Abstract
Protective immunity to the pig roundworm, Ascaris suum, has been demonstrated by immunization of pigs with antigens derived from the parasite's larval stages. We identified a protective antigen commonly expressed in the human and pig Ascaris infections as a 16-kDa protein (As16), which has no similarity at the amino acid level to mammalian proteins but has some similarity to those of the filarial parasites and Caenorhabditis elegans gene product. Localization analysis revealed that the native As16 was highly expressed in the adult worm intestine, hypodermis, and cuticles. In addition, As16 was detected in the parasite excretory and secretory products. Mice intranasally vaccinated with Escherichia coli-expressed recombinant As16 (rAs16), coupled with cholera toxin B subunit, generated a significant increase in the level of rAs16-specific immunoglobulin G (IgG) and IgE in serum. Mucosal IgA levels were also increased. The recombinant protein evoked a mixed (both Th1 and Th2) type of immune response characterized by elevated levels of gamma interferon and interleukin-10 in the culture supernatants of activated spleen cells. An increased level of IgG1 and IgG2a in serum was also observed. The vaccinated mice showed a reduction by 58% in the recovery of challenged larvae compared to a nonvaccinated control. These results suggest the possibility of developing a mucosal vaccine for human and pig ascariasis.
The Ascaris roundworm is a major geohelminthic parasite that is widely distributed among both humans and animals worldwide. It is estimated that more than 1.5 billion people are infected with Ascaris parasites, mainly in tropical and subtropical areas (6). The ascarids Ascaris and Toxocara are responsible for significant morbidity and economic loss in animals (9). One of these roundworms, Ascaris suum, was originally identified as a ubiquitous, pathogenic parasite of swine and is a biochemically well-characterized parasite (52). Studies on A. suum are believed to provide important information about the biology of other ascarid nematodes, especially human ascarids. A. suum infection is established orally by third-stage larvae (L3) after their development from embryonated eggs. The L3 invade into the small intestine of the host, migrate into the liver and the lung, and finally reach the cecum and/or proximal colon, where they develop into adult worms. Recent studies have revealed that A. suum of swine origin can develop in humans, indicating its zoonotic importance (4, 44). Although numerous studies have been carried out thus far to characterize the two species of parasites on a morphological, immunological, or biochemical basis, species discrimination between Ascaris lumbricoides and A. suum has been controversial (1, 29, 33, 38). Controlling pig ascariasis may help to decrease the incidence of human ascariasis.
Prior studies have shown that pigs can be rendered immune to A. suum infection by immunization with radiation-attenuated infective larvae or by chemically abbreviated infection (18, 26, 60). In addition, crude larval antigens can induce protective immunity (61). Similar findings were observed in an A. suum laboratory animal infection model (8, 22, 49). Passive transfer of sera from immune animals is effective for larval killing and stunting in guinea pigs (27). These data suggest that the larval stages possess antigens that induce protective immunity against infection and that the A. suum mouse infection model can be used to identify immunoprotective molecules.
Intranasal or oral routes for vaccination are among the convenient routes for immunization against pathogenic organisms. The initial phase of A. suum infection occurs in the mucosal surface of the cecum and/or proximal colon of the host, and this is followed by the tissue migratory phase. It has been shown that local antibodies present at the site where the infective L3 enter the host can induce partial protection against A. suum infection in mice (23). Thus, intestinal immunity should be an important initial defense against the invasion of A. suum larval stages into the host, whereas systemic immunity mediated by serum antibodies may protect the host against larval migration. Previous animal studies have demonstrated that mucosal administration of several antigens fused to cholera toxin B subunit (CTB) can induce vigorous mucosal and systemic immune responses (11, 62). Thus, CTB is used to induce protective immunity as a mucosal adjuvant in a variety of antigens from virus to parasite origins (33). In fact, the possibility of using CTB as a mucosal adjuvant in humans has been reported (7, 24, 28). Our aim in the present study was to identify vaccine molecules whose mucosal administration could induce protection against A. suum infection.
Although several antigens have been identified from a variety of parasites as vaccine candidates, these mainly include the possibility of autoimmune responses since they are homologous with any known host proteins. Recent studies indicate that vaccine molecules for parasitic infections are desired for use as parasite-specific antigens with no similarity to mammalian proteins (2, 15). In the present study, several cDNAs encoding antigens were identified by immunoscreening an A. suum infective L3 expression library by using sera raised from rabbits immunized with A. suum embryonated eggs. We selected a cDNA encoding a 16-kDa protein with a low similarity to mammalian protein from the current database. Surprisingly, the human roundworm, A. lumbricoides, has a 16-kDa protein consisting of the same amino acid sequences as the 16-kDa A. suum protein (As16). We performed embryonated egg challenge infection with CTB as a mucosal adjuvant in a mouse A. suum model. Mice immunized with Escherichia coli-expressed recombinant As16 (rAs16) coupled with CTB showed protection against challenge infection with A. suum embryonated eggs; the recovery of larvae from the lung with mucosal and systemic immune responses was reduced in mice. Based on these data, we suggest rAs16 as a mucosal vaccine candidate for human and pig ascariasis caused by ascarid nematodes.
MATERIALS AND METHODS
Parasites.
Adult female A. lumbricoides worms were obtained from patients after treatment with piperazine in Bac Gian, Vietnam. Adult A. suum were obtained from infected pigs at a slaughterhouse in Shimotsuma, Japan. Adult Toxocara canis organisms were recovered from an infected dog in Miyazaki, Japan. Unembryonated and embryonated eggs were obtained essentially as described elsewhere (57). Infective L3 and lung-stage L3 were obtained as previously described (14, 57). Excretory and secretory (ES) products from the larval stages and adult worms were collected as previously described (57). In brief, the living larvae or worms were cultured at 37°C for 48 h in RPMI 1640 medium supplemented with penicillin (100 μg/ml) and streptomycin (100 μg/ml). The supernatant was concentrated by using Biomax 5 (molecular weight cutoff, 5,000; Millipore, Bedford, Mass.) and then dialyzed against phosphate-buffered saline (PBS) in an Aside-A-Lyzer dialysis cassette (Pierce, Rockford, Ill.). The protein concentrations were measured with Micro-BCA protein assay reagent (Pierce), and the supernatant was stored at −80°C. RNA was isolated from embryonated eggs by using an RNA isolation kit (Clontech, Palo Alto, Calif.). Poly(A)+ mRNA was prepared from total RNA by using a polytract mRNA isolation kit (Clontech), and first-strand cDNA synthesis was performed by using a cDNA synthesis kit and an oligo(dT)15 primer (Amersham Pharmacia Biotech, Piscataway, N.J.). Ascaris cDNA expression libraries were constructed in UniZap XR vector (Stratagene, La Jolla, Calif.) according to the manufacturer's instructions as previously described (58). Protein concentrations of PBS-soluble parasite antigens and ES products were measured by using Micro-BCA protein assay reagent (Pierce).
Production of rabbit immune sera.
Antibodies to the larval stages of A. suum and A. lumbricoides were generated in specific-pathogen-free rabbits by repeated inoculation with infective stages. Japanese white rabbits (SLC, Hamamatsu, Japan) were inoculated orally with 2 × 103 eggs containing the infective stage, and this was repeated fortnightly for a total of four inoculations. The rabbits were bled 7 days after the final inoculation, and the sera were stored at −20°C until use. The corresponding preimmune serum was used as a control at the same dilution as the antiserum.
Cloning of cDNA encoding As16.
