuma. 2001. Taxonomy and identification of the Burkholderia cepacia complex. J. Clin. Microbiol. 39:3427-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Corbett, C. R., M. N. Burtnick, C. Kooi, D. E. Woods, and P. A. Sokol. 2003. An extracellular zinc metalloprotease gene of Burkholderia cepacia. Microbiology 149:2263-2271. [DOI] [PubMed]
- 9.D'Argenio, D. A., L. A. Gallagher, C. A. Berg, and C. Manoil. 2001. Drosophila as a model host for Pseudomonas aeruginosa infection. J. Bacteriol. 183:1466-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Govan, J. R., J. E. Hughes, and P. Vandamme. 1996. Burkholderia cepacia: medical, taxonomic and ecological issues. J. Med. Microbiol. 45:395-407. [DOI] [PubMed] [Google Scholar]
- 11.Handelsman, J., S. Raffel, E. H. Mester, L. Wunderlich, and C. R. Grau. 1990. Biological control of damping-off of alfalfa seedlings with Bacillus cereus UW85. Appl. Environ. Microbiol. 56:713-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henry, D. A., M. E. Campbell, J. J. LiPuma, and D. P. Speert. 1997. Identification of Burkholderia cepacia isolates from patients with cystic fibrosis and use of a simple new selective medium. J. Clin. Microbiol. 35:614-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes, A., R. Nolan, R. Taylor, R. Finley, M. Riley, R. Z. Jiang, S. Steinbach, and R. Goldstein. 1999. An epidemic of Burkholderia cepacia transmitted between patients with and without cystic fibrosis. J. Infect. Dis. 179:1197-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jander, G., L. G. Rahme, and F. M. Ausubel. 2000. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J. Bacteriol. 182:3843-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamblin, A. F., J. A. Crow, J. E. Johnson, K. A. Silverstein, T. M. Kunau, A. Kilian, D. Benz, M. Stromvik, G. Endre, K. A. VandenBosch, D. R. Cook, N. D. Young, and E. F. Retzel. 2003. MtDB: a database for personalized data mining of the model legume Medicago truncatula transcriptome. Nucleic Acids Res. 31:196-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LiPuma, J. J., S. E. Dasen, D. W. Nielson, R. C. Stern, and T. L. Stull. 1990. Person-to-person transmission of Pseudomonas cepacia between patients with cystic fibrosis. Lancet 336:1094-1096. [DOI] [PubMed] [Google Scholar]
- 17.LiPuma, J. J., T. Spilker, L. H. Gill, P. W. Campbell III, L. Liu, and E. Mahenthiralingam. 2001. Disproportionate distribution of Burkholderia cepacia complex species and transmissibility markers in cystic fibrosis. Am. J. Respir. Crit. Care Med. 164:92-96. [DOI] [PubMed] [Google Scholar]
- 18.Mahajan-Miklos, S., L. G. Rahme, and F. M. Ausubel. 2000. Elucidating the molecular mechanisms of bacterial virulence using non-mammalian hosts. Mol. Microbiol. 37:981-988. [DOI] [PubMed] [Google Scholar]
- 19.Mahenthiralingam, E., T. Coenye, J. W. Chung, D. P. Speert, J. R. Govan, P. Taylor, and P. Vandamme. 2000. Diagnostically and experimentally useful panel of strains from the Burkholderia cepacia complex. J. Clin. Microbiol. 38:910-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahenthiralingam, E., P. Vandamme, M. E. Campbell, D. A. Henry, A. M. Gravelle, L. T. Wong, A. G. Davidson, P. G. Wilcox, B. Nakielna, and D. P. Speert. 2001. Infection with Burkholderia cepacia complex genomovars in patients with cystic fibrosis: virulent transmissible strains of genomovar III can replace Burkholderia multivorans. Clin. Infect. Dis. 33:1469-1475. [DOI] [PubMed] [Google Scholar]
- 21.McKevitt, A. I., S. Bajaksouzian, J. D. Klinger, and D. E. Woods. 1989. Purification and characterization of an extracellular protease from Pseudomonas cepacia. Infect. Immun. 57:771-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohman, D. E., J. C. Sadoff, and B. H. Iglewski. 1980. Toxin A-deficient mutants of Pseudomonas aeruginosa PA103: isolation and characterization. Infect. Immun. 28:899-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parke, J. L., and D. Gurian-Sherman. 2001. Diversity of the Burkholderia cepacia complex and implications for risk assessment of biological control strains. Annu. Rev. Phytopathol. 39:225-258. [DOI] [PubMed] [Google Scholar]
- 24.Rahme, L. G., F. M. Ausubel, H. Cao, E. Drenkard, B. C. Goumnerov, G. W. Lau, S. Mahajan-Miklos, J. Plotnikova, M. W. Tan, J. Tsongalis, C. L. Walendziewicz, and R. G. Tompkins. 2000. Plants and animals share functionally common bacterial virulence factors. Proc. Natl. Acad. Sci. USA 97:8815-8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahme, L. G., E. J. Stevens, S. F. Wolfort, J. Shao, R. G. Tompkins, and F. M. Ausubel. 1995. Common virulence factors for bacterial pathogenicity in plants and animals. Science 268:1899-1902. [DOI] [PubMed] [Google Scholar]
