Abstract
Ehrlichia chaffeensis and Anaplasma phagocytophilum are agents of human monocytic and granulocytic ehrlichioses, respectively. They are extremely sensitive to mechanical stress and are pleomorphic gram-negative bacteria. Membrane incorporation of cholesterol from the eukaryotic host is known to be essential for other fragile and pleomorphic bacteria and mycoplasmas that lack a cell wall. Thus, we tested whether cholesterol is required for E. chaffeensis and A. phagocytophilum. Using a freeze fracture technique and biochemical analysis, these bacteria were found to contain significant levels of membrane cholesterol. These bacteria lack genes for cholesterol biosynthesis or modification. However, host cell-free bacteria had the ability to take up directly exogenous cholesterol or NBD-cholesterol, a fluorescent cholesterol derivative. Treatment of the bacteria with cholesterol extraction reagent methyl-β-cyclodextrin caused their ultrastructural changes. Furthermore, pretreatment of the bacteria with methyl-β-cyclodextrin or NBD-cholesterol deprived these bacteria of the ability to infect leukocytes, thus killing these obligate intracellular bacteria. Analysis of E. chaffeensis and A. phagocytophilum genome sequences revealed that these bacteria lack all genes for the biosynthesis of lipid A and most genes for the biosynthesis of peptidoglycan, which confer structural strength to gram-negative bacteria. Taken together, these results suggest that human ehrlichiosis agents became cholesterol dependent due to the loss of these genes. As the first report of gram-negative bacteria incorporating cholesterol for survival, these findings offer insight into the unique nature of their parasitism and imply that cholesterol is important in the control of human ehrlichioses.
Ehrlichioses are emerging infectious diseases caused by gram-negative obligate intracellular bacteria in the family Anaplasmataceae. Five species in this family are known to infect humans (31). Among them, the best documented are the recently discovered Ehrlichia chaffeensis, the agent of human monocytic ehrlichiosis (HME), and Anaplasma phagocytophilum, the agent of human granulocytic ehrlichiosis (HGE). Human ehrlichioses are severe influenza-like febrile illnesses that frequently require hospitalization, and 2 to 4% of reported cases were fatal (3, 12, 41). Because of the disease's potential, in 1998 human ehrlichioses became one of the nationally reportable diseases (18).
Like Lyme disease and Rocky Mountain spotted fever infections, infections of these pathogens are acquired by humans by the bite of infected ticks from wild-animal reservoirs (3, 12, 26, 41). Once transmitted to humans, E. chaffeensis and A. phagocytophilum have the remarkable ability to parasitize first-line immune defensive cells (monocytes/macrophages and neutrophils, respectively) as their exclusive survival sites (31). These bacteria then replicate in membrane-bound inclusions in the host cytoplasm secluded from host immune surveillance and destruction by lysosomes and reactive oxygen intermediates (4, 30, 31, 42). Although several host cell signals required for infection by these bacteria have been elucidated (17, 31), the details of the mechanism(s) by which these bacteria evade powerful microbicidal activity of the host remain to be discovered.
Unlike other gram-negative bacteria or even the closely related obligate intracellular bacterium Rickettsia, members of the family Anaplasmataceae display several unique characteristics. They are extremely sensitive to mechanical stress such as sonication, freezing and thawing, and osmolarity changes (36; our personal observations). In addition, they are highly pleomorphic and enveloped with a rippled thin outer membrane which lacks thickening of the inner or outer leaflet and shows no sign of a peptidoglycan layer or lipopolysaccharide (LPS), as reported in previous studies (29, 32-35). These unique physical characteristics suggest the unusual cell wall compositions found in bacteria of the family Anaplasmataceae. Mycoplasmas, although they lack a cell wall and are extracellular parasitic bacteria, share some physical characteristics with members of the family Anaplasmataceae, such as being extremely fragile and pleomorphic. Mycoplasmas are the only prokaryotes to date known to incorporate cholesterol or related sterols from hosts or environments to stabilize the cytoplasmic membrane, while they lack genes to synthesize or modify sterols (10). Therefore, we examined whether E. chaffeensis and A. phagocytophilum can incorporate exogenous cholesterol into their membranes and tested the role of bacterium-incorporated cholesterol in infecting host cells. We also determined whether genes homologous to those required for the biosynthesis of LPS and peptidoglycan in Rickettsia, the most closely related bacterium, are present in human ehrlichiosis agents.
MATERIALS AND METHODS
Organisms.
E. chaffeensis strain Arkansas and A. phagocytophilum strain HZ were cultivated in human leukemia cell lines (THP-1 and HL-60 cells, respectively) in RPMI 1640 supplemented with 10% fetal bovine serum and 2% l-glutamine at 37°C in 5% CO2 and 95% air (6, 35). No antibiotic was used throughout the study.
To prepare host cell-free E. chaffeensis and A. phagocytophilum with minimum damage to the bacteria, infected host cells were homogenized in sucrose-potassium buffer (0.2 M sucrose, 0.02 M potassium phosphate buffer, pH 7.4) for 20 strokes with a loose-fitted Dounce homogenizer. After removing nuclei and unbroken cells by low-speed centrifugation, the host cell-free organisms in the supernatant were pelleted by centrifugation at 10,000 × g for 10 min.
Filipin labeling and freeze fracture.
Host cell-free bacteria were fixed in 3% glutaraldehyde for 30 min and labeled with 50 μg of filipin III (Sigma, St. Louis, Mo.)/ml for 1 h at room temperature with rotation (37). The same volume of dimethyl sulfoxide (0.5% final concentration), a solvent of filipin, was added to control groups. Bacteria were prepared for freeze fracture as described previously (37). Briefly, bacteria were washed three times in 0.1 M sodium cacodylate buffer and then cryoprotected with 30% glycerol-0.1 M cacodylate buffer for 1 h on ice prior to freeze fracturing. Samples were then gently pelleted, the thick slurry of the bacteria was mounted in a gold-plated specimen carrier, and the specimen was quickly frozen in liquid ethane cooled by liquid nitrogen. Freeze fracture was performed in a Balzer 400T freeze fracture apparatus, and samples were immediately shadowed with platinum at a 45° angle and coated with carbon. Replicas were cleaned with Clorox and examined with a Philips CM12 transmission electron microscope (TEM) operated at 60 kV.