An infective L3 cDNA library constructed in UniZap XR vector (Stratagene) according to the manufacturer's instructions was used for immunoscreening. The library was screened with a 1:200 dilution of the rabbit A. suum immune serum as described by Sambrook et al. (48). The nucleotide sequences of the cDNAs were determined by the Sanger dideoxy chain termination method by using a Prism Ready dye terminator cycle sequencing kit (Perkin-Elmer, Foster City, Calif.). DNA samples were analyzed by using an automated DNA sequencer (373A; Applied Biosystems, Foster City, Calif.). BLASTX (National Center for Biotechnology Information [NCBI], National Institutes of Health, Bethesda, Md.) searches were performed to obtain cDNA clones coding low similarity against mammalian proteins stored in the current database. The GENETYX-WIN DNA sequence analysis software system (Software, Inc., Tokyo, Japan) and the BLAST (3) network server of the NCBI were used to analyze the nucleotides and deduce the amino acid sequences to determine similarities with previously reported sequences in GenBank. A clone designated L2R37 that contains an entire open reading frame (ORF) was selected for further analysis in the present study. A primary sequence motif was identified by using the PROSITE (5) network server at the European Molecular Biology Laboratory. Analysis of the signal sequence (39) was performed by using SignalP V1.1 at the Center for Biological Sequence Analysis (http://www.cbs.dtu.dk/services/SignalP/index.html).
Expression and purification of rAs16 fusion protein.
A coding region of As16 cDNA, except for the signal peptides, was amplified by PCR as previously described (59). A sense primer (5′-CCG AGC TCG AGA GAC AAA CAC CAT CAC GCG TAC CAC CCT-3′) containing an XhoI (Promega, Madison, Wis.) site upstream of the start codon and an antisense primer (5′-CCG AAT TCT CAC TTA CGC ACC GCC AGC GAT TGC CTT-3′) containing an EcoRI (Promega) site just downstream of amino acid residue 150 were used. The PCR fragments were digested with XhoI and EcoRI and then ligated into the plasmid expression vector pTrcHisB (Invitrogen, Carlsbad, Calif.), which was also digested with the same enzymes as described in the manufacturer's protocol. The resultant plasmid was transferred into Escherichia coli strain TOP10F′ (Invitrogen). Transformed cells were grown to an optical density at 600 nm (OD600) of 1.0 at 37°C in SOC medium supplemented with 50 μg of ampicillin/ml. IPTG (isopropyl-β-d-thiogalactopyranoside) was then added to a final concentration of 1 mM, and the culture was grown for an additional 4 h at 37°C. The E. coli cells were pelleted and resuspended in a lysis buffer (50 mM NaH2PO4 [pH 8.0], 10 mM Tris-HCl [pH 8.0], 100 mM NaCl). Lysozyme was added to 100 μg/ml, and the cell suspension was incubated on ice for 20 min. The suspension was disrupted with an ultrasonic processor on ice. The lysate was centrifuged at 25,000 × g for 60 min at 4°C. rAs16 protein in the supernatant was purified by using metal chelation chromatography (Invitrogen) under nondenaturing conditions as described in the manufacturer's protocol. Protein eluted with imidazole was concentrated by using Centrisart I (molecular weight cutoff, 10,000; Sartorius, Gottingen, Germany) and then dialyzed against PBS in an Aside-A-Lyzer dialysis cassette (Pierce). The purification process was monitored by standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (30) and immunoblotting (56) with a T7 Taq monoclonal antibody (Novagen, Madison, Wis.).
Production of an antiserum to rAs16.
Antiserum to rAs16 was prepared by subcutaneous injection of BALB/c mice with 50 μg of the recombinant protein mixed with TiterMax Gold (CytRx, Norcross, Ga.), followed by another injection 2 weeks later in the same adjuvant. The mice were bled 2 weeks after the second injection. The antisera from the mice were mixed and stored at −20°C until use.
cDNA cloning of A. lumbricoides 16-kilodalton protein.
An adult A. lumbricoides cDNA library was constructed in UniZap XR vector (Stratagene) according to the manufacturer's instructions. A cDNA encoding A. lumbricoides 16-kDa protein was obtained from the cDNA library immunoscreening with a 1:500 dilution of sera from mice immunized with rAs16 as described above.
Immunoblot analysis.
Immunoblot analysis was performed as previously described (57). Parasite antigens or rAs16 separated by SDS-PAGE were transferred onto nitrocellulose membranes, and the membranes were incubated for 30 min with 5% skim milk. For detection of parasite-derived As16, the membranes were incubated with the mouse anti-rAs16. Pig sera from animals with drug-abbreviated infection or mouse or rabbit sera from animals repeatedly inoculated with A. suum embryonated eggs were used for detection of the antigenicity of rAs16. After the membranes were washed with Tris-buffered saline-Tween 20, they were incubated with alkaline phosphatase-conjugated goat anti-mouse, anti-pig, or anti-rabbit immunoglobulin G (IgG; ICN Pharmaceuticals, Costa Mesa, Calif.) as a secondary antibody. After the membranes were washed, the proteins bound to the secondary antibody were visualized with 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium (NBT/BCIP; Gibco-BRL, Rockville, Md.).
Immunohistochemistry.
Parasites were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer and embedded in paraffin. The sections on glass slides were blocked for 1 h in 1% H2O2 in PBS containing 10% ethanol to inactivate endogenous peroxidase. For immunolocalization, the slides were treated with PBS containing 10% goat serum for 1 h at room temperature. They were then incubated with anti-mouse rAs16 (1:40) overnight at 4°C. The slides were rinsed thoroughly with PBS and reacted with a peroxidase-labeled mouse IgG secondary antibody and the substrate 3′,3′-diaminobenzidine tetrahydrochloride (Sigma Fast DAB set; Sigma, St. Louis, Mo.). After color development, the slides were dehydrated in a graded series of alcohol, cleared in xylene, and then covered with coverslips and observed under a microscope (Axiophot; Carl Zeiss, Jena, Germany).
Challenge infection and sampling.
Six-week-old female BALB/c mice (SLC) from a pathogen-free colony were used for challenge infection studies. The mice were divided into four groups of 10 animals each. CTB used as mucosal adjuvant was purchased from Sigma (purity, ≥95% [C-9903]). For preparation of conjugation, rAs16 was coupled with CTB in darkness at 4°C for 16 h. The immunized group was inoculated intranasally with 25 μg of rAs16 coupled with 20 μg of CTB under light ether anesthesia. On day 21, a booster inoculation of 15 μg of rAs16 coupled with 10 μg of CTB was given. A final boost of 15 μg of rAs16 coupled with 10 μg of CTB was given on day 35. The second and third groups were inoculated with the same dose of CTB or rAs16 alone, respectively, on the same days as the immunized group. The fourth group was given PBS alone. At 2 weeks after the final immunization, five animals from each group were inoculated orally with 2,500 A. suum infective embryonated eggs. The mice were euthanized on day 7. Their lungs were removed and minced with a surgical knife, and larvae were recovered by the method of Baermann as described by Slotved et al. (50, 51) and counted under a microscope. The five remaining animals in each group were euthanized on day 14 after the final immunzation and used for cytokine, serum antibody, and mucosal antibody assay. The small intestine was removed and put on an ice pack, and mucous tissues were removed with a surgical knife. The mucosal tissues were placed in an equal volume of PBS containing a protein inhibitor cocktail (Complete; Boehringer Mannheim, Mannheim, Germany) and then vortexed until the tissues were disrupted. The mixture was centrifuged at 26,000 × g for 60 min at 4°C, and the supernatant was stored at −80°C.
Cytokine assay.