- 26.Rahme, L. G., M. W. Tan, L. Le, S. M. Wong, R. G. Tompkins, S. B. Calderwood, and F. M. Ausubel. 1997. Use of model plant hosts to identify Pseudomonas aeruginosa virulence factors. Proc. Natl. Acad. Sci. USA 94:13245-13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sajjan, U., G. Thanassoulis, V. Cherapanov, A. Lu, C. Sjolin, B. Steer, Y. J. Wu, O. D. Rotstein, G. Kent, C. McKerlie, J. Forstner, and G. P. Downey. 2001. Enhanced susceptibility to pulmonary infection with Burkholderia cepacia in Cftr−/− mice. Infect. Immun. 69:5138-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sajjan, U., Y. Wu, G. Kent, and J. Forstner. 2000. Preferential adherence of cable-piliated Burkholderia cepacia to respiratory epithelia of CF knockout mice and human cystic fibrosis lung explants. J. Med. Microbiol. 49:875-885. [DOI] [PubMed] [Google Scholar]
- 29.Schwab, U., M. Leigh, C. Ribeiro, J. Yankaskas, K. Burns, P. Gilligan, P. A. Sokol, and R. Boucher. 2002. Patterns of epithelial cell invasion by different species of the Burkholderia cepacia complex in well-differentiated human airway epithelia. Infect. Immun. 70:4547-4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silo-Suh, L., S. J. Suh, P. A. Sokol, and D. E. Ohman. 2002. A simple alfalfa seedling infection model for Pseudomonas aeruginosa strains associated with cystic fibrosis shows AlgT (sigma-22) and RhlR contribute to pathogenesis. Proc. Natl. Acad. Sci. USA 99:15699-15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson, I. N., J. Finlay, D. J. Winstanley, N. Dewhurst, J. W. Nelson, S. L. Butler, and J. R. Govan. 1994. Multi-resistance isolates possessing characteristics of both Burkholderia (Pseudomonas) cepacia and Burkholderia gladioli from patients with cystic fibrosis. J. Antimicrob. Chemother. 34:353-361. [DOI] [PubMed] [Google Scholar]
- 32.Sokol, P. A., P. Darling, S. Lewenza, C. R. Corbett, and C. D. Kooi. 2000. Identification of a siderophore receptor required for ferric ornibactin uptake in Burkholderia cepacia. Infect. Immun. 68:6554-6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sokol, P. A., P. Darling, D. E. Woods, E. Mahenthiralingam, and C. Kooi. 1999. Role of ornibactin biosynthesis in the virulence of Burkholderia cepacia: characterization of pvdA, the gene encoding l-ornithine N5-oxygenase. Infect. Immun. 67:4443-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sokol, P. A., C. Kooi, R. S. Hodges, P. Cachia, and D. E. Woods. 2000. Immunization with a Pseudomonas aeruginosa elastase peptide reduces severity of experimental lung infections due to P. aeruginosa or Burkholderia cepacia. J. Infect. Dis. 181:1682-1692. [DOI] [PubMed] [Google Scholar]
- 35.Sokol, P. A., and D. E. Woods. 1984. Relationship of iron and extracellular virulence factors to Pseudomonas aeruginosa lung infections. J. Med. Microbiol. 18:125-133. [DOI] [PubMed] [Google Scholar]
- 36.Speert, D. P., D. Henry, P. Vandamme, M. Corey, and E. Mahenthiralingam. 2002. Epidemiology of Burkholderia cepacia complex in patients with cystic fibrosis, Canada. Emerg. Infect. Dis. 8:181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tabata, S. 2002. Impact of genomics approaches on plant genetics and physiology. J. Plant Res. 115:271-275. [DOI] [PubMed] [Google Scholar]
- 38.Tan, M. W., S. Mahajan-Miklos, and F. M. Ausubel. 1999. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. USA 96:715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan, M. W., L. G. Rahme, J. A. Sternberg, R. G. Tompkins, and F. M. Ausubel. 1999. Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc. Natl. Acad. Sci. USA 96:2408-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vandamme, P., D. Henry, T. Coenye, S. Nzula, M. Vancanneyt, J. J. LiPuma, D. P. Speert, J. R. Govan, and E. Mahenthiralingam. 2002. Burkholderia anthina sp. nov. and Burkholderia pyrrocinia, two additional Burkholderia cepacia complex bacteria, may confound results of new molecular diagnostic tools. FEMS Immunol. Med. Microbiol. 33:143-149. [DOI] [PubMed] [Google Scholar]
- 41.Vandamme, P., B. Holmes, M. Vancanneyt, T. Coenye, B. Hoste, R. Coopman, H. Revets, S. Lauwers, M. Gillis, K. Kersters, and J. R. Govan. 1997. Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. nov. Int. J. Syst. Bacteriol. 47:1188-1200. [DOI] [PubMed] [Google Scholar]