Abstract
In this paper we show that Clostridium perfringens epsilon-toxin accumulates predominantly in the mouse kidney, where it is distributed mainly in glomeruli, capillaries, and collecting ducts. Although some pycnotic and exfoliated epithelial cells were observed in distal tubuli and collecting ducts, there were no findings indicative of severe renal injury. Bilateral nephrectomy increased the mouse lethality of the toxin, suggesting that the kidney contributes to the host defense against the lethal toxicity of epsilon-toxin.
Epsilon-toxin produced by Clostridium perfringens types B and D is a potent toxin that is responsible for rapidly fatal enterotoxemia in livestock (17, 22). The toxin has been well defined in terms of the proteolytic activation of epsilon-protoxin (7, 10), its pore-forming ability in Madin-Darby canine kidney (MDCK) cell membranes (12, 18) and artificial lipid bilayers (19), and its heptamerization in detergent-insoluble glycosphingolipid-enriched microdomains (10, 11). However, the pathogenic mechanisms involved in the lethality of epsilon-toxin remain largely unknown, except that it exhibits toxicity towards neuronal cells (2, 8, 9) and blood vessels in the brains of mice and rats (1, 3, 4). Besides massive necrosis in the brain, pulpy kidneys are noticeable in animals that have died due to enterotoxemia (22). Epsilon-toxin exhibits cytotoxicity towards MDCK cells derived from dog renal distal tubules or collecting ducts but not towards any other cell lines (17, 21, 24). Moreover, epsilon-toxin was reported to be most abundant in the kidneys when intravenously (i.v.) administered to mice (13). However, nothing is known about its nephrotoxicity.
The aim of this study was to determine the distribution of epsilon-toxin in the mouse kidney and also its nephrotoxicity. Male ddY mice (4 weeks old; SLC Japan, Shizuoka, Japan) were used. The epsilon-protoxin and epsilon-toxin used in this study were recombinant toxins, which were purified, activated, and labeled with [γ-35S]ATP as described previously (11). The intravenous 50% lethal dose of epsilon-toxin was determined to be approximately 20 ng per mouse. The time to death after challenge estimated for mice susceptible to 15 ng of epsilon-toxin was 8.3 ± 0.4 h (mean ± standard error [SE], n = 5), and that for mice receiving 500 ng of epsilon-toxin was 0.35 ± 0.01 h (mean ± SE, n = 5).
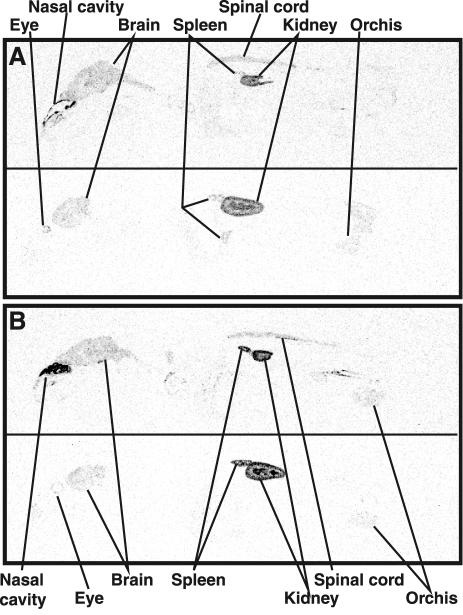
First, we examined, by whole-body autoradiography (WBA), whether epsilon-toxin is accumulated predominantly in the mouse kidney in accordance with the results obtained by others using a different methodology (13). One hundred nanograms of 35S-epsilon-toxin or 35S-epsilon-protoxin was i.v. injected into a mouse through a tail vein. The mouse given 35S-epsilon-toxin was frozen in dry ice-acetone at approximately 1 h postinjection (p.i.) immediately after death. The mouse given 35S-epsilon-protoxin was sacrificed by cervical dislocation at 1 h p.i., followed by freezing in dry ice-acetone. Frozen sections (50 μm each) were prepared with an autocryotome (NA-500F; Nakagawa Seisakusho, Tokyo, Japan), dried at −20°C, and then exposed to an imaging plate (Fuji Photo Film, Kanagawa, Japan) for 2 weeks. WBA involving epsilon-toxin and epsilon-protoxin showed the same distribution profile, indicating that their putative receptor(s) is the same (Fig. 1). The toxins were detected most abundantly in the kidneys and fairly abundantly in the brain and spinal cord. In the kidneys the toxins were distributed in the outer and inner regions but not in the intermediate one. They were also detected in other organs, such as the spleen and eyes. Surprisingly, the toxins were deposited densely in the nasal turbinates, suggesting that they are enriched in an epsilon-toxin receptor.