Cholesterol assay of purified bacteria.
The host cell-free bacteria were further purified by Percoll density gradient centrifugation at 61,900 × g for 30 min at 4°C with or without the addition of 20 μg of water-soluble cholesterol (Sigma)/ml (24). The purified bacteria were washed three times in phosphate-buffered saline (PBS; pH 7.4), lysed in PBS containing 1% NP-40 and 0.1% sodium dodecyl sulfate, and sonicated for 10 s to shear the DNA. Escherichia coli or uninfected host cells cultured in the same medium and lysed by the same procedure were used as controls. Using an Amplex Red cholesterol assay kit according to the instructions of the manufacturer (Molecular Probes, Eugene, Oreg.), the cholesterol contents were determined with a Gemini XS spectropfluorometer (Molecular Devices, Sunnyvale, Calif.). The total cholesterol content was normalized for the total protein concentration determined with bicinchoninic acid reagent (Pierce, Rockford, Ill.).
Fluorescence labeling of bacteria.
For filipin labeling, host cell-free bacteria were fixed in 3% paraformaldehyde for 15 min and incubated with 50 μg of filipin III/ml in PBS for 1 h at room temperature. To test the incorporation of NBD-cholesterol (22-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-23,24-bisnor-5-cholen-3β-ol; Molecular Probes), bacteria were incubated with 10 μg of NBD-cholesterol/ml at 37°C for 30 min, washed twice with PBS, and fixed in 3% paraformaldehyde. To test the specificity of NBD-cholesterol incorporation, host cell-free bacteria were also incubated with 10 μg of NBD-ceramide {N-[12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]dodecanoyl]-ceramide} (Sigma)/ml at 37°C for 30 min. Filipin-, NBD-cholesterol-, or NBD-ceramide-labeled bacteria were then surface labeled with dog anti-E. chaffeensis serum preadsorbed with THP-1 cells or horse anti-A. phagocytophilum serum preadsorbed with HL-60 cells for 1 h at room temperature. After two washes with PBS, bacteria were incubated with lissamine rhodamine-conjugated anti-dog or anti-horse secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, Pa.) for 30 min. As negative controls, infected THP-1 or HL-60 cells were incubated with secondary conjugated antibodies alone or with preimmune dog or horse serum and rhodamine-conjugated anti-dog immunoglobulin G or anti-horse immunoglobulin G, respectively (5). Fluorescence-labeled bacteria were observed under a Nikon Eclipse E400 fluorescent microscope with a xenon-mercury light source.
Bacterial treatments and examination of ultrastructure and infectivities.
Host cell-free bacteria were preincubated with 10 mM methyl-β-cyclodextrin (MβCD) (Sigma)-20 μg of water-soluble cholesterol/ml or -10 μg of NBD-cholesterol/ml at 37°C for 5 to 30 min. After being washed with PBS, the bacteria were added to their respective host cells and the infection was determined after 3 days of culture growth by counting the bacterial numbers in 100 cells in triplicate wells (17). TEM of bacteria treated with MβCD was performed as previously described (34). Briefly, bacteria were fixed in 3% glutaraldehyde-2% formaldehyde-0.02% trinitrophenol-0.1 M sodium cacodylate buffer (pH 7.4), stained at 4°C in reduced osmium tetraoxide (1% OsO4 and 1% potassium ferrocyanide) for 1 h, and rinsed three times with cold 0.1 M cacodylate buffer. After uranyl acetate block staining, the bacteria were then dehydrated with a graded series of ethanol. Ultra-thin sections (60 nm) were stained with uranyl acetate and lead citrate and observed under a Philips EM 300 TEM.
Genes for the biosynthesis of LPS and peptidoglycan in the family Anaplasmataceae or other related bacteria.
Data pertaining to essential genes required for the biosyntheses of lipid A (the essential component of LPS), murein sacculus (the essential component of peptidoglycan), and diaminopimelate (an amino acid unique to peptidoglycan) in Rickettsia prowazekii (GenBank accession no. AJ235269) (2) were used to search microbial genome databases. Homology searches were performed using BLASTP (or TBLASTN if annotated protein sequences were not available), with default settings as provided at the National Center for Biotechnology Information (NCBI) website (http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi?). Microbial genome database data used included incomplete sequences of a Wolbachia endosymbiont of Drosophila melanogaster (GenBank accession number NC_002978) (∼85% completion as of 10 January 2003) and completed genome sequences of Rickettsia conorii (AE006914), Coxiella burnetii (AE016828), Agrobacterium tumefaciens (AE008688 and AE008689), Mesorhizobium loti (BA000012), Sinorhizobium meliloti (AL591688), Brucella suis (AE014291), and Chlamydia trachomatis (AE001273) from NCBI. In addition, completed genome sequences of E. chaffeensis (NC_004127) and Neorickettsia sennetsu (NC_004620) and an incomplete sequence of A. phagocytophilum (>98% completion) (NC_004351) were obtained from The Institute for Genomic Research (http://www.tigr.org). Only sequences of genes with expect values (E values) of <10−5 were considered significant homologous sequences.
