Abstract
The inoculation of live, nonattenuated Leishmania major to produce a lesion in a selected site that heals, referred to as leishmanization, is to date the only vaccine against leishmaniasis that has proven to be effective in humans. Its use has been restricted or abandoned entirely, however, due to safety concerns. In an attempt to develop a leishmanization protocol that minimizes pathology while maintaining long-term protection, live parasites were coinjected with CpG-containing immunostimulatory oligodeoxynucleotides (CpG ODNs) alone or in combination with whole-cell lysates of heat-killed L. major promastigotes bound to alum (ALM). C57BL/6 mice infected intradermally by using L. major plus CpG ODN with or without ALM developed few or no dermal lesions and showed an early containment of parasite growth, while mice infected with L. major with or without ALM developed sizable dermal lesions that required up to 10 weeks to heal. The CpG ODNs provoked a transient inflammation that included an early recruitment and accumulation of gamma interferon-producing CD4+ lymphocytes in the site. Attenuation of the live vaccine did not compromise its ability to confer long-term immunity, as mice receiving L. major and CpG ODN plus ALM were totally protected against reinfection with L. major for up to 6 months. By comparison, the immunity elicited by two efficient nonlive vaccines began to wane by 6 months. Our results suggest that immune modulation using CpG ODNs might be a practical approach to improving the safety of a highly effective live vaccine that has already been widely applied.
Leishmania major is the causative agent of zoonotic cutaneous leishmaniasis, the most widely distributed form of cutaneous leishmaniasis in the Old World. Cutaneous leishmaniasis due to L. major is self-limiting, and healing is associated with complete and lifelong immunity to reinfection. Using the mouse model, researchers have tried numerous approaches to developing safe, nonlive vaccines against L. major which have in common their use of appropriate adjuvants, such as interleukin 12 (IL-12) or Corynebacterium parvum, to elicit type 1 responses against a variety of recombinant Leishmania antigens or killed parasites (2, 15, 18, 32, 49). Other strategies have involved the use of live recombinant vectors, such as Salmonella spp., Mycobacterium bovis BCG, or vaccinia virus (1, 13, 51); live attenuated parasites (20, 45); or plasmid DNA encoding single or multiple parasite antigens (10, 16, 29, 36, 40, 50). While each of these studies has indicated some level of protection, complete, long-lived protection has rarely been demonstrated. The demonstration that antigen persistence and IL-12 production are necessary to maintain vaccine efficacy in experimental models (15, 17, 43) may explain, at least in part, the consistent failure of a safe, nonlive vaccine made up of whole-cell killed L. major inoculated with BCG as adjuvant to confer protection against cutaneous or visceral leishmaniasis in human trials (22, 31, 39).
In contrast to the experience with killed vaccines, live vaccination, or leishmanization as it is called, involving the inoculation of virulent organisms in the arm to protect against the development of severe or multiple lesions, especially on the face, provides virtually complete and lifelong protection. Large-scale leishmanization trials were carried out in the former Soviet Union (21), Israel (14), and Iran (33) with highly successful results. However, leishmanization has had a number of problems, most notably an unacceptable frequency of large ulcerating lesions that are slow to heal or, in rare cases, nonhealing (30). Thus, while it remains the only vaccine with proven efficacy in humans, its widespread use has been discontinued in each of these regions where leishmaniasis is endemic. Leishmanization might again be adopted as a primary intervention strategy were its pathological consequences made more predictably benign, as for instance by using live, attenuated parasites. An alternative approach might be to use a live, nonattenuated vaccine containing an appropriate adjuvant that promotes a more rapid onset of antileishmanial immunity and more rapid healing of cutaneous lesions.
The ability of CpG-containing immunostimulatory oligodeoxynucleotides (CpG ODNs) to induce both innate and adaptive cellular immune responses has made them a prospective prophylactic and therapeutic vaccine adjuvant for diseases requiring cellular immunity. CpG ODNs have been shown to stimulate macrophages and dendritic cells to synthesize several cytokines, including IL-12, IL-18, tumor necrosis factor alpha, alpha interferon (IFN-α), IFN-β, and IFN-γ, to upregulate costimulatory molecules such as CD40 and major histocompatibility complex class II and to enhance the ability of dendritic cells to present soluble protein to class I-restricted T cells (reviewed in references 24 and 26). The use of CpG ODNs as an effective adjuvant in whole-cell killed and subunit vaccines against L. major (37, 41, 48) and as immunotherapy to promote resistance in L. major-infected BALB/c mice has already been demonstrated (47, 52). CpG ODNs have not, however, been tested in the context of live vaccination against L. major. In addition to the use of CpG ODNs as adjuvant, the inclusion of heat-killed Leishmania in a live vaccine might help to prevent the development of active lesions, or promote their more rapid healing, in a manner similar to its use in conjunction with BCG as immunotherapy for cutaneous leishmaniasis (11).