FIG. 1.
Whole-body autoradiograms for a mouse at approximately 1 h p.i. with 100 ng of 35S-epsilon-toxin (A) and one at 1 h p.i. with 100 ng of 35S-epsilon-protoxin (B). Upper and lower panels, sections at median and sagittal (5 to 6 mm lateral of the median line) planes, respectively.
For immunostaining of epsilon-toxin, anti-epsilon-protoxin antibodies were generated with formalinized epsilon-protoxin in a female New Zealand White rabbit (SLC). Anti-epsilon-protoxin immunoglobulin G (IgG) was purified by means of epsilon-toxin immobilized on Sepharose 4B and protein A-Sepharose (Amersham Biosciences, Little Chalfont, Buckinghamshire, United Kingdom). The purified IgG (0.7 mg/ml) was checked for specificity and sensitivity by enzyme-linked immunosorbent assay and the Western blot procedure. Nonspecific control IgG (0.6 mg/ml) was purified from rabbit preimmune serum by means of protein A-Sepharose. Mice given i.v. 500 ng of epsilon-toxin or the vehicle (1% Bacto Peptone-0.25% NaCl solution) were anesthetized with ether at 20 min p.i., followed by perfusion with 5 ml of phosphate-buffered saline (PBS) and then 20 ml of fixative consisting of 4% paraformaldehyde, 2.5% polyvinylpyrrolidone, 2% sucrose, and 0.1 M sodium phosphate buffer (pH 7.4). The cryostat sections of 5-μm thickness were prepared as described by Pavelka and Ellinger (16) except that treatment with ammonium chloride was omitted. To amplify the immunohistochemical signal, two signal amplification systems, a dextran polymer visualization system (EnVision+; Dako, Carpinteria, Calif.) and a tyramide signal amplification system (NEN Life Science Products, Boston, Mass.), were used in combination with some modifications of the suppliers' instructions. Endogenous peroxidase activity was inhibited by treatment with 3% H2O2 in TBST (50 mM Tris-HCl [pH 7.4], 300 mM NaCl, 0.1% Tween 20), and nonspecific binding of primary antibodies was blocked with blocking buffer (5% goat serum, 1% Triton X-100 in antibody dilution buffer [Dako]). After incubation with primary antibodies (rabbit anti-epsilon-protoxin IgG or nonspecific control IgG, each diluted to 2.3 μg/ml in blocking buffer), the sections were subjected to amplification with EnVision+ (goat anti-rabbit IgG and horseradish peroxidase-immobilized dextran polymer; Dako). The immunohistochemical signal was further amplified with a TSA fluorescence system (fluorescein; NEN Life Science Products). The sections were mounted on glass slides using a ProLong antifade kit (Molecular Probes, Eugene, Oreg.) and then viewed under a laser scanning confocal microscope (Olympus Optical, Nagano, Japan).