Mice spleen cells were isolated by using the mechanical dissociation method by teasing through stainless steel screens (Sigma) and suspended in RPMI 1640 supplemented with 10% heat-activated fetal calf serum, 10 mM HEPES buffer, 0.2 mM l-glutamine, and 5 μg of gentamicin/ml. Cells were cultured at 37°C and 5% CO2 in 24-well plates at a final concentration of 4 × 106 in a final volume of 1.0 ml/well. The cells were stimulated with 5 μg of rAs16/ml for 72 h, and culture supernatant was stored at −80°C. Cytokine analysis was performed by sandwich enzyme-linked immunosorbent assays (ELISAs; eBioscience, San Diego, Calif.) for interleukin-2 (IL-2), IL-4, IL-10, and gamma interferon (IFN-γ) according to the manufacturer's protocol.
Antibody assays.
Measurement of mucosal IgA, serum IgG, and serum IgE and IgG subclass-specific antibody to rAs16 was performed by ELISA. The wells of polystyrene microplates (AE1640; Sumitomo, Tokyo, Japan) were coated with 100 μl of a 2-μg/ml concentration of rAs16 in 0.1 M carbonate buffer (pH 9.6). The plates were incubated at 4°C for 16 h and washed three times with PBS containing 0.05% Tween 20 (PBS-T). Wells were blocked with 100 μl of PBS-1% bovine serum albumin (Sigma) for 1 h at 37°C. After the wells were washed five times with PBS-T, serial dilutions of the mucosal extract from the small intestine or the serum were added, followed by incubation for 1 h at 37°C. After the incubation, the wells were washed five times with PBS-T, and 100 μl of horseradish peroxidase (HRP)-conjugated anti-mouse IgA or IgG (Bethyl Laboratories, Montgomery, Tex.) diluted 1:10,000 was added to the wells. The plates were incubated for 1 h at 37°C and washed five times with PBS-T. Detection was performed at 37°C with 100 μl of 2,2′azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS; Kirkegaard & Perry Laboratories, Gaithersburg, Md.), and the color reaction was terminated by the addition of 100 μl of 1% SDS. Plates were read at 405 nm in a microplate reader (Spectrafluor; Wako, Tokyo, Japan).
The IgE response was examined by using plates incubated with the concentration of rAs16 described above. After the antigens were bound to the plates, a diluted serum sample and rat monoclonal antibody anti-mouse IgE (American Research Products, Belmont, Mass.) diluted 1:10,000 were added, followed by incubation for 1 h at 37°C. After the plates were washed five times with PBS-T, they were incubated with HRP-conjugated goat anti-rat IgG (Bethyl Laboratories) at 37°C for 1 h. The plates were washed five times with PBS-T, and then color development was performed as described above. IgG subclass responses were detected by using plates incubated with the concentration of rAs16 described above. After the antigens were bound to the plates, a diluted serum sample and rabbit anti-mouse IgG1, IgG2a, IgG2b, or IgG3 (Bethyl Laboratories) were added, followed by incubation for 1 h at 37°C. After the plates were washed five times with PBS-T, they were incubated with HRP-conjugated goat anti-rabbit IgG at 37°C for 1 h. The plates were washed five times with PBS-T, and then color development was performed as described above. The endpoint titer was determined as the reciprocal log2 of the last dilution that gave an OD405 value above the OD405 value of the negative control samples from the fourth experimental group.
Statistical analysis.
The data are expressed as means ± standard deviations for the experimental groups. Comparisons between the experimental groups were performed by using the two-tailed Student t test.
Nucleotide sequence accession number.
The nucleotide sequence data reported in the present study are cited in the DDBJ/EMBL/GenBank database under accession number AB089179.
RESULTS
Molecular characterization of the cDNAs encoding As16.
Several cDNA clones were obtained by immunoscreening 5 × 105 plaques, and their DNA sequences were determined. BLASTX searches were performed to obtain cDNA clones coding low similarity against mammalian proteins stored in the current database. A clone designated L2R37 was therefore selected for further analysis in the present study. Sequence analysis showed that L2R37 was 615 bp long with 18 A's at the 3′ end. The ATG initiation codon is predicted to be at nucleotides 76 to 78 and is followed by a region encoding a hydrophobic sequence of 18 amino acids, which may function as a signal peptide. An entire ORF of the L2R37 cDNA encodes a putative polypeptide of 150 amino acids with a molecular mass of 16,426 Da (Fig. 1). Removal of the signal peptide resulted in a putative mature protein with a molecular mass of 14,429 Da and a pI of 5.23. A database search for the protein conducted with the information obtained from the NCBI revealed that As16 shared 34% similarity with the amino acid sequence of an Ov-17 immunodominant hypodermal antigen (GenBank accession no. P36991) from the human filarial parasite Onchocerca volvulus and WB14 antigen from Wuchereria bancrofti (AF063940). As16 also shared some homology with SXP-1 protein from Brugia malayi (AAA27864) and a Caenorhabditis elegans gene product (T15428). As16 had no significant amino acid sequence similarity with any known sequence except with those of filarial antigens and the C. elegans gene product, suggesting that the As16 antigen predicted by As16 cDNA is a nematode-specific gene product.
FIG. 1.
Comparison of the deduced amino acid sequences of As16 with the amino acid sequences of selected homologous molecules. The sequences are from W. bancrofti (WB-14; AF063940), O. volvulus (Ov-17; P36991), B. malayi (SPX-1; AAA27864), and C. elegans (Ce; T15428). Identical residues are marked with an asterisk, and conserved residues are marked with a dot. Gaps, marked by hyphens, were introduced for better alignment. The predicated signal peptides are shown in boldface.
Characterization of the recombinant As16.
The ORF of As16, except for the signal peptide sequence, was subcloned into the pTrcHisB protein expression vector. rAs16 was expressed in E. coli and found to migrate as a 20-kDa fusion protein with a hexa-histidine tag on SDS-PAGE. Immunoblot analysis was performed with T7 tag monoclonal antibody directed against the amino-terminal fusion peptide of rAs16. The epitope tag fusion peptide in rAs16 is ca. 4 kDa. Thus, rAs16 has an approximate molecular mass of 16 kDa, a finding similar to that predicted from the amino acid sequence of As16. rAs16 was purified by metal chelation chromatography under native conditions. Approximately 500 μg of purified rAs16 was obtained from a liter of bacterial culture. The rAs16 was 99% pure as determined by SDS-PAGE analysis (data not shown). The purified rAs16 was used for the production of polyclonal antibodies in mice and for a vaccine trial by using a mouse-A. suum infection model.
The human roundworm expresses As16.
Both A. suum and A. lumbricoides are well known as closely related ascarid species. We performed immunoblot analysis of A. lumbricoides infective L3 extract to identify the As16 homologue in ascarid nematodes. Figure 2 shows that a specific band reacted with anti-As16 antibody in the parasite extract of A. lumbricoides. No band was seen in the extract of T. canis. These results suggest that A. lumbricoides possesses the As16 homologue. In order to isolate cDNA encoding the As16 homologue in A. lumbricoides, we immunoscreened an A. lumbricoides infective L3 cDNA library by using mouse anti-As16 serum. Several positive clones were obtained by immunoscreening 2 × 105 plaques of the adult female A. lumbricoides cDNA library. Their DNA sequence analyses revealed that the ORF consisting of 450 bp almost matched except that two nucleotides; G and T at nucleotide positions 147 and 282, respectively, in the ORF of As16 were replaced with A and A in the ORF of A. lumbricoides. The deduced amino acid sequences were completely identical to that of As16. Thus, these studies indicate that the roundworms infecting humans and pigs possess a 16-kDa antigen consisting of the same amino acid sequences. Information on the immunogenicity of As16 may lead to further understanding of pig ascariasis, as well as human ascariasis.