RESULTS
E. chaffeensis and A. phagocytophilum have membrane cholesterol.
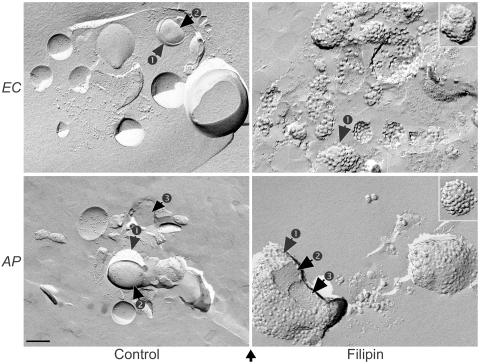
To determine whether these bacteria contain cholesterol in their membranes, we first employed a freeze fracture technique. When bacteria are incubated with filipin, a polyene compound which binds specifically to cholesterol (38), a filipin-cholesterol complex can be visualized by this technique as intramembranous protuberances at diameters of around 20 to 25 nm if cholesterol is present in the membrane (37). Results showed that characteristics of filipin-cholesterol complexes were distributed evenly throughout the outer membranes of both E. chaffeensis and A. phagocytophilum (Fig. 1, right panels). This protuberance was specific to filipin-treated bacteria, since bacteria without any treatment (32) and control bacteria treated with the same concentration of the solvent (dimethyl sulfoxide) did not show such membrane protuberances, although membrane protein particles of much smaller sizes were visible (Fig. 1, left panels).
FIG. 1.
Freeze fracture of filipin-labeled host cell-free E. chaffeensis and A. phagocytophilum. Freeze-fractured replicas of filipin-labeled E. chaffeensis (EC) and A. phagocytophilum (AP) were examined by TEM. Intramembranous protuberances of filipin-cholesterol complexes in diameters of around 20 to 25 nm can be seen in the outer membranes (arrow 1) of filipin-treated EC and AP but are absent from the inner membrane (arrow 2) and the cytosol (arrow 3) of the bacteria. The bottom arrow indicates the direction of shadowing. Bar, 0.2 μm.
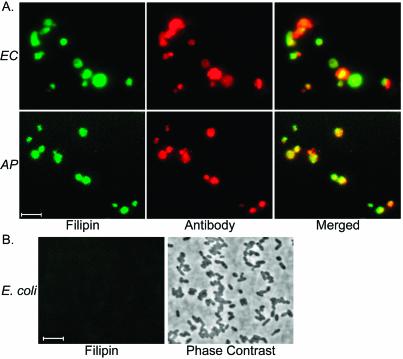
To confirm this observation, we labeled filipin-incubated host cell-free bacteria with anti-E. chaffeensis or anti-A. phagocytophilum antibody to examine the colocalization of cholesterol with bacterial-surface antigens. Cholesterol-bound filipin exhibits a strong fluorescence at 480 nm when excited at 360 nm in low-polarity environments, allowing it to be detected by fluorescence microscopy (11). We found colocalization of cholesterol with bacterial-surface antigens in both E. chaffeensis and A. phagocytophilum (Fig. 2A) The binding of filipin was specific to these bacteria, as there was no binding of filipin to another gram-negative bacterium (E. coli) under the same incubation conditions (Fig. 2B). In addition, negative controls (described in Materials and Methods) did not label E. chaffeensis or A. phagocytophilum or any cellular structures.
FIG. 2.
Fluorescence microscopy of filipin-labeled E. chaffeensis and A. phagocytophilum (A) and E. coli (B). (A) Fluorescence emitted by filipin-labeled E. chaffeensis (EC) and A. phagocytophilum (AP) was localized in the bacterial surface labeled with bacterium-specific antibodies. (B) Fluorescence emitted by filipin is undetectable in E. coli (E. coli was visualized by phase contrast). For easier viewing, the blue fluorescence of filipin was converted to green pseudo-color in Adobe Photoshop. Bars, 5 μm.
The amounts of cholesterol were biochemically determined in bacteria purified by Percoll density-gradient centrifugation. This procedure generates highly purified bacteria free of membrane debris and host cell organelles, as previously characterized by TEM (24). As shown in Table 1 these two bacterial strains had higher levels of total cholesterol content per milligram of protein than those seen with their host cells (∼130 μg of total cholesterol/mg of protein in bacteria compared to ∼80 μg of total cholesterol/mg of protein in host cells). Of note, the percentages of unesterified cholesterol in the total cholesterol of bacteria were very similar to those of their respective host cells (∼91% in E. chaffeensis and its host THP-1 cells; ∼82% in A. phagocytophilum and its host HL-60 cells), suggesting that these bacteria take up cholesterol or its derivatives directly from their host cells without modifications. In contrast, cholesterol was undetectable in E. coli by this assay method (data not shown).
TABLE 1.
Cholesterol contents of purified E. chaffeensis and A. phagocytophilum and of their respective host THP-1 and HL-60 cells
| Host cell and bacterium | Total cholesterol content (μg/mg of protein)a | % Unesterified cholesterol in total cholesterolb |
|---|---|---|
| HL-60 | 79.3 ± 5.7 | 82.3 |
| A. phagocytophilum | 128.4 ± 2.9 | 81.8 |
| A. phagocytophilum + cholesterolc | 464.3 ± 1.6 | 98.4 |
| THP-1 | 81.9 ± 2.9 | 91.2 |
| E. chaffeensis | 130.8 ± 0.5 | 91.4 |
| E. chaffeensis + cholesterolc | 549.8 ± 2.7 | 99.2 |
Cholesterol contents are expressed as micrograms of total cholesterol (esterified and unesterified cholesterol) per milligram of total protein. Data are expressed as means ± standard deviations (n = 3) and are representative of three independent experiments with similar results.
Unesterified cholesterol contents were determined in an Amplex Red cholesterol assay system without the addition of cholesterol esterase.
Exogenous water-soluble unesterified cholesterol was added to the Percoll-SPK buffer used during bacterial purification.