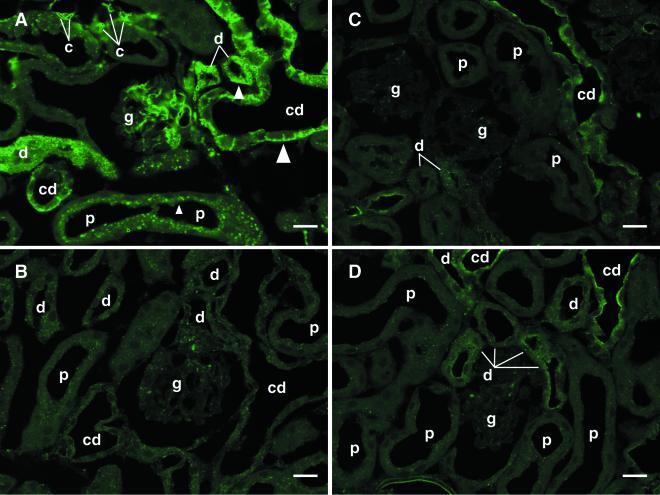
Strong labeling was found in the kidney sections from epsilon-toxin-injected mice but not in those from vehicle-injected ones when stained with epsilon-protoxin-specific IgG (Fig. 2A and B). Nonspecific control IgG gave weak signal intensity to the collecting ducts in both sections (Fig. 2C and D). However, the immunoreactivity of nonspecific control IgG was restricted to the apical side, differing from that of epsilon-protoxin-specific IgG, which gave strong signal intensity to the basolateral side in epsilon-toxin-injected mice (Fig. 2A). More importantly, collecting ducts per se were not reactive with epsilon-protoxin-specific IgG (Fig. 2B). Thus, we concluded that the immunostaining involving epsilon-protoxin-specific IgG allows the specific detection of epsilon-toxin distributed in the kidneys. At low magnification, epsilon-toxin was detected in the whole kidney except for the outer medulla (data not shown), this being consistent with the doubly stained WBA profile of the kidney. Epsilon-toxin was detected most prominently in the glomeruli, capillaries, and collecting ducts (Fig. 2A). It was also detected to a large extent in the epithelial cells of the distal tubules and to a lesser extent but significantly in those of the proximal tubules. In the epithelial cells of the proximal tubules epsilon-toxin was mainly detectable on the luminal side, while in the epithelial cells of the distal tubules and collecting ducts it was on the basolateral side (Fig. 2A).
FIG. 2.
Epsilon-toxin immunostaining of the renal cortex from a mouse given i.v. 500 ng of epsilon-toxin (A and C) or the vehicle alone (B and D) and fixed at 20 min p.i. Sections were stained with either epsilon-protoxin-specific (A and B) or nonspecific (C and D) IgG, as described in the text. The glomeruli (g), capillaries (c), and collecting ducts (cd) were heavily stained. The renal epithelial cells of the distal tubules (d) were fairly densely stained, and those of the proximal tubules (p) were weakly stained. Note that the toxin was detected on the apical side of the proximal tubules (small arrowhead) and the basolateral side of the distal tubules (medium arrowhead) and collecting ducts (large arrowhead). Bars, 20 μm.
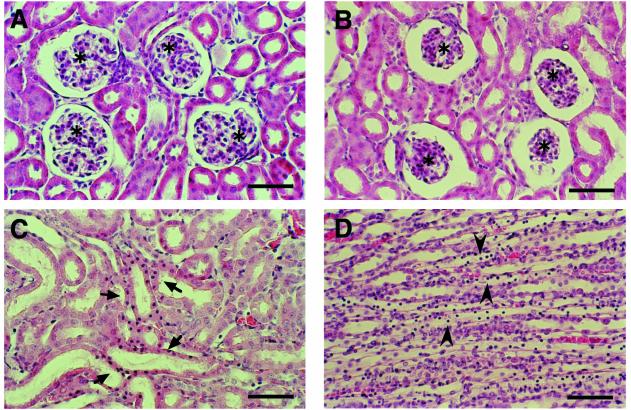
To examine toxin-induced morphological changes in the mouse kidney, mice were i.v. given 20 to 500 ng of epsilon-toxin, followed by transcardial perfusion with 10 ml of PBS and then 20 ml of 4% paraformaldehyde in PBS. The kidneys were cut into small pieces, which were placed in the fixative for 2 days. After routine dehydration and embedding, 2-μm cut sections were stained with hematoxylin-eosin. A histological comparison between mice in the same dose group showed that renal pathological changes became more evident as fixation time was delayed. To detect maximal but not postmortem changes, only mice surviving at 6.5 to 7.5 h after challenge with 20 to 50 ng of epsilon-toxin were fixed prior to death. Figure 3 shows morphological changes detected in both the cortex and the medulla of the kidneys from a mouse given 50 ng of toxin (Fig. 3). In the cortex, glomeruli showed apparent shrinkage resulting in dilatation of Bowman's space. Proximal tubules were almost intact, while distal tubules and collecting ducts exhibited degenerative changes: a decrease in the height of epithelial cells with a dilated lumen and cellular degeneration with karyopycnosis and cellular exfoliation into the lumen. Since the mouse given 50 ng of the toxin showed the most prominent renal histological changes among those given 20 to 50 ng of the toxin, the severity and extent of these pathological changes may also be dependent on the toxin dose. However, they were less prominent in the kidneys from mice given 100 or 200 ng of epsilon-toxin. Moreover, there was no obvious histological finding in the kidneys from mice given 500 ng of the toxin, even in those from a mouse surviving exceptionally long and fixed at 30 min p.i. (data not shown). Therefore, the changes described above seem to be the maximal premortem histological changes, although they are not very severe.