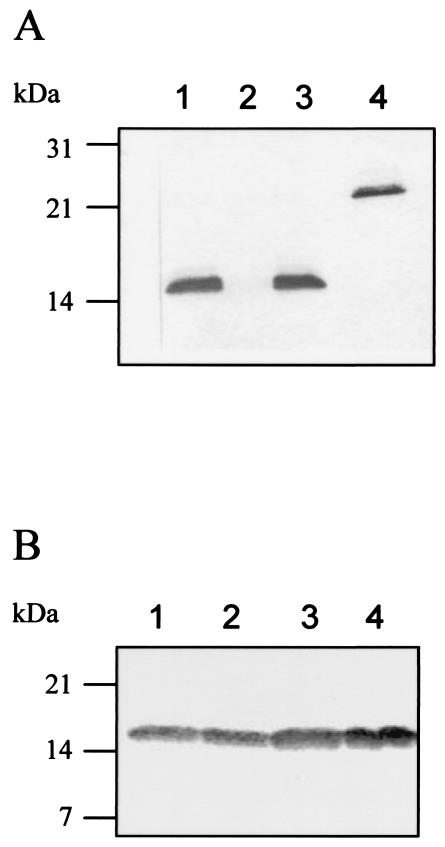
FIG. 2.
(A) As16 homologues in ascarid nematodes. Lane 1, A. lumbricoides; lane 2, T. canis; lane 3, A. suum; lane 4, rAs16 (50 ng). Forty micrograms of each parasite extract was used for immunoblot analysis with mouse anti-rAs16 serum. The As16 homologues bound to anti-rAs16 serum were detected by using NBT/BCIP. (B) Native As16 is secreted into ES products. Fifty micrograms of protein per lane was subjected to SDS-12% PAGE and then blotted onto a nitrocellulose membrane. The native As16 bound to anti-rAs16 serum was detected by using NBT/BCIP. Lane 1, L3; lane 2, lung-stage larvae; lane 3, adult female worm; lane 4, adult male worm.
Identification of native antigen corresponding to rAs16.
The native antigen corresponding to rAs16 was identified at various developmental stages of A. suum. The expression of the parasite-derived form corresponding to rAs16 was evaluated by immunoblot analysis with parasite extracts prepared from embryonated eggs, infective L3, lung-stage L3, and female and male adult worms. Mouse anti-rAs16 serum bound strongly to a 16-kDa parasite-derived antigen in parasite extracts from all stages (data not shown). We performed two-dimensional immunoblot analysis of the adult female extract to evaluate how much was produced in the parasite (data not shown). Antigens that reacted with mouse anti-rAs16 serum were detected as major spots, suggesting that native As16 was abundantly expressed. In addition, the native As16 was secreted to the outside of the parasites (Fig. 2). Serum from a preimmune mouse did not react with any antigens in the parasite extract (data not shown). We determined the anatomic location of native As16 by immunohistochemistry using anti-rAs16 antibody. Examination of a flat section showed strong reactivity in the hypodermis and cuticle, suggesting that native As16 is abundantly expressed there (Fig. 3). The epithelium of the intestine and the ovary also reacted with the antibody. Immunohistochemical localization of As16 in the human-associated roundworm was also performed with mouse anti-rAs16 antibody. Positive labelings observed in A. suum were seen in the same position of A. lumbricoides (data not shown).
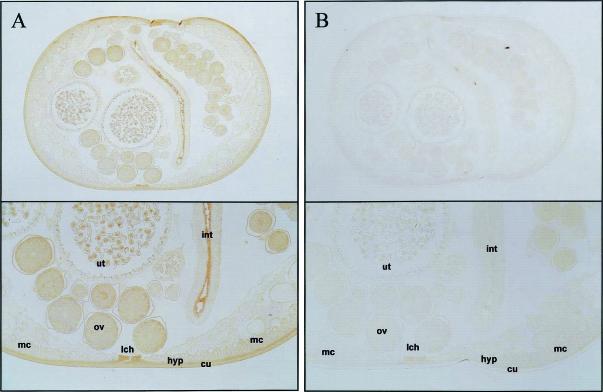
FIG. 3.
Immunohistochemical localization of As16 in adult female A. suum. Parasites were fixed in paraformaldehyde and embedded in paraffin as described in Materials and Methods. The sections were incubated with mouse anti-rAs16 sera at 1:150 (A) or preimmune sera at 1:150 (B) diluted in PBS, and the antibody binding was detected by using an HRP-labeled mouse IgG secondary antibody and Sigma Fast tablet. After color development, the slides were dehydrated in a graded series of alcohol and cleared in xylene. The slides then were covered with coverslips and observed with an Axiophot microscope. Upper panels show cross-sections of whole worm; lower panels show a magnified part of the cross-section. Abbreviations: hyp, hypodermis; int, intestine; lch, lateral chord; mc, muscle cell; ov, ovary; ut, uterus.
Reactivity of rAs16 with various immune sera.
The reactivity of rAs16 with serum from pigs with flubendazole-abbreviated infection was examined by using immunoblot analysis. The serum reacted with rAs16, suggesting that rAs16 was antigenic in the natural host (Fig. 4). Sera from rabbits and mice repeatedly inoculated with A. suum embryonated eggs or A. lumbricoides embryonated eggs also reacted with rAs16. We also performed ELISA of rAs16 with the same immune sera described above. The ELISA titer of the pig sera (14.0 ± 3.5 [expressed as the reciprocal log2 titer ± the standard deviation], n = 4) was significantly higher than that of the pig preimmune sera (<7, n = 5). Sera from rabbits immunized with A. suum or A. lumbricoides showed titers of 13.2 ± 2.8 (n = 3) and 13.0 ± 3.0 (n = 3), respectively. Sera from mice immunized with A. suum or A. lumbricoides showed titers of 11.2 ± 2.8 (n = 5) and 10.2 ± 3.0 (n = 5), respectively. The ELISA titer of both rabbit and mice preimmune sera was <7.
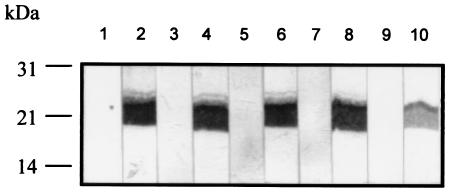
FIG. 4.
Reactivity of recombinant As16 with sera from rabbits, mice, and pigs repeatedly infected with A. suum (lanes 1 to 6) or A. lumbricoides (lanes 7 to 10) infective embryonated eggs. Fifty nanograms of protein in each lane was subjected to SDS-14% PAGE and then blotted onto a nitrocellulose membrane. The recombinant As16 bound to serum samples was detected by using NBT/BCIP. Lane 1, preimmune rabbit; lane 2, immune rabbit; lane 3, preimmune mouse; lane 4, immune mouse; lane 5, preimmune pig; lane 6, immune pig; lane 7, preimmune rabbit; lane 8, immune rabbit; lane 9, preimmune mouse; lane 10, immune mouse.
Immune response to intranasal vaccination.
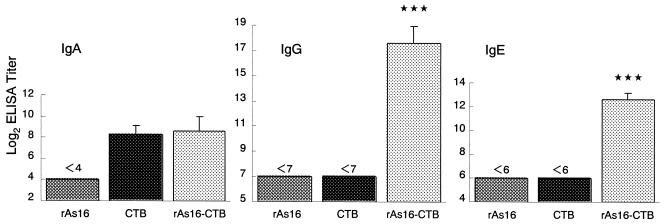
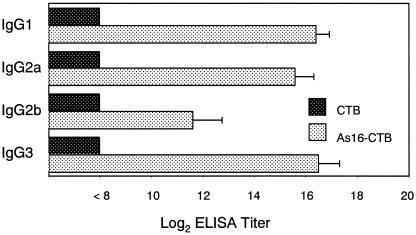
We measured the levels of rAs16-specific antibody responses in the mucous fluid from the small intestine and sera from mice intranasally immunized with rAs16-CTB. As shown in Fig. 5, predominant rAs16-specific IgA responses in the mucosal extracts, and IgG and IgE responses in the serum were noted. No detectable anti-As16 IgA antibody responses in the mucosal extracts or anti-As16 IgG and anti-As16 IgE antibody responses in the sera were seen in mice immunized with rAs16 alone or CTB alone. Furthermore, we examined rAs16-specific serum IgG subclass responses. All of the rAs16-specific IgG subclass titers were significantly higher in mice immunized with rAs16-CTB than those immunized with rAs16 alone or CTB alone (Fig. 6). No detectable IgG subclass anti-As16 antibody response was seen in mice immunized with CTB alone.