E. chaffeensis and A. phagocytophilum can take up exogenous cholesterol.
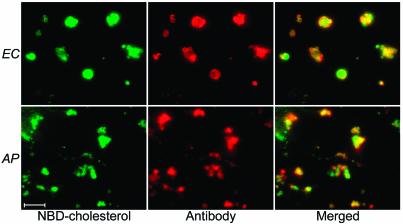
According to comparisons (using BLASTX with default settings as provided by the NCBI website [http://www.ncbi.nlm.nih.gov/BLAST/]) of the genome sequences of E. chaffeensis and A. phagocytophilum from all available protein sequence databases, there are no genes related to the biosynthesis or modification of cholesterol or related sterols in these bacteria (data not shown). This information, and the identical percentages of unesterified cholesterol in the total cholesterol of bacteria and host cells (Table 1), suggested that cholesterol in these bacteria can be taken up from the environment. To test this hypothesis, water-soluble unesterified cholesterol was added to the Percoll-sucrose-potassium buffer used during the purification of the bacteria. Compared to bacteria purified without the addition of cholesterol, the cholesterol amount in bacteria purified with cholesterol supplementation increased by threefold (>400 μg of cholesterol/mg of protein), and almost all the cholesterol content was unesterified cholesterol (98 to 99%) (Table 1). The uptake of exogenous cholesterol was specific to these bacteria, since under the same incubation conditions there was no cholesterol uptake by another gram-negative bacterium, E. coli (data not shown). To confirm this observation, host cell-free E. chaffeensis and A. phagocytophilum were incubated with NBD-cholesterol, a fluorescent cholesterol derivative that can be incorporated in membranes of eukaryotic cells (21). Fluorescence microscopy showed that NBD-cholesterol was indeed localized in these bacteria (Fig. 3) NBD-ceramide, a fluorescently labeled precursor of sphingolipid used as a negative control, did not label host cell-free E. chaffeensis or A. phagocytophilum, indicating that NBD-cholesterol incorporation was a consequence of the presence of cholesterol but not of NBD (data not shown). This observation is consistent with the previous result of Mott et al., which showed that neither inclusions of A. phagocytophilum nor the bacterium itself accumulated NBD-ceramide (20). In contrast, sphingomyelin (endogenously synthesized from NBD-ceramide) is transported to the inclusions of C. trachomatis and incorporated into the bacterial membrane (13). Taken together, these results demonstrated that E. chaffeensis and A. phagocytophilum can directly take up exogenous cholesterol or its derivatives with no measurable modification of the incorporated cholesterol.
FIG. 3.
NBD-cholesterol was directly incorporated into E. chaffeensis (EC) and A. phagocytophilum (AP). Fluorescence microscopy showed that the green fluorescence emitted by NBD-cholesterol was localized in the bacterial surface labeled with bacterium-specific antibodies, indicating the direct uptake of NBD-cholesterol by these bacteria. Bar, 5 μm.
Cholesterol is required in Ehrlichia spp. for maintenance of structural integrity.
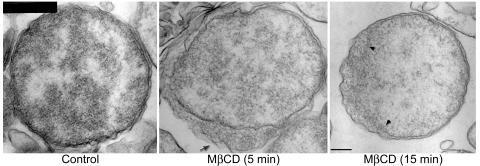
Since mycoplasmas undergo irreversible lysis when treated with certain sterol binding agents such as digitonin and streptolysin-O (7, 28), we tested whether cholesterol is required for maintaining the structural integrity of E. chaffeensis. For this study, MβCD, a cage-like compound that is known to selectively extract cholesterol from the plasma membrane, was used at a concentration that does not reduce eukaryotic cellular viability (25). Host cell-free E. chaffeensis was treated with 10 mM MβCD at 37°C for 5 or 15 min, and the changes in the ultrastructure and the time period required to elicit a detectable effect were determined. In contrast to results showing the tightly spaced inner and outer membranes characteristic of host cell-free E. chaffeensis in the control group, MβCD induced irregular dilations (filled with amorphous materials presumably derived from the cytoplasm) of the periplasmic space in ∼50% of bacteria as early as 5 min after treatment (Fig. 4). When the treatment with MβCD was extended to 15 min, the bacterial cytoplasmic content became sparse due to the exudation of ribosomes and other cytoplasmic contents to the periplasm (through the apparent discontinuity of the inner membrane of E. chaffeensis organisms) in ∼50% of bacteria (Fig. 4). This result demonstrated that membrane cholesterol plays a critical role in maintaining the physical integrity of these bacteria.
FIG. 4.
The ultrastructure of E. chaffeensis was impaired by MβCD treatment. Host cell-free E. chaffeensis was treated with 10 mM MβCD at 37°C for 5 or 15 min, and the control group was incubated with RPMI medium, a solvent of MβCD, in the same conditions for 15 min. The arrow in the middle panel indicates irregular dilations of the periplasmic space in E. chaffeensis treated with MβCD for 5 min. The area between the two arrowheads in the right panel shows the discontinuity of the inner membrane in E. chaffeensis treated with MβCD for 15 min. More than 50% of the treated bacteria showed changes (indicated by arrow and arrowheads) compared to bacteria in the control group. Bar, 0.2 μm.
The role of cholesterol in E. chaffeensis and A. phagocytophilum in host leukocyte infections.
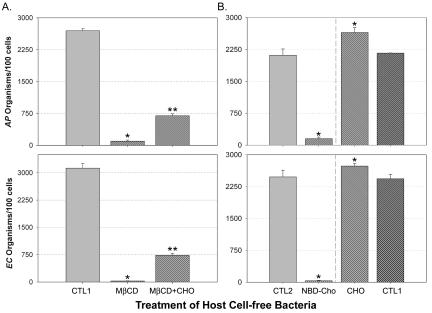
To determine whether the ultrastructural changes resulting from the removal of cholesterol affect the ability of these bacteria to infect host cells, E. chaffeensis and A. phagocytophilum were pretreated with MβCD for various periods. After washes were performed to prevent residual MβCD from removing cholesterol from host cells, bacteria were incubated with their host cells. Results showed that 30 min of MβCD pretreatment was required to effectively block these bacteria from infection of their host cells (Fig. 5A). At 15 min of MβCD treatment, ∼50% inhibition of the bacterial infection was observed (data not shown). Such inhibition could be partially reversed by supplementing E. chaffeensis and A. phagocytophilum with 20 μg of water-soluble cholesterol/ml during preincubation (Fig. 5A). This result suggests that cholesterol is essential in the membranes of E. chaffeensis and A. phagocytophilum for infection of their host cells and thus for the survival of the infecting bacteria.