FIG. 3.
Hematoxylin-eosin-stained sections of a mouse kidney at 6.5 h p.i. with the vehicle alone (A) or 50 ng of epsilon-toxin (B to D). Note the shrinkage of the glomerulus (asterisks), epithelial cells exhibiting karyopycnosis (arrows), and cells exfoliated into the lumen (arrowheads) in the distal tubules and collecting ducts in the toxin group. Bars, 50 μm.
The renal pathological changes and accumulation of epsilon-toxin may suggest the following possibilities. First, the nephrotoxicity of epsilon-toxin may be involved in its lethal toxicity not primarily but still somehow. Second, the kidneys may contribute to the decrease in the amount of circulating epsilon-toxin and thereby protect the host from its lethal toxicity. In order to assess these possibilities, the effect of nephrectomy on the lethal toxicity of epsilon-toxin was examined. Mice were anesthetized with ether, and then bilateral nephrectomy was accomplished by tying a silk suture securely around the hilus renalis containing the artery, vein, and ureter, followed by resection of the kidneys. The abdominal incision was closed, immediately followed by injection with the vehicle alone or 50 or 100 ng of epsilon-toxin through a tail vein. The bilateral nephrectomy clearly shortened the time required for the toxin to kill mice (Table 1), indicating that the kidneys protect the host from the lethal toxicity of epsilon-toxin. The kidneys, however, could filter any circulating toxins, and the effect of nephrectomy may reflect such a general feature of the kidneys, not being specific to epsilon-toxin. To address this issue, we examined the lethality for nephrectomized mice of two other toxins, C. perfringens alpha-toxin, which is similar in molecular size but not neurotoxicity to epsilon-toxin, and botulinus type A neurotoxin, which is larger and more neurotoxic than epsilon-toxin. Nephrectomy did not shorten the time to death in either case (Table 1). These results exclude the generality of the effect observed for epsilon-toxin intoxication and suggest that the accumulation of epsilon-toxin in the kidneys through specific binding causes the effect.
TABLE 1.
Lethality of epsilon-toxin, alpha-toxin, and botulinus toxin for nephrectomized and unnephrectomized mice
| Toxin | Amt of toxin (ng or MLDa) | Nephrectomy | Time required for killing miceb (h)
|
|
|---|---|---|---|---|
| Mean ± SE | Range | |||
| None | 0 | + | 21 ± 1 | 20-23 |
| Epsilon-toxin | 50 | − | 5.8 ± 0.5 | 4.4-6.8 |
| 50 | + | 1.0 ± 0.1 | 0.83-1.2 | |
| 100 | − | 1.1 ± 0.1 | 0.88-1.3 | |
| 100 | + | 0.63 ± 0.05 | 0.05-0.77 | |
| Alpha-toxinc | 100 | − | 2.2 ± 0.1 | 1.9-2.4 |
| 100 | + | 2.5 ± 0.2 | 2.1-3.3 | |
| Botulinus neurotoxind | 2 × 105 | − | 1.8 ± 0.1 | 1.5-2.0 |
| 2 × 105 | + | 2.3 ± 0.1 | 1.9-2.5 | |
The amount of botulinum toxin is expressed as MLD (minimal lethal dose) determined by intraperitoneal injection into mice.
Mice with and without nephrectomy were i.v. given toxins (n = 5 for each group) or the vehicle alone (n = 3), and then the time of animal death was recorded.
Alpha-toxin was purified from recombinant E. coli (23), and its specific activity was determined by p-nitrophenylphosphorylcholine hydrolysis assay (6) to be 303 nmol/min/mg.