FIG. 5.
Antigen-specific mucosal IgA, serum IgG, and IgE antibody titers to rAs16 in mice intranasally immunized with rAs16. Mice were immunized as described in Materials and Methods. The titers are given as the reciprocal log2 of the highest dilution of serum that gave an OD405 greater than that seen with control (no serum added to the well). The value is expressed as the reciprocal log2 titer ± the standard deviation of the mean for each group of five mice. Asterisks indicate that the mean value was significantly higher than that of the rAs16-alone or CTB alone group (P < 0.001).
FIG. 6.
Serum IgG subclass responses to rAs16. The titer of each subclass is shown as the reciprocal log2 as described in Materials and Methods. Data are expressed as the mean values ± the standard deviations of the mean for each group of five mice.
Cytokine responses in vaccinated animals.
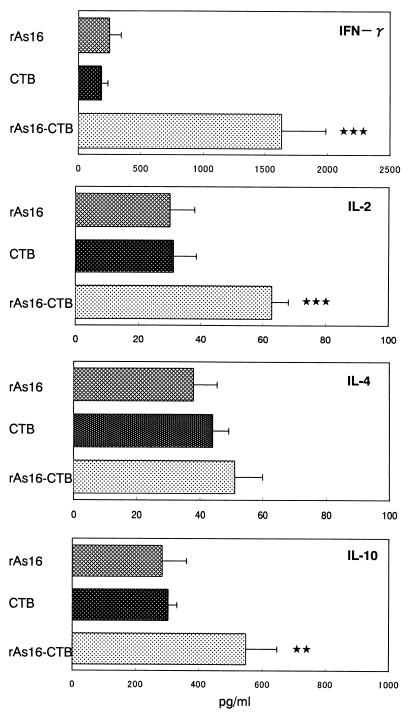
Cytokine secretion by rAs16-stimulated splenocytes was confirmed and quantified by ELISA. Splenocytes from mice immunized with rAs16-CTB secreted a significantly higher level of IFN-γ, IL-2, and IL-10 when stimulated with rAs16 than did splenocytes derived from mice immunized with rAs16 and CTB alone (Fig. 7). The release of IL-4 was not significantly higher in the mice immunized with rAs16-CTB than in the rAs16 and the CTB alone groups.
FIG. 7.
Levels of cytokine produced by splenic lymphocytes from mice intranasally vaccinated with rAs16. Cells were stimulated with rAs16 for 48 h, and the concentrations of cytokines in the supernatant were determined by ELISA. Data are expressed as the mean values ± the standard deviations of the mean for each group of five mice. Each sample was examined in triplicate. Asterisks indicate that the mean value was significantly higher than that of the group immunized with CTB alone (✽✽✽, P < 0.001; ✽✽, P < 0.01).
Intranasal vaccination against A. suum embryonated eggs.
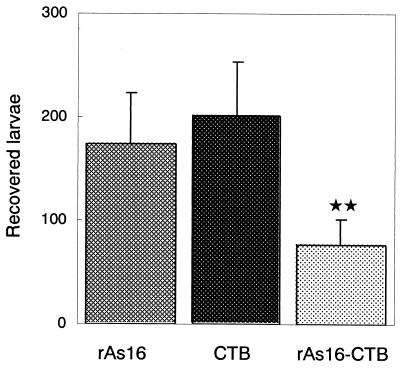
In our preliminary experiments, we observed an apparent reduction in the recovery of larvae from the lung in mice subcutaneously injected with rAs16-precipitated Freund complete adjuvant (rAs16-FCA). Mice which were immunized with rAs16-FCA (titers of 185 ± 58, n = 5) and which received two booster doses at a 2-week interval showed a 50% reduction in recovered larvae from the lungs after the challenge infection compared to either the nonimmunized group of mice or mice that received FCA alone (92 ± 36, n = 5). In the group of mice orally immunized with A. suum embryonated eggs, a 99% reduction in recovered larvae was found compared to the number in the nonimmunized or FCA-alone groups. A significant rAs16-specific IgG titer in the serum was found in both groups of mice immunized with rAs16-FCA (18.2 ± 1.2 [expressed as the reciprocal log2 titer ± the standard deviation], n = 5) and A. suum embryonated eggs (13.2 ± 2.2, n = 5), suggesting that protection against A. suum L3 infection may be immunologically induced in preliminary experiments. The test result pertaining to the nasal administration of rAs16-CTB is presented in Fig. 8. The group of mice inoculated intranasally with rAs16-CTB showed a significant reduction in the recovery of larvae from the lung compared to the CTB-alone or rAs16-alone groups after challenge with A. suum embryonated eggs. The same level of larval reduction was also observed in the repeatedly challenged experiments.
FIG. 8.
Number of larvae recovered from mice immunized with rAs16. Mice were immunized as described in Materials and Methods. Data are expressed as the mean values ± the standard deviations in each group of five mice. Asterisks indicate that the mean value was significantly lower than that of the group immunized with rAs16 or CTB alone (P < 0.01).
DISCUSSION
Numerous reports have shown that protective immune responses to A. suum infection can be achieved in pigs by immunization with irradiated A. suum infective L3 or by chemically abbreviated larval infection (18, 54, 60). These evidences suggest an important role of larval antigens in protective immunity against infection with A. suum. In order to isolate the immune-reactive antigens from various larval stages, we used a permissive host rabbit to raise antibodies against larvae by inoculating the rabbit repeatedly with A. suum embryonated eggs. Serum from the rabbit reacted with several recombinant clones in the A. suum infective L3 cDNA library. Among the several cDNA clones that reacted with the rabbit immune sera, clone L2R37 was selected due to its low similarity with mammalian proteins in the current database. We produced a recombinant protein derived from the ORF of L2R37 and determined its native form by immunoblot analysis of A. suum parasite extract with an antibody raised in mice against the recombinant protein. The antibody reacted with the 16-kDa antigen, which is now designated As16.
As16 displays a similar amino acid sequence to antigens from parasitic nematodes (46). However, extensive database searches failed to detect any similarity with any protein of known function. Parasite-specific antigens with no similarity to host proteins are desirable as parasite vaccine antigens because antibodies against them should not cross-react with the host proteins (2). Recently, abundant larval transcript (ALT) antigen, a highly immunoprotective antigen, was identified from the human filarial B. malayi (15). A vaccination study demonstrated that ALT gave the highest protection among recombinant antigens that have been cloned from parasitic filarial nemotodes. ALT has no similarity to mammalian proteins, suggesting that it is a parasite-specific molecule.
Analysis of the developmental stage-specific expression of As16 showed that As16 might be ubiquitously expressed at all life stages of A. suum. In addition, the present localization revealed that As16 was expressed at the hypodermis and cuticle as well as the epithelium of the intestine. As16 was also seen in ES products, suggesting that As16 is released outside of the parasite. The nematode cuticle is a complex extracellular structure that is secreted by the underlying syncytium of the hypodermis cells. Recent studies have shown that the cuticle of parasitic nematodes is a dynamic structure with important absorptive, secretory, and enzymatic activities (31) and not merely an inert protective covering as was once believed. Previous studies suggested that ES products from nematode parasitic stages changed the host physiology and suppressed host immune responses (10, 16, 42). In addition, ES products are tightly regulated as parasite specific and are likely to be involved in invasion or parasitism. Some investigators believe that these products may be associated with parasite survival (17, 63). Additional studies are needed to determine the biological function of As16 in Ascaris parasites.