Following intradermal inoculation of L. major, C57BL/6 (B/6) mice develop a cutaneous lesion that resolves spontaneously after 8 to 10 weeks. Self-cure is associated with a low-level, persistent infection and with the development of long-lasting immunity to reinfection. L. major infection in B/6 mice, therefore, seems to be an appropriate model to study the pathology and immunity associated with leishmanization in humans. In the present paper, we investigated the potential of CpG ODNs, delivered with or without killed parasites at the site of live vaccination with L. major in B/6 mice, to enhance primary immunity and thereby moderate the pathology associated with leishmanization without compromising the potency or durability of protection that live vaccination normally confers.
MATERIALS AND METHODS
Mice.
C57BL/6 (B/6) mice were purchased from the Division of Cancer Treatment, National Cancer Institute (Frederick, Md.). All mice were maintained in the National Institute of Allergy and Infectious Diseases animal care facility under pathogen-free conditions.
Live vaccination protocol.
L. major clone V1 (MHOM/IL/80/Friedlin) promastigotes were grown at 26°C in medium 199 supplemented with 20% Hi-FCE (HyClone, Logan, Utah), 100 U of penicillin/ml, 100 μg of streptomycin/ml, 2 mM l-glutamine, 40 mM HEPES, 0.1 mM adenine (in 50 mM HEPES), 5 mg of hemin (in 50% triethanolamine)/ml, and 1 mg of 6-biotin (M199/S)/ml. Infective-stage promastigotes (metacyclics) of L. major were isolated from stationary cultures (4 to 5 days old) by negative selection of infective forms using peanut agglutinin (Vector Laboratories Inc, Burlingame, Calif.). Mice were infected in the ear dermis by using a 27.5-gauge needle in a volume of 10 μl. Mice were injected in both ears or in only the right ear with 104 L. major metacyclic promastigotes alone or with a combination of two CpG ODNs (50 μg), freeze-thaw lysates of autoclaved L. major promastigotes bound to aluminum hydroxide (ALM; 50 μg) prepared as previously described (19), or the combination of both. CpG ODNs were synthesized at the Center for Biologics Evaluation and Research Core Facility (Bethesda, Md.). The sequences of the immunostimulatory ODNs (5′ to 3′) were TCAACGTTGA and GCTAGACGTTAGCGT, with each containing a phosphorothioate backbone, as previously described (25).
DNA and killed vaccination protocols.
For DNA vaccination, mice were injected in the right ear with 10 μg of total DNA of a mixture of plasmid DNAs encoding the antigens LACK, LmSTI1, and TSA suspended in sterile phosphate-buffered saline as previously described (28). Some mice were vaccinated with a mixture of ALM plus CpG ODNs by using the same dose employed in the live vaccination protocol. Vaccinated mice were given booster injections 2 weeks later using the same regimen.
L. major challenge.
Protection against L. major challenge was evaluated in mice immunized in the right ear only. Fifteen weeks or 6 months after vaccination, 500 L. major metacyclic promastigotes in a volume of 10 μl were inoculated intradermally into the opposite (left) ear by using a 27.5-gauge needle. The evolution of the lesions was monitored by measuring the diameter of the induration with a direct-reading vernier caliper (Thomas, Swedesboro, N.J.).
Parasite quantitation.
Parasite loads in the ears were determined as previously described (5). Briefly, the ventral and dorsal sheets of the infected ears were separated, deposited dermal-side down in Dulbecco's modified Eagle medium containing 100 U of penicillin/ml, 100 μg of streptomycin/ml, and Liberase CI enzyme blend (50 μg/ml; Boehringer Mannheim). Ears were incubated for 2 h at 37°C. The sheets were cut into small pieces and homogenized using a Teflon-coated microtissue grinder in a microcentrifuge tube containing 100 μl of M199/S. The tissue homogenates were filtered using a 70-μm-pore-size cell strainer (Falcon Products Inc., St. Louis, Mo.), serially diluted in a 96-well flat-bottom microtiter plate containing biphasic medium, prepared by using 50 μl of NNN medium containing 20% defibrinated rabbit blood, and overlaid with 100 μl of M199/S (5). The number of viable parasites in each ear was determined from the highest dilution at which promastigotes could be grown out after 7 days of incubation at 26°C. The number of parasites was also determined in the local draining lymph nodes (retromaxillar). The lymph nodes were recovered and mechanically dissociated using a pellet pestle and then serially diluted as described above.