Botulinus type A neurotoxin, which had been purified as described elsewhere (5), was provided by K. Oguma (Department of Bacteriology, Okayama University Graduate School of Medicine and Dentistry, Okayama, Japan).
Epsilon-toxin is accumulated predominantly in the kidneys, which attenuates its lethality. This apparently paradoxical finding implies the biological significance of the epsilon-toxin accumulation in the kidneys. We propose that the kidneys contribute to the host defense against epsilon-toxin by trapping the toxin and thereby protecting more susceptible organs, e.g., the brain, from its lethal toxicity. Our hypothesis is of interest in the context of the host defense against bacterial infection. Another example of specialized host defense in kidneys is the protection from urinary tract infection by Tamm-Horsfall glycoprotein secreted from the surface of cells of the Henle’s loop, which binds to type 1 fimbriated Escherichia coli, preventing infection by the organism (14, 15, 20). Interestingly, both C. perfringens and E. coli are intestinal commensal bacteria. Mammals might have developed defense mechanisms specific to individual bacteria or toxins during their interaction with commensal bacteria.
Acknowledgments
We thank K. Oguma (Department of Bacteriology, Okayama University Graduate School of Medicine and Dentistry, Okayama, Japan) for providing purified botulinus neurotoxin and J. Wada (Department of Medicine and Clinical Science, Okayama University Graduate School of Medicine and Dentistry) for advice and help on nephrectomy.
This work was supported by a grant-in-aid from the Japan Society for the Promotion of Science and also by the Sasagawa Scientific Research Grant from Japan Science Society.
Editor: A. D. O'Brien
REFERENCES
- 1.Finnie, J. W. 1984. Ultrastructural changes in the brain of mice given Clostridium perfringens type D epsilon toxin. J. Comp. Pathol. 94:445-452. [DOI] [PubMed] [Google Scholar]
- 2.Finnie, J. W., P. C. Blumbergs, and J. Manavis. 1999. Neuronal damage produced in rat brains by Clostridium perfringens type D epsilon toxin. J. Comp. Pathol. 120:415-420. [DOI] [PubMed] [Google Scholar]
- 3.Finnie, J. W., P. C. Blumbergs, J. Manavis, T. D. Utteridge, V. Gebski, J. G. Swift, B. Vernon-Roberts, and T. R. Kuchel. 2001. Effect of global system for mobile communication (gsm)-like radiofrequency fields on vascular permeability in mouse brain. Pathology 33:338-340. [PubMed] [Google Scholar]
- 4.Ghabriel, M. N., C. Zhu, P. L. Reilly, P. C. Blumbergs, J. Manavis, and J. W. Finnie. 2000. Toxin-induced vasogenic cerebral oedema in a rat model. Acta Neurochir. Suppl. 76:231-236. [DOI] [PubMed] [Google Scholar]
- 5.Inoue, K., Y. Fujinaga, T. Watanabe, T. Ohyama, K. Takeshi, K. Moriishi, H. Nakajima, K. Inoue, and K. Oguma. 1996. Molecular composition of Clostridium botulinum type A progenitor toxins. Infect. Immun. 64:1589-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katayama, S.-I., O. Matsushita, J. Minami, S. Mizobuchi, and A. Okabe. 1993. Comparison of the alpha-toxin genes of Clostridium perfringens type A and C strains: evidence for extragenic regulation of transcription. Infect. Immun. 61:457-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minami, J., S. Katayama, O. Matsushita, C. Matsushita, and A. Okabe. 1997. Lambda-toxin of Clostridium perfringens activates the precursor of epsilon-toxin by releasing its N- and C-terminal peptides. Microbiol. Immunol. 41:527-535. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto, O., J. Minami, T. Toyoshima, T. Nakamura, T. Masada, S. Nagao, T. Negi, T. Itano, and A. Okabe. 1998. Neurotoxicity of Clostridium perfringens epsilon-toxin for the rat hippocampus via the glutamatergic system. Infect. Immun. 66:2501-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyamoto, O., K. Sumitani, T. Nakamura, S.-I. Yamagami, S. Miyata, T. Itano, T. Negi, and A. Okabe. 2000. Clostridium perfringens epsilon-toxin causes excessive release of glutamate in the mouse hippocampus. FEMS Microbiol. Lett. 189:109-113. [DOI] [PubMed] [Google Scholar]