FIG. 5.
MβCD (A) and NBD-cholesterol (B) blocked the infection of E. chaffeensis and A. phagocytophilum in host cells. Host cell-free E. chaffeensis (EC) and A. phagocytophilum (AP) were incubated with 10 mM MβCD, 20 μg of water-soluble cholesterol (CHO)/ml, or 10 μg of NBD-cholesterol (NBD-Cho)/ml at 37°C for 30 min. Infectivities were determined at day 3 postinfection. CTL1, bacteria treated with same volume of RPMI as the control groups for MβCD and cholesterol; CTL2, bacteria treated with same volume of methanol (0.05% final concentration in RPMI) as a control for NBD-cholesterol. One asterisk (*) indicates a statistical difference (P < 0.05) from the control groups and two asterisks (**) indicate a statistical difference (P < 0.05) from the MβCD-treated groups, as determined by Student's t test. Data are representative of three experiments.
Since secondary membrane damages caused by cholesterol extraction or an associated loss of viability may reduce the infectivity of Ehrlichia and Anaplasma spp., we next examined the role of cholesterol in the bacterial infection without extracting cholesterol. The cholesterol derivative NBD-cholesterol was incorporated into the membrane in place of cholesterol, thus preserving the intact membrane; however, due to the presence of a bulky and polar NBD group, NBD-cholesterol often fails to mimic cholesterol functions faithfully (21). NBD-cholesterol was incorporated directly into the membranes of E. chaffeensis and A. phagocytophilum, as shown in Fig. 3, but this incorporation rendered these bacteria completely unable to infect or survive in host cells (Fig. 5B). In contrast, the preincubation of water-soluble cholesterol with host cell-free E. chaffeensis and A. phagocytophilum promoted the infection of these bacteria, suggesting a critical role for cholesterol in the bacterial membrane in infections of host cells (Fig. 5B).
E. chaffeensis and A. phagocytophilum lack all genes for lipid A biosynthesis and most genes for murein sacculus biosynthesis.
In gram-negative bacteria, LPS and peptidoglycan are essential cell wall components, having significant roles in providing strength to the outer membrane and maintaining overall structural integrity (22, 27). Unique physical characteristics and previously determined physical and molecular evidence (29, 32-35, 42) suggest the absence of LPS and peptidoglycan from members of the family Anaplasmataceae. Thus, when draft genome sequences of E. chaffeensis and A. phagocytophilum became available, we examined whether genes for the biosynthesis of lipid A (an essential component of LPS) and peptidoglycan were present in these bacteria.
R. prowazekii and R. conorii, the closest relatives of the family Anaplasmataceae, have all of the genes for the biosynthesis of lipid A and peptidoglycan in their genomes (2, 23). Thus, we searched in all four genera (Ehrlichia, Anaplasma, Neorickettsia, and Wolbachia) of the family Anaplasmataceae and other related bacteria for sequences homologous to sequences of the biosynthesis genes for LPS and peptidoglycan in R. prowazekii. As shown in Table 2 none of the genes for the biosynthesis of lipid A and few of the genes for the biosynthesis of murein sacculus were found in the genome sequences of E. chaffeensis, A. phagocytophilum, and N. sennetsu. Wolbachia spp. (endosymbionts of invertebrates in the family Anaplasmataceae) also lacked lipid A biosynthesis genes but retained nearly all of the genes for the biosynthesis of diaminopimelate and murein sacculus (Table 2). However, since the genome sequences of A. phagocytophilum and Wolbachia spp. are presently incomplete, a few genes that belong to these categories might be present in the unsequenced region. Further analysis (comparing the sequences of all genes in the finished genome sequence of E. chaffeensis to those in the database of clusters of orthologous groups of proteins [COG] provided at the NCBI website [http://www.ncbi.nlm.nih.gov/COG/]) of pertinent sequences confirmed that there are no genes in functional categories related to the biosynthesis of lipid A or peptidoglycan (data not shown). In contrast, nearly all genes for the biosynthesis of lipid A and peptidoglycan homologous to those in R. prowazekii were found in other members of the α-Proteobacteria, to which the family Anaplasmataceae belongs, including R. conorii, A. tumefaciens, S. meliloti, M. loti, and B. suis, and in other obligate intracellular bacteria such as C. burnetii and C. trachomatis (Table 2).
TABLE 2.
Genes for the biosynthesis of lipid A and peptidoglycan in selected members of the α-Proteobacteria family and other obligate intracellular bacteriaa
| Biosynthesis gene for cell wall componentb | α-Proteobacteria results
|
Results for other obligate intracellular bacteria | |||
|---|---|---|---|---|---|
| Rickettsiaceae |
Anaplasmataceae
|
Rhizobiaceae
|
|||
| R. prowazekii and R. conorii | E. chaffeensis, A. phagocytophilum,c and N. sennetsu | Wolbachia sp.c | S. meliloti, M. loti, A. tumefaciens, and B. suis | C. burnetii and C. trachomatis | |
| LPS | |||||
| Lipid A | + | − | − | + | + |
| Peptidoglycan | |||||
| Murein sacculus | + | −d | ±e | + | + |
| Diaminopimelate | + | ±f | + | + | + |
Genes for the biosynthesis of LPS and peptidoglycan in R. prowazekii, the bacterium most closely related to E. chaffeensis and A. phagocytophilum, were used for BLAST searches of genome sequence databases for homologous genes in selected members of the α-Proteobacteria family and other intracellular bacteria. Only genes with E values of ≤10−5 were considered homologous genes.
The gene names (GenBank accession number) for the biosynthesis genes of cell wall components of R. prowazekii are listed below: for lipid A, lpxA (A71708), lpxB (C71688), lpxC (CAA14716), lpxD (CAA14482), kdsA (F71714), kdsB (D71695), kdtA (H71717), and htrB (E71631); for diaminopimelate, asd (F71687), dapA (CAA14886), dapB (A71725), dapD (E71730), dapE (A71650), dapF (F71699), and lysC (E71635); and for murein sacculus, alr (F71718), dacF (D71696), ddlB (CAA14711), glmU (D71704), mraY1 (E71664), mraY2 (B71644), mrcA (A71642), murA (H71662), murB (D71679), murC (C71679), murD (A71699), murE (G71664), murF (F71664), murG (C71699), phpA1 (C71661), pbpA2 (E71661), pbpE (G71680), and slt (G71697). For complete gene names and functional categories of these genes in R. prowazekii, refer to Andersson et al. (2).
Incomplete genome sequences: A. phagocytophilum, >98% completion; Wolbachia endosymbiont of D. melanogaster, ∼85% completion.
E. chaffeensis has the homologous gene dacF, A. phagocytophilum has dacF and murB, and N. sennetsu has murB, murE, and murF.
The Wolbachia endosymbiont of D. melanogaster lacks genes alr, mrcA, pbpE, and slt.
E. chaffeensis has all homologous genes for the biosynthesis of diaminopimelate. However, only the homologous genes asd and dapA were found in A. phagocytophilum and N. sennetsu.
DISCUSSION
We have made novel observations that E. chaffeensis and A. phagocytophilum can incorporate exogenous cholesterol into their membranes and lack genes for LPS and peptidoglycan biosynthesis. Membrane cholesterol is required for the survival of these bacteria, since the depletion of membrane cholesterol or alteration in the structure of cholesterol disrupts their structural integrity and renders them unable to infect their host cells.
In gram-negative bacteria, LPS and peptidoglycan are known to provide strength to the outer membrane and maintain overall structural integrity (22, 27). Studies showed that a conditional lipid A synthesis mutant of E. coli is extremely fragile (40). In mycoplasmas, viruses, and eukaryotes, cholesterol or related sterols provide stability and lipid bilayer fluidity to the cytoplasmic membrane (10, 19). Thus, to compensate for the loss of the mechanical strength provided by LPS and peptidoglycan, Ehrlichia and Anaplasma spp. may become cholesterol dependent. The use of cholesterol in place of LPS and peptidoglycan might also explain the unusual morphology of these bacteria, including such characteristics as an extremely thin outer membrane, a highly pleomorphic nature, and a level of fragility unusual for gram-negative bacteria.
Unlike the situation seen with other obligatory intracellular bacteria (e.g., Rickettsia, Chlamydia, and Coxiella), monocytes/macrophages and granulocytes are the exclusive host cells of E. chaffeensis, N. sennetsu, and A. phagocytophilum in mammals. All of the obligatory intracellular bacteria examined, including Rickettsia, Coxiella, and Chlamydia (but excluding members of the family Anaplasmataceae), have genes encoding lipid A and peptidoglycan biosynthesis. Thus, the loss of genes for lipid A biosynthesis may have been a critical event during the evolution of ancestors of the family Anaplasmataceae, some of whose descendants became the present-day obligate intracellular bacteria of primary host defensive cells. Specifically, monocytes/macrophages or neutrophils express pattern recognition receptors such as Toll-like receptors that can bind to conserved pathogen-associated molecular patterns, such as those of LPS or peptidoglycan, which are shared by groups of microorganisms.
Such binding elicits profound innate immune responses in these cells, including phagocytosis, phagosome-lysosome fusion, release of reactive oxygen intermediates, and the secretion of various proinflammatory mediators to eliminate the invading microorganisms (1, 8, 39). Since the loss of genes for biosynthesis of LPS and peptidoglycan from leukocytes eliminates the possibility of triggering these microbicidal activities (8, 39), such a loss is expected to increase the chances of the intraleukocytic survival of Ehrlichia and Anaplasma spp. Furthermore, Ehrlichia and Anaplasma spp. are required to survive and replicate inside midgut and salivary gland epithelial cells of ixodid tick vectors. In the absence of an adaptive immune response, insect cells have a primitive innate defense mechanism responsive to the presence of LPS (15, 16). Thus, loss of the ability to synthesize LPS gave these bacteria an advantage to survive in insect cells. Loss of most of peptidoglycan biosynthesis genes might be advantageous, particularly for infection of vertebrates, since Wolbachia in the family Anaplasmataceae, which is not known to infect vertebrates, retained nearly all of the genes for the biosynthesis of peptidoglycan.
In mycoplasmas that lack a cell wall, cholesterol or other sterols are required for maintenance of bacterial structural integrity, but more importantly, they are involved in several membrane processes and cellular functions, such as ion transport, control of cell volume, activities of membrane bound enzymes, and distribution of membrane proteins through protein-sterol interactions (10). It is possible that cholesterol also plays some of these roles in E. chaffeensis and A. phagocytophilum. After treatment with MβCD, the ultrastructure of E. chaffeensis showed initial leakage in the inner membrane followed by swelling and lysis of bacteria, suggesting that cholesterol is required for support of the inner membrane. These bacteria internalize into host leukocytes by receptor-mediated endocytosis but not by phagocytosis (14, 31). The loss of infectivity induced in E. chaffeensis and A. phagocytophilum by the replacement of cholesterol with NBD-cholesterol, which has an additional polar group, suggests that cholesterol not only provides mechanical strength but is also involved in binding bacterial ligands or triggering the receptor-mediated endocytosis of these bacteria into the host cells.
Fishbein et al. and Bakken et al. (3) originally pointed out that more-severe illness is associated with increased age in HME and HGE patients, respectively. A more recent study confirmed the unusually high median ages of HME and HGE patients (53 and 51 years, respectively) (12a). The dependency of E. chaffeensis and A. phagocytophilum on cholesterol for infection and survival may partially account for this age association of the illness, because cholesterol levels were reported to increase with increasing age (9). For patients with other tick-borne infectious diseases, the median age is much lower. For example, the median age for patients with Lyme disease is 39 years and the median age for patients with Rocky Mountain spotted fever caused by R. rickettsii is 38 years (12a). The difference is not due to different tick vectors or reservoir hosts, since Borrelia burgdorferi and A. phagocytophilum are transmitted by the same Ixodes scapularis tick. To our knowledge, this possibility has been investigated neither in a population-based study nor in laboratory settings. In summary, as the first report of gram-negative bacteria incorporating cholesterol for survival, these observations provide new perspectives for understanding the mechanism of obligate parasitism of human ehrlichiosis agents.
Acknowledgments
We thank K. Wolken for her excellent technical assistance in freeze fracture and C. Brooks and K. Hoyt for the access to fluorescence microplate readers. We also thank K. Gibson for assistance with preparation of the manuscript.
This work (other than the genome sequencing) is supported by the NIH grant R01 AI30010 to Y.R. E. chaffeensis, A. phagocytophilum, and N. sennetsu genome sequences were obtained at the Institute for Genomic Research with support by National Institutes of Health (NIH) grant R01 AI47885 to Y.R.
Editor: B. B. Finlay
REFERENCES
- 1.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, S. G., A. Zomorodipour, J. O. Andersson, T. Sicheritz-Ponten, U. C. Alsmark, R. M. Podowski, A. K. Naslund, A. S. Eriksson, H. H. Winkler, and C. G. Kurland. 1998. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 396:133-140. [DOI] [PubMed] [Google Scholar]
- 3.Bakken, J. S., J. Krueth, C. Wilson-Nordskog, R. L. Tilden, K. Asanovich, and J. S. Dumler. 1996. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA 275:199-205. [PubMed] [Google Scholar]
- 4.Banerjee, R., J. Anguita, D. Roos, and E. Fikrig. 2000. Cutting edge: infection by the agent of human granulocytic ehrlichiosis prevents the respiratory burst by down-regulating gp91phox. J. Immunol. 164:3946-3949. [DOI] [PubMed] [Google Scholar]
- 5.Barnewall, R. E., N. Ohashi, and Y. Rikihisa. 1999. Ehrlichia chaffeensis and E. sennetsu, but not the human granulocytic ehrlichiosis agent, colocalize with transferrin receptor and up-regulate transferrin receptor mRNA by activating iron-responsive protein 1. Infect. Immun. 67:2258-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnewall, R. E., and Y. Rikihisa. 1994. Abrogation of gamma interferon-induced inhibition of Ehrlichia chaffeensis infection in human monocytes with iron-transferrin. Infect. Immun. 62:4804-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernheimer, A. W., and M. Davidson. 1965. Lysis of pleuropneumonia-like organisms by staphylococcal and streptococcal toxins. Science 148:1229-1231. [DOI] [PubMed] [Google Scholar]
- 8.Beutler, B. 2000. Tlr4: central component of the sole mammalian LPS sensor. Curr. Opin. Immunol. 12:20-26. [DOI] [PubMed] [Google Scholar]
- 9.Chung, S. J. 1992. Relationship among age, serum cholesterol level and population percentile in adults. Int. J. Bio-Med. Comput. 31:99-116. [DOI] [PubMed] [Google Scholar]
- 10.Dahl, J. 1993. The role of cholesterol in mycoplasma membranes. Subcell. Biochem. 20:167-188. [DOI] [PubMed] [Google Scholar]
- 11.Drabikowski, W., E. Lagwinska, and M. G. Sarzala. 1973. Filipin as a fluorescent probe for the location of cholesterol in the membranes of fragmented sarcoplasmic reticulum. Biochim. Biophys. Acta 291:61-70. [DOI] [PubMed] [Google Scholar]
- 12.Dumler, J. S., and J. S. Bakken. 1998. Human ehrlichioses: newly recognized infections transmitted by ticks. Annu. Rev. Med. 49:201-213. [DOI] [PubMed] [Google Scholar]
- 12a.Gardner, S. L., R. C. Holman, J. W. Krebs, R. Berkelman, and J. E. Childs. 2003. National surveillance for the human ehrlichioses in the United States, 1997-2001, and proposed methods for evaluation of data quality. Ann. N. Y. Acad. Sci. 990:80-89. [DOI] [PubMed]
- 13.Hackstadt, T., M. A. Scidmore, and D. D. Rockey. 1995. Lipid metabolism in Chlamydia trachomatis-infected cells: directed trafficking of Golgi-derived sphingolipids to the chlamydial inclusion. Proc. Natl. Acad. Sci. USA 92:4877-4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herron, M. J., C. M. Nelson, J. Larson, K. R. Snapp, G. S. Kansas, and J. L. Goodman. 2000. Intracellular parasitism by the human granulocytic ehrlichiosis bacterium through the P-selectin ligand, PSGL-1. Science 288:1653-1656. [DOI] [PubMed] [Google Scholar]
- 15.Kim, Y. S., S. J. Han, J. H. Ryu, K. H. Choi, Y. S. Hong, Y. H. Chung, S. Perrot, A. Raibaud, P. T. Brey, and W. J. Lee. 2000. Lipopolysaccharide-activated kinase, an essential component for the induction of the antimicrobial peptide genes in Drosophila melanogaster cells. J. Biol. Chem. 275:2071-2079. [DOI] [PubMed] [Google Scholar]
- 16.Lehane, M. J., D. Wu, and S. M. Lehane. 1997. Midgut-specific immune molecules are produced by the blood-sucking insect Stomoxys calcitrans. Proc. Natl. Acad. Sci. USA 94:11502-11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin, M., M. X. Zhu, and Y. Rikihisa. 2002. Rapid activation of protein tyrosine kinase and phospholipase C-γ2 and increase in cytosolic free calcium are required by Ehrlichia chaffeensis for internalization and growth in THP-1 cells. Infect. Immun. 70:889-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McQuiston, J. H., C. D. Paddock, R. C. Holman, and J. E. Childs. 1999. The human ehrlichioses in the United States. Emerg. Infect. Dis. 5:635-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore, N. F., E. J. Patzer, Y. Barenholz, and R. R. Wagner. 1977. Effect of phospholipase C and cholesterol oxidase on membrane integrity, microviscosity, and infectivity of vesicular stomatitis virus. Biochemistry 16:4708-4715. [DOI] [PubMed] [Google Scholar]
- 20.Mott, J., R. E. Barnewall, and Y. Rikihisa. 1999. Human granulocytic ehrlichiosis agent and Ehrlichia chaffeensis reside in different cytoplasmic compartments in HL-60 cells. Infect. Immun. 67:1368-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukherjee, S., X. Zha, I. Tabas, and F. R. Maxfield. 1998. Cholesterol distribution in living cells: fluorescence imaging using dehydroergosterol as a fluorescent cholesterol analog. Biophys. J. 75:1915-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikaido, H. 1996. Outer membrane, p. 29-47. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Resnikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C.
- 23.Ogata, H., S. Audic, P. Renesto-Audiffren, P. E. Fournier, V. Barbe, D. Samson, V. Roux, P. Cossart, J. Weissenbach, J. M. Claverie, and D. Raoult. 2001. Mechanisms of evolution in Rickettsia conorii and R. prowazekii. Science 293:2093-2098. [DOI] [PubMed] [Google Scholar]
- 24.Ohashi, N., N. Zhi, Y. Zhang, and Y. Rikihisa. 1998. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect. Immun. 66:132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohtani, Y., T. Irie, K. Uekama, K. Fukunaga, and J. Pitha. 1989. Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. Eur. J. Biochem. 186:17-22. [DOI] [PubMed] [Google Scholar]
- 26.Paddock, C. D., and J. E. Childs. 2003. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin. Microbiol. Rev. 16:37-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park, J. T. 1996. The murein sacculus, p. 48-57. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Resnikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C.
- 28.Razin, S., and M. Argaman. 1963. Lysis of mycoplasma, bacterial protoplasts, spheroplasts, and L-forms by various agents. J. Gen. Microbiol. 30:155-172. [DOI] [PubMed] [Google Scholar]
- 29.Rikihisa, Y. 1999. Clinical and biological aspects of infection caused by Ehrlichia chaffeensis. Microbes Infect. 1:367-376. [DOI] [PubMed] [Google Scholar]
- 30.Rikihisa, Y. 2000. Ehrlichial strategy for survival and proliferation in leukocytes. Subcell. Biochem. 33:517-538. [DOI] [PubMed] [Google Scholar]
- 31.Rikihisa, Y. 2003. Mechanisms to create a safe haven by members of the family Anaplasmataceae. Ann. N. Y. Acad. Sci. 548-555. 990: [DOI] [PubMed]
- 32.Rikihisa, Y. 1991. The tribe Ehrlichieae and ehrlichial diseases. Clin. Microbiol. Rev. 4:286-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rikihisa, Y. 1990. Ultrastructure of Rickettsia with special emphasis on Ehrlichia, p. 22-31. In J. C. Williams and I. Kakoma (ed.), Ehrlichiosis: a vector-borne disease of animals and humans, vol. 54. Kluwer Publishing Co., Norwell, Mass.
- 34.Rikihisa, Y., B. D. Perry, and D. O. Cordes. 1985. Ultrastructural study of ehrlichial organisms in the large colons of ponies infected with Potomac horse fever. Infect. Immun. 49:505-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rikihisa, Y., N. Zhi, G. P. Wormser, B. Wen, H. W. Horowitz, and K. E. Hechemy. 1997. Ultrastructural and antigenic characterization of a granulocytic ehrlichiosis agent directly isolated and stably cultivated from a patient in New York State. J. Infect. Dis. 175:210-213. [DOI] [PubMed] [Google Scholar]
- 36.Ristic, M., and J. P. Kreier. 1984. Genus I Anaplasma Theiler 1970, 7AL, p. 720-722. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, 1st ed., vol. 1. Williams and Wilkins, Baltimore, Md.
- 37.Robinson, J. M., and M. J. Karnovsky. 1980. Evaluation of the polyene antibiotic filipin as a cytochemical probe for membrane cholesterol. J. Histochem. Cytochem. 28:161-168. [DOI] [PubMed] [Google Scholar]
- 38.Severs, N. J. 1997. Cholesterol cytochemistry in cell biology and disease. Subcell. Biochem. 28:477-505. [DOI] [PubMed] [Google Scholar]
- 39.Ulevitch, R. J., and P. S. Tobias. 1994. Recognition of endotoxin by cells leading to transmembrane signaling. Curr. Opin. Immunol. 6:125-130. [DOI] [PubMed] [Google Scholar]
- 40.Vuorio, R., and M. Vaara. 1992. The lipid A biosynthesis mutation lpxA2 of Escherichia coli results in drastic antibiotic supersusceptibility. Antimicrob. Agents Chemother. 36:826-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker, D. H., and J. S. Dumler. 1996. Emergence of the ehrlichioses as human health problems. Emerg. Infect. Dis. 2:18-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webster, P., J. W. IJdo, L. M. Chicoine, and E. Fikrig. 1998. The agent of human granulocytic ehrlichiosis resides in an endosomal compartment. J. Clin. Investig. 101:1932-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]