In vivo recall response.
Mice were injected in both ears with a combination of living and killed antigens consisting of 106 metacyclic L. major promastigotes and 12.5 μg of soluble leishmanial antigen prepared from freeze-thawed (three times) stationary-phase L. major promastigotes. Forty-eight hours later, mice were sacrificed and cells from the ear dermis and local draining lymph nodes (three or four mice) were obtained. Single-cell suspensions from the ear dermis were obtained as described above. For the analysis of surface markers and intracytoplasmic staining for IFN-γ, cells were stimulated with L. major-infected bone marrow-derived murine dendritic cells (BMDDC) as a source of antigen for 16 h, at which time brefeldin A was added (10 μg/ml) (8). The cells were cultured for an additional 6 h and then fixed in 4% paraformaldehyde. Prior to staining, cells were incubated with an anti-Fc γ III/II (Pharmingen) receptor and 10% normal mouse serum in phosphate-buffered saline containing 0.1% bovine serum albumin and 0.01% NaN3. Cells were permeabilized and stained for the surface marker CD3 (145-2 C11; fluorescein isothiocyanate labeled; Pharmingen), CD4, or CD8 (RM4-5 and 53-6.7; cychrome conjugated; Pharmingen) and for the cytokine IFN-γ conjugated to R-phycoerythrin (JE56-5H4; Pharmingen). Incubations were carried out for 30 min on ice. The isotype controls used were rat immunoglobulin G2b (IgG2b; A95-1; Pharmingen) and rat IgG2a (R35-95; Pharmingen). The frequency of CD4+ and CD8+ T cells was determined by gating on CD3+ cells. For each sample, at least 100,000 cells were analyzed. The data were collected and analyzed using CELLQuest software and a FACScalibur flow cytometer (Becton Dickinson, San Jose, Calif.).
For cytokine measurement in culture supernatants, pooled cells from draining lymph nodes were resuspended in RPMI medium containing fetal bovine serum-penicillin-streptomycin at 6 × 106 cells/ml, and 0.1 ml was plated in U-bottom 96-well plates. Cells were incubated at 37°C in an atmosphere of 5% CO2 with uninfected or L. major-infected BMDDC. IFN-γ and IL-10 production in 48-h culture supernatants was quantitated by enzyme-linked immunosorbent assay.
Statistical analysis.
Statistical tests were performed with SigmaStat (Jandel Software). Because most comparisons derived from data with nonhomogeneous variances, Kruskal-Wallis analysis of variance on ranks was performed and multiple comparisons were done by the Dunn method. Dual comparisons were made with the Mann-Whitney rank sum test. All data from parasite numbers were log transformed before statistical tests were conducted.
RESULTS
CpG ODNs administered with or without ALM at the time and site of live vaccination moderate lesion development.