- 10.Miyata, S., O. Matsushita, J. Minami, S. Katayama, S. Shimamoto, and A. Okabe. 2001. Cleavage of a C-terminal peptide is essential for heptamerization of Clostridium perfringens epsilon-toxin in the synaptosomal membrane. J. Biol. Chem. 276:13778-13783. [DOI] [PubMed] [Google Scholar]
- 11.Miyata, S., J. Minami, E. Tamai, O. Matsushita, S. Shimamoto, and A. Okabe. 2002. Clostridium perfringens epsilon-toxin forms a heptameric pore within the detergent-insoluble microdomains of MDCK cells and rat synaptosomes. J. Biol. Chem. 277:39463-39468. [DOI] [PubMed] [Google Scholar]
- 12.Nagahama, M., S. Ochi, and J. Sakurai. 1998. Assembly of Clostridium perfringens epsilon-toxin on MDCK cell membrane. J. Nat. Toxins 7:291-302. [PubMed] [Google Scholar]
- 13.Nagahama, M., and J. Sakurai. 1991. Distribution of labeled Clostridium perfringens epsilon toxin in mice. Toxicon 29:211-217. [DOI] [PubMed] [Google Scholar]
- 14.Pak, J., Y. Pu, Z. T. Zhang, D. L. Hasty, and X. R. Wu. 2001. Tamm-Horsfall protein binds to type 1 fimbriated Escherichia coli and prevents E. coli from binding to uroplakin Ia and Ib receptors. J. Biol. Chem. 276:9924-9930. [DOI] [PubMed] [Google Scholar]
- 15.Parkkinen, J., R. Virkola, and T. K. Korhonen. 1988. Identification of factors in human urine that inhibit the binding of Escherichia coli adhesins. Infect. Immun. 56:2623-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavelka, M., and A. Ellinger. 1989. Pre-embedding labeling techniques applicable to intracellular binding site, p. 199-214. In H. Plattner (ed.), Electron microscopy of subcellular dynamics. CRC Press, Inc., Boca Raton, Fla.
- 17.Payne, D., and E. Oyston. 1997. The Clostridium perfringens ɛ-toxin, p. 439-447. In J. I. Rood, B. A. McClane, J. G. Songer, and R. W. Titball (ed.), The clostridia: molecular biology and pathogenesis. Academic Press, London, United Kingdom.
- 18.Petit, L., M. Gibert, D. Gillet, C. Laurent-Winter, P. Boquet, and M. R. Popoff. 1997. Clostridium perfringens epsilon-toxin acts on MDCK cells by forming a large membrane complex. J. Bacteriol. 179:6480-6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petit, L., E. Maier, M. Gibert, M. R. Popoff, and R. Benz. 2001. Clostridium perfringens epsilon toxin induces a rapid change of cell membrane permeability to ions and forms channels in artificial lipid bilayers. J. Biol. Chem. 276:15736-15740. [DOI] [PubMed] [Google Scholar]
- 20.Reinhart, H. H., N. Obedeanu, and J. D. Sobel. 1990. Quantitation of Tamm-Horsfall protein binding to uropathogenic Escherichia coli and lectins. J. Infect. Dis. 162:1335-1340. [DOI] [PubMed] [Google Scholar]
- 21.Shortt, S. J., R. W. Titball, and C. D. Lindsay. 2000. An assessment of the in vitro toxicology of Clostridium perfringens type D epsilon-toxin in human and animal cells. Hum. Exp. Toxicol. 19:108-116. [DOI] [PubMed] [Google Scholar]
- 22.Songer, J. G. 1996. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9:216-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsutsui, K., J. Minami, O. Matsushita, S. Katayama, Y. Taniguchi, S. Nakamura, M. Nishioka, and A. Okabe. 1995. Phylogenetic analysis of phospholipase C genes from Clostridium perfringens types A to E and Clostridium novyi. J. Bacteriol. 177:7164-7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uzal, F. A., B. E. Rolfe, N. J. Smith, A. C. Thomas, and W. R. Kelly. 1999. Resistance of ovine, caprine and bovine endothelial cells to Clostridium perfringens type D epsilon toxin in vitro. Vet. Res. Commun. 23:275-284. [DOI] [PubMed] [Google Scholar]