It has been controversial for many years and is still a matter of debate as to whether A. lumbricoides Linnaeus 1758 from humans represents a different species or strain than A. suum Goeze 1782 from pigs. Humans or pigs can be infected with either of the two species, and various pathological abnormalities in humans are reportedly associated with A. suum infections (20, 32, 55). A number of attempts have been made to characterize the two species for diagnostic purposes but with little success (29, 33, 38). In the present study, it was found that As16 is well conserved in both human and pig ascariasis, indicating that As16 can be substituted as a molecule for protective antigens in human ascariasis. The use of the As16 molecule as a model to study various aspects of human diseases related to ascariasis is further justified from the present findings.
The generation of protective immune responses at the mucosal surface by nasal or oral administration is a critical goal in the development of a vaccine against intestinal pathogens. The present immunoblot analyses with sera from a variety of hosts immunized against A. suum embryonated eggs showed that rAs16 was antigenic. We examined whether vaccination with rAs16 induced protection in a mouse-A. suum model (22, 23, 49) in order to evaluate the use of rAs16 as a new vaccine candidate for parasitic diseases caused by Ascaris nematodes. Since the mucosal surface of the small intestine is the initial site of the A. suum infection, it is important to establish protective immunity there (23). It has been reported that administration of A. suum embryonated eggs to animals results in the induction of an A. suum infective L3-specific IgA response in the small intestine. However, a major problem with the delivery of antigens to the intestinal mucosa is that oral administration of soluble proteins gives rise either to no immune response or to the development of tolerance (62). In contrast, numerous reports have demonstrated that CTB induces both mucosal and systemic immunity after oral or nasal immunization (34, 47, 64). CTB is a nontoxic binding moiety of cholera toxin; it is composed of a ring of five identical polypeptides that bind with high affinity to GM1 and other ganglioside cell surface receptors and promote the entry of the A subunit into the cell (53). Oral or intranasal immunization has been shown to successfully produce protective immunity in a variety of viral, bacterial, and parasitic infections (33). In the present study, we performed nasal immunization with CTB as a mucosal adjuvant in a BALB/c mouse A. suum model. The number of larvae recovered from the lung was reduced by ca. 58% compared to that recovered from the control group, indicating that nasal immunization with rAs16 prevents the migration of larvae to the lung. We found that mice vaccinated with rAs16-CTB had high titers of rAs16-specific mucosal IgA and serum IgG, suggesting that rAs16-CTB induced both local and systemic protective immune responses against A. suum. However, no significant difference between rAs16-CTB and CTB-vaccinated mice was found. The degree of protection in mice immunized with rAs16-CTB was higher than that in mice parenterally immunized with rAs16 with FCA. Further analysis of rAs16-specific mucosal IgA antibody in mice vaccinated with rAs16-CTB will help to delineate the role of IgA in protection against invasion of A. suum infective L3.
It is also worth noting that an elevation of rAs16-specific IgE titer was seen in Th2 responder BALB/c mice vaccinated with rAs16-CTB. Similar findings with respect to antigen-specific IgE antibody associated with protection or resistance to reinfection have been shown in a variety of helminthic infections (12, 40). In human ascariasis, it has also been shown that Ascaris-specific IgE are associated with protection against human ascariasis (35). Recently, recombinant filarial tropomyosin antigen, a promising vaccine candidate, was found to induce protective immunity with a high titer of human IgE antibody response to the vaccinated antigen (21). Interestingly, its IgE epitope was shared with the IgG epitope of tropomyosin, suggesting that the protection against filarial infection is mediated by a hypersensitivity reaction. Studies are under way to examine the B-cell epitope of the As16 molecule by using sera from rAs16-vaccinated mice and pig immune sera against A. suum infection.
Mice immunized with rAs16 coupled with CTB had a high level of both anti-rAs16 IgG1 antibody and anti-rAs16 IgG2a antibody, suggesting that rAs16-CTB vaccination resulted in stimulating both type I and type II immune responses against rAs16 (36). In fact, immunization of rAs16 coupled with CTB elicited dominant IFN-γ and IL-2 production, markers of type I response, together with IL-10 production, a type II marker. However, IL-4 production, a marker of type II immune responses, was low. It has been reported that selected cytokines (IL-6 and IL-10) from type II cells likely play a compensatory role in the regulation of mucosal IgA responses (41). A high level of IFN-γ production might have led to reduced IL-4 production by splenic CD4+ T cells in mice mucosally immunized with rAs16-CTB, since IFN-γ and IL-4 affect each other as antagonists (43). On the other hand, the life cycle of ascarid nematodes involves two different phases consisting of larval and adult stages. Specific events in these two phases may provoke different host immune responses against the larval stages in the tissues and the adult worms in the small intestine of the natural host. The development of A. suum in mice includes passage through the larval stages before the development to adult worms. Recently, it was demonstrated that the migratory phase that occurs between L3 and the larval stage resulted in protective immunity against A. suum infection, but not against adult worms, in pigs (25). Mice vaccinated with rAs16 were protected against migration of A. suum larvae, suggesting that rAs16 is capable of killing the larval stages of A. suum by immune responses. Further analysis of mice immunized with As16-CTB may provide insight into the immunological mechanisms involved in host resistance against infection at the ascarid larval stage. In fact, immune responses against tissue-dwelling helminths are different from those against gastrointestinal parasites (13, 19).
Complete protection induced by immune responses with a single recombinant antigen has not been established in the field of parasitic infections. It seems reasonable to assume that protective immunity against parasites is suggestive of an interaction between several antigens associated with host immunity. Recently, immunization consisting of three protective antigens was performed in mice against challenge infection with the human filarial parasite O. volvulus (2). The ratio of protective immunity induced by the cocktail immunization with FCA as an adjuvant was the same as that observed when any of the single-antigen immunizations were tested. Systemic immunity might be a combination of peripheral and mucosal immunity, or it might be dominated by an incomplete form of immunity dictated by specific antigens (37). It has been shown that mucosal immunization with CTB is capable of inducing protective immunity due to mucosal immunity characterized by the release of antigen-specific IgA into the secretions, and peripheral immunity characterized by the appearance of antigen-specific IgG in the blood, although parental immunization does not significantly induce mucosal immunity (45). In the present study, the pathology associated with recombinant As16 (rAs16) and CTB, including tissue reactions at the site of adminstration, was not found (data not shown), although the nature of responses to rAs16 and rAs14 are not fully clarified in mucosally immunized animals. One of the current goals in the field of human and veterinary vaccines is the development of a noninvasive and practical route for administration via the mucosal surfaces. We previously developed a nasal immunization technique with rAs14 that involves protective immune responses against A. suum infection (58). Optimization with both rAs14 and rAs16-CTB by the mucosal immunization route may provide better protection against Ascaris infection in mice and should extend our understanding of the induction of protective immunity against human and pig ascariasis.
Acknowledgments
We thank Y. Ando and T. Fujisawa for excellent technical assistance.
This study was supported in part by a grant (Edible vaccine) from the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN).
Editor: J. M. Mansfield
REFERENCES
- 1.Abebe, W., N. Tsuji, H. Kasuga-Aoki, T. Miyoshi, T. Isobe, T. Arakawa, Y. Matsumoto, and S. Yoshihara. 2002. Lung-stage protein profile and antigenic relationship between Ascaris lumbricoides and Ascaris suum. J. Parasitol. 88:811-816. [DOI] [PubMed] [Google Scholar]
- 2.Abraham, D., O. Leon, S. Leon, and S. Lustigman. 2001. Development of a recombinant antigen vaccine against infection with the filarial worm Onchocerca volvulus. Infect. Immun. 69:262-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul, S. F., W. Gish, W. W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, T. J., M. E. Romero-Abal, and J. Jaenike. 1993. Genetic structure and epidemiology of Ascaris populations: patterns of host affiliation in Guatemala. Parasitology 107:319-334. [DOI] [PubMed] [Google Scholar]
- 5.Bairoch, A. 1992. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 20:2013-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, M. S. 1997. The global burden of intestinal nematode infections: fifty years on. Parasitol. Today 13:438-443. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, D., N. Orr, M. Haim, S. Ashkenazi, G. Robin, M. S. Green, M. Ephros, T. Sela, R. Slepon, I. Ashkenazi, D. N. Taylor, A. M. Svennerholm, A. Eldad, and J. Shemer. 2000. Safety and immunogenicity of two different lots of the oral, killed enterotoxigenic Escherichia coli-cholera toxin B subunit vaccine in Israeli young adults. Infect. Immun. 68:4492-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crandall, C. A., and R. B. Crandall. 1971. Ascaris suum: immunoglobulin responses in mice. Exp. Parasitol. 30:426-437. [DOI] [PubMed] [Google Scholar]
- 9.Cromptom, D. W. T. 2001. Ascaris and ascariasis. Adv. Parasitol. 48:285-375. [DOI] [PubMed] [Google Scholar]
- 10.Deehan, M. R., M. M. Harnett, and W. Harnett. 1997. A filarial nematode secreted product differentially modulates expression and activation of protein kinase C isoforms in B lymphocytes. J. Immunol. 159:6105-6111. [PubMed] [Google Scholar]
- 11.Eriksson, K., and J. Holmgren. 2002. Recent advances in mucosal vaccines and adjuvants. Curr. Opin. Immunol. 14:666-672. [DOI] [PubMed] [Google Scholar]
- 12.Faulkner, H., J. Turner, J. Kamgno, S. D. Pion, M. Boussinesq, and J. E. Bradley. 2002. Age- and infection intensity-dependent cytokine and antibody production in human trichuriasis: the importance of IgE. J. Infect. Dis. 185:665-672. [DOI] [PubMed] [Google Scholar]
- 13.Garside, P., M. W. Kennedy, D. Wakelin, and C. E. Lawrence. 2000. Immunopathology of intestinal helminth infection. Parasite Immunol. 22:605-612. [DOI] [PubMed] [Google Scholar]
- 14.Geenen, P. L., J. Bresciani, J. Boes, A. Pedersen, L. Eriksen, H. P. Fagerholm, and P. Nansen. 1999. The morphogenesis of Ascaris suum to the infective third-stage larvae within the egg. J. Parasitol. 85:616-622. [PubMed] [Google Scholar]
- 15.Gregory, W. F., A. k. Atmadja, J. E. Allen, and R. M. Maizels. 2000. The abundant larval transcript-1 and -2 genes of Brugia malayi encode stage-specific candidate vaccine antigens for filariasis. Infect. Immun. 68:4174-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harnett, W., and M. M. Harnett. 1999. Phosphorylcholine: friend or foe of the immune system? Immunol. Today 20:125-129. [DOI] [PubMed] [Google Scholar]
- 17.Hawdon, J. M., B. F. Jones, D. R. Hoffman, and P. J. Hotez. 1993. Cloning and characterization of Ancylostoma-secreted protein. A novel protein associated with the transition to parasitism by infective hookworm larvae. J. Biol. Chem. 271:6672-6678. [DOI] [PubMed] [Google Scholar]
- 18.Hill, D. E., R. H. Fetterer, R. D. Romanowski, and J. F. Urban, Jr. 1994. The effect of immunization of pigs with Ascaris suum cuticle components on the development of resistance to parenteral migration during a challenge infection. Vet. Immunol. Immunopathol. 42:161-169. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann, K. F., A. W. Cheever, and T. A. Wynn. 2000. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 164:6406-6416. [DOI] [PubMed] [Google Scholar]
- 20.Inatomi, Y., T. Murakami, M. Tokunaga, K. Ishiwata, Y. Nawa, and M. Uchino. 1999. Encephalopathy caused by visceral larva migrans due to Ascaris suum. J. Neurol. Sci. 164:195-199. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins, R. E., M. J. Taylor, N. J. Gilvary, and A. E. Bianco. 1998. Tropomyosin implicated in host protective responses to microfilariae in onchocerciasis. Proc. Natl. Acad. Sci. USA 95:7550-7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeska, E. L., J. F. Williams, and D. F. Cox. 1969. Ascaris suum: larval returns in rabbits, guinea pigs and mice after low-dose exposure to eggs. Exp. Parasitol. 26:187-192. [DOI] [PubMed] [Google Scholar]
- 23.Jeska, E. L., and M. Stankiewicz. 1989. Responses of NFR/N inbred mice to very low-dose infections with Ascaris suum. Int. J. Parasitol. 19:85-89. [DOI] [PubMed] [Google Scholar]
- 24.Johansson, E. L., L. Wassen, J. Holmgren, M. Jertborn, and A. Rudin. 2001. Nasal and vaginal vaccinations have differential effects on antibody responses in vaginal and cervical secretions in humans. Infect. Immun. 69:7481-7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jungersen, G., L. Eriksen, A. Roepstorff, P. Lind, E. N. Meeusen, T. Rasmussen, and P. Nansen. 1999. Experimental Ascaris suum infection in the pig: protective memory response after three immunizations and effect of intestinal adult worm population. Parasite Immunol. 21:619-630. [DOI] [PubMed] [Google Scholar]
- 26.Kelly, G., and D. P. Nayak. 1964. Acquired immunity to migrating larvae of Ascaris suum induced in pigs by repeated oral inoculation of infective eggs. J. Parasitol. 50:499-503. [PubMed] [Google Scholar]
- 27.Khoury, P. B., B. E. Stromberg, and E. J. Soulsby. 1977. Immune mechanisms to Ascaris suum in inbred guinea-pigs. I. Passive transfer of immunity by cells or serum. Immunology 32:405-411. [PMC free article] [PubMed] [Google Scholar]
- 28.Kozlowski, P. A., S. B. Williams, R. M. Lynch, T. P. Flanigan, R. R. Patterson, S. Cu-Uvin, and M. R. Neutra. 2002. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J. Immunol. 169:566-574. [DOI] [PubMed] [Google Scholar]
- 29.Kurimoto, H. 1974. Morphological, biochemical and immunological studies on the differences between Ascaris lumbricoides Linneaus, 1758 and Ascaris suum Goeze, 1782. Jpn. J. Parasitol. 23:251-267. [Google Scholar]
- 30.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 31.Maizels, R. M., and M. L. Blaxter, and M. E. Selkirk. 1993. Forms and functions of nematode surfaces. Exp. Parasitol. 77:380-384. [DOI] [PubMed] [Google Scholar]
- 32.Maruyama, H., Y. Nawa, S. Noda, T. Mimori, and W. Y. Choi. 1996. An outbreak of visceral larva migrans due to Ascaris suum in Kyushu, Japan. Lancet 347:1766-1767. [DOI] [PubMed] [Google Scholar]
- 33.Maung, M. 1973. Ascaris lumbricoides Linne, 1758 and Ascaris suum Goeze 1782: morphological differences between specimens obtained from man and pig. Southeast Asian J. Trop. Med. Pub. Health 1:41-45. [PubMed] [Google Scholar]
- 34.McGhee, J. R., and H. Kiyono. 1999. The mucosal immune system, p. 909-945. In W. E. Paul (ed.), Fundamental immunology, 4th ed. Lippincott-Raven, Philadelphia, Pa.
- 35.McSharry, C., Y, Xia, C. V. Holland, and M. W. Kennedy. 1999. Natural immunity to Ascaris lumbricoides associated with immunoglobulin E antibody to ABA-1 allergen and inflammation indicators in children. Infect. Immun. 67:484-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosmann, T. R., and R. L. Coffman. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145-173. [DOI] [PubMed] [Google Scholar]
- 37.Mosmann, T. R., and S. Sad. 1996. The expanding universe of T-cell subsets: Th1, Th2, and more. Immunol. Today 17:138-146. [DOI] [PubMed] [Google Scholar]
- 38.Nadler, S. A. 1987. Biochemical and immunological systematics of some Ascaridoid nematodes: genetic divergence between congeners. J. Parasitol. 73:811-816. [PubMed] [Google Scholar]
- 39.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 40.Nyindo, M., T. M. Kariuki, P. W. Mola, I. O. Farah, L. Elson, R. E. Blanton, and C. L. King. 1999. Role of adult worm antigen-specific immunoglobulin E in acquired immunity to Schistosoma mansoni infection in baboons. Infect. Immun. 67:636-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okahashi, N., M. Yamamoto, L. Vancott, S. N. Chatfield, M. Roberts, H. Bluethmann, T. Hiroi, H. Kiyono, and J. R. McGhee. 1996. Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+ Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses. Infect. Immun. 64:1516-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pastrana, D. V., N. Raghavan, P. FitzGerald. S. W. Eisinger, C. Metz, R. Bucala, R. P. Schleimer, C. Bickel, and A. L. Scott. 1998. Filarial nematode parasites secrete a homologue of the human cytokine macrophage migration inhibitory factor. Infect. Immun. 66:5955-5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pene, J., F. Rousset, F. Briere. I. Chretien, X. Paliard, J. Banchereau, H. Spits, and J. E. De Vries. 1988. IgE production by normal human B cells induced by alloreactive T-cell clones is mediated by IL-4 and suppressed by IFN-γ. J. Immunol. 141:1218-1224. [PubMed] [Google Scholar]
- 44.Peng, W., T. J. Anderson, X. Zhou, and M. W. Kennedy. 1988. Genetic variation in sympatric Ascaris populations from humans and pigs in China. Parasitology 117:355-361. [DOI] [PubMed] [Google Scholar]
- 45.Pizza, M., M. M. Giuliani, M. R. Fontana, E. Monaci, G. Douce, G. Dougan, K. H. Mills, R. Rappuoli, and G. Del Giudice. 2001. Mucosal vaccines: nontoxic derivatives of LT and CT as mucosal adjuvants. Vaccine 19:2534-2541. [DOI] [PubMed] [Google Scholar]
- 46.Rao, K. V., M. Eswaran, V. Ravi, B. Gnanasekhar, R. B. Narayanan, P. Kaliraj, K. Jayaraman, A. Marson, N. Raghavan, and A. L. Scott. 2000. The Wuchereria bancrofti orthologue of Brugia malayi SXP1 and the diagnosis of bancroftian filariasis. Mol. Biochem. Parasitol. 107:71-80. [DOI] [PubMed] [Google Scholar]
- 47.Ruedl, C., C. Rieser, N. Kofler, G. Wick, and H. Wolf. 1996. Humoral and cellular immune responses in the murine respiratory tract following oral immunization with cholera toxin or Escherichia coli heat-labile enterotoxin. Vaccine 14:792-798. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, New York, N.Y.
- 49.Slotved, H. C., L. Eriksen, K. D. Murrell, and P. Nansen. 1998. Early Ascaris suum migration in mice as a model for pigs. J. Parasitol. 84:16-18. [PubMed] [Google Scholar]
- 50.Slotved, H. C. L. Eriksen, K. D. Murrell, and P. Nansen. 1997. Comparison of methods for recovery of Ascaris suum larvae from tissues of mice. Int. J. Parasitol. 27:1305-1310. [DOI] [PubMed] [Google Scholar]
- 51.Slotved, H. C., A. Roepstorff, E. H. Barnes, L. Eriksen, and P. Nansen. 1996. Comparison of two methods for recovering migrating Ascaris suum larvae from the liver and lungs of pigs. J. Parasitol. 82:612-615. [PubMed] [Google Scholar]
- 52.Soulsby, E. J. L. 1986. Helminths, arthropods and protozoa of domesticated animals, 7th ed. Bailliere Tindall, London, England.
- 53.Spangler, B. D. 1992. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol. Rev. 56:622-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stankiewicz, M., and E. L. Jeska. 1990. Evaluation of pyrantel-tartrate abbreviated Ascaris suum infections for the development of resistance in young pigs against migrating larvae. Int. J. Parasitol. 20:77-81. [DOI] [PubMed] [Google Scholar]
- 55.Takeyama, Y., M. Kamimura, J. Suzumiya. K. Oh, M. Okumura, H. Akahane, H. Maruyama, Y. Nawa, T. Ohkawara, and M. Kikuchi. 1997. Case report: eosinophilic colitis with high antibody titer against Ascaris suum. J. Gastroenterol. Hepatol. 12:204-206. [DOI] [PubMed] [Google Scholar]
- 56.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsuji, N., H. Kasuga-Aoki, H., T. Isobe, and S. Yoshihara. 2000. Cloning and characterisation of a peroxiredoxin from the swine roundworm Ascaris suum. Int. J. Parasitol. 30:125-128. [DOI] [PubMed] [Google Scholar]
- 58.Tsuji, N., K. Suzuki, H. Kasuga-Aoki, Y. Matsumoto, T. Arakawa, K. Ishiwata, and T. Isobe. 2001. Intranasal immunization with recombinant Ascaris suum 14-kilodalton antigen coupled with cholera toxin B subunit induces protective immunity to A. suum infection in mice. Infect. Immun. 69:7285-7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsuji, N., T. H. Morales, V. V. Ozols, A. B. Carmody, and R. Chandrashekar. 1998. Molecular characterization of a calcium-binding protein from the filarial parasite Dirofilaria immitis. Mol. Biochem. Parasitol. 97:69-79. [DOI] [PubMed] [Google Scholar]
- 60.Urban, J. F., Jr., and F. G. Tromba. 1982. Development of immune responsiveness to Ascaris suum antigens in pigs vaccinated with ultraviolet-attenuated eggs. Vet. Immunol. Immunopathol. 3:399-409. [DOI] [PubMed] [Google Scholar]
- 61.Urban, J. F., Jr., and R. D. Romanowski. 1985. Ascaris suum: protective immunity in pigs immunized with products from eggs and larvae. Exp. Parasitol. 60:245-254. [DOI] [PubMed] [Google Scholar]
- 62.van Ginkel, F. W., H. H. Nguyen, and J. R. McGhee. 2000. Vaccines for mucosal immunity to combat emerging infectious diseases. Emerg. Infect. Dis. 6:123-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whelan, M., M. M. Harnett, K. M. Houston, V. Patel, W. Harnett, and K. P. Rigley. 2000. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J. Immunol. 164:6453-6460. [DOI] [PubMed] [Google Scholar]
- 64.Yamamoto, M., J. R. McGhee, Y. Hagiwara, S. Otake, and H. Kiyono. 2001. Genetically manipulated bacterial toxin as a new generation mucosal adjuvant. Scand. J. Immunol. 53:211-217. [DOI] [PubMed] [Google Scholar]