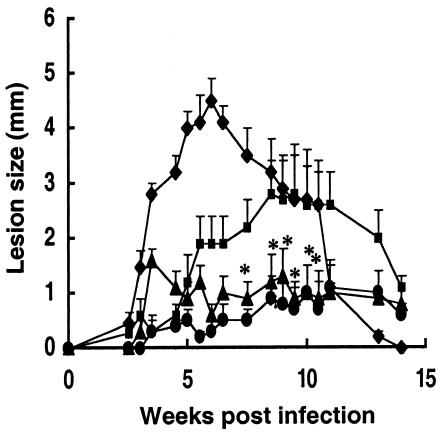
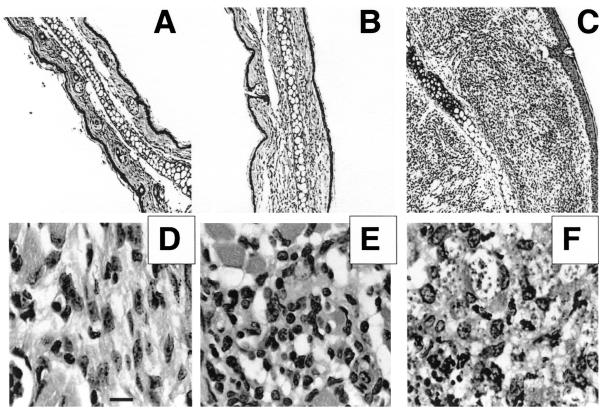
For the purposes of this study, the primary infection in one ear was employed as the live vaccine while reinfection in the opposite ear was employed as the vaccine challenge. In preliminary studies to optimize any effect that CpG ODN might have in moderating lesion development, the timing and site of CpG ODN administration relative to L. major infection were investigated. Mice treated with CpG ODN 3 days before or at the same time and in the same site as infection in the skin in each case reduced the size of the active lesion, while CpG ODN administered 7 or 21 days after infection or in a distal site had relatively minor effects (data not shown). Accordingly, all subsequent studies employed coinjection of live L. major with CpG ODN and/or ALM. In the experiment shown in Fig. 1, B/6 mice were vaccinated by inoculation of 104 live, metacyclic promastigotes in the ear dermis with or without either 50 μg of CpG ODNs, 50 μg of ALM, or a combination of 50 μg of CpG ODNs plus 50 μg of ALM. The mice that were infected with L. major plus CpG ODNs or the combination of CpG ODNs plus ALM showed a striking reduction in dermal pathology compared with the mice infected with L. major alone. These differences were statistically significant at multiple time points during infection (P < 0.05). Mice infected with live parasites plus CpG ODNs displayed a transient inflammation that resolved in a few days, and the moderation of lesion development was comparable to that achieved using CpG ODNs plus ALM. The combination of live parasites and ALM without CpG ODNs resulted in partial reduction in lesion scores during the first 5 weeks, although the lesions resolved more slowly than for the rest of the groups. The histopathology associated with live vaccination at 4 weeks postinfection is shown in Fig. 2. The lesions in the mice receiving live parasites alone were associated with a massive, diffusely organized cellular infiltrate composed of granulocytes, lymphocytes, and infected macrophages (Fig. 2C and F), whereas the moderated lesions in the CpG ODN-injected mice contained a substantially reduced infiltrate composed primarily of lymphocytes and very few infected macrophages (Fig. 2B and E).
FIG. 1.
Live vaccination with CpG ODN with or without ALM attenuates lesion development in C57BL/6 mice. Mice were vaccinated in the ear dermis with 104 metacyclic promastigotes alone (⧫) or in combination with either 50 μg of ALM (▪), 50 μg of CpG ODNs (▴), or 50 μg of ALM and 50 μg of CpG ODNs (•). Data shown represent the mean induration ± standard deviation (SD) for 10 mice per group. Asterisks indicate statistically significant differences compared with results for mice vaccinated with parasites alone or with parasites plus ALM (P < 0.05).
FIG. 2.
Sections of ears from mice receiving live vaccine with or without CpG ODN. Ears were harvested at 4 weeks postvaccination and were processed for hematoxylin and eosin staining. Ear sections were prepared from healthy C57BL/6 mice (A and D) or from mice vaccinated with 104 metacyclic promastigotes alone (C and F) or with 50 μg of CpG ODN (B and E). Magnification, ×10 (A, B, and C) and ×100 (D, E, and F). Bar, 10 μm.
CpG ODNs elicit an early containment of parasite growth and recruitment of CD4+ cells to the site of immunization.
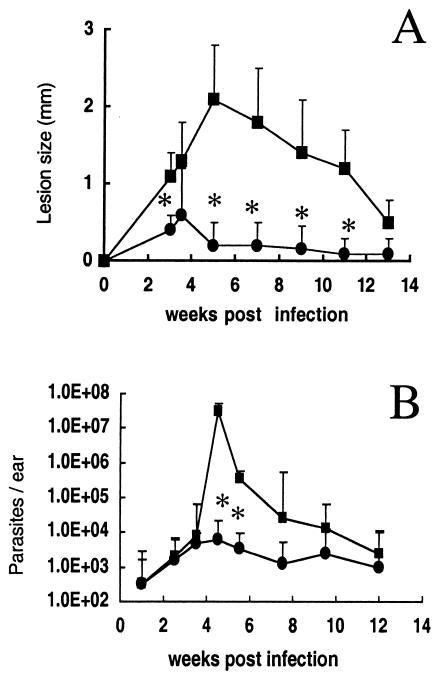
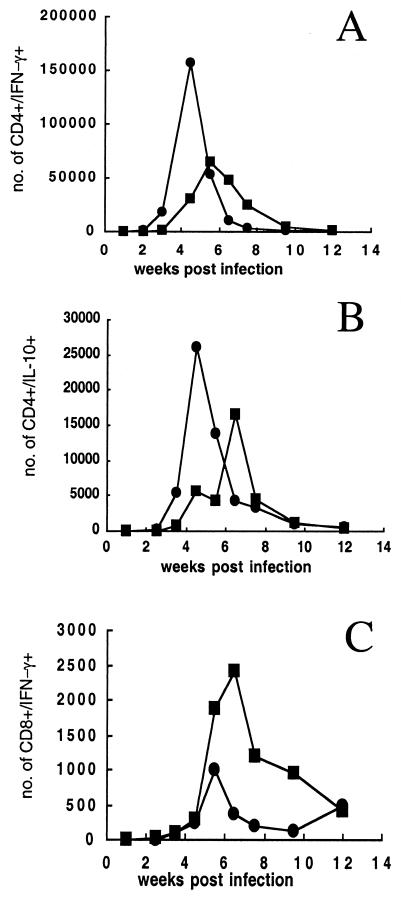
To further study the mechanism by which CpG ODNs moderated the pathology associated with live vaccination, a kinetic analysis of parasite growth and immune response in the site was carried out in mice vaccinated in the ear with 104 L. major metacyclics alone or in combination with 50 μg of CpG ODNs. Mice vaccinated with live parasites plus CpG ODNs displayed a transient inflammation at 3 to 5 weeks, after which time little or no dermal pathology was observed (Fig. 3A). Parasite numbers were determined in the two experimental groups over the course of the infection (Fig. 3B). As described previously (6), the parasite number in ears of mice infected with L. major typically peak just prior to the appearance of the lesion, in this case at approximately 5 weeks, with >10 million parasites per ear. In mice infected with L. major plus CpG ODNs, the number of parasites that grew in the site was reduced up to 1,000-fold compared with mice infected with L. major alone (P < 0.001). The transient inflammation and rapid control of parasite growth were associated with an early increase in the absolute number of CD4+ T lymphocytes recruited to the site that produced high amounts of IFN-γ following restimulation with infected BMDDC in vitro (Fig. 4A). The number of CD4+ IFN-γ-producing cells at 4.5 weeks was fivefold higher than that in mice infected with parasites alone (156,400 versus 30,300). The resolution of the transient inflammation in the parasite- and CpG ODN-infected mice coincided with the decrease of CD4+ lymphocytes in the site, whereas these cells began to accumulate appreciably in the ears of mice infected with parasites alone by 6 weeks (>60,000 cells). The IL-10-producing CD4+ lymphocytes migrated to the infection site of mice receiving live parasites plus CpG ODNs with a similar kinetic as the IFN-γ-producing CD4+ T cells, although their absolute numbers were less. Interestingly, the transient inflammation and parasite control were not associated with an increase in CD8+ IFN-γ-producing T cells in the site, which (Fig. 4C) at 4.5 weeks remained low, at around 300 cells in both groups. The number of IFN-γ-producing CD8+ T cells was in fact higher in the mice infected with parasites alone, reaching a peak at 6 weeks postinfection (approximately 2,500 cells).
FIG. 3.
Live vaccination with CpG ODNs promotes early containment of parasite growth in the site. Mice were vaccinated in the ear dermis with 104 metacyclic promastigotes alone (▪) or in combination with 50 μg of CpG ODNs (•). (A) Lesion size, expressed as mean induration ± SD for 3 to 10 mice (6 to 20 ears) per group. (B) Parasite number per ear, expressed as geometric mean ± SD (six ears per group). Asterisks indicate statistically significant difference compared with results for mice vaccinated with parasites alone (P < 0.001).
FIG. 4.
Live vaccination with CpG ODNs promotes early recruitment of IFN-γ-producing CD4+ T cells to the site. Mice were vaccinated in the ear dermis with 104 metacyclic promastigotes alone (▪) or with 50 μg of CpG ODNs (•). Graphs show the total number of IFN-γ+ CD4+ (A), IL-10+ CD4+ (B), or IFN-γ+ CD8+ (C) T cells in the inoculation site of B/6 mice at weekly intervals following vaccination. Staining for surface markers and intracytoplasmic staining for IFN-γ or IL-10 were analyzed on dermal cells pooled from three mice (six ears) following in vitro restimulation with L. major-infected BMDDC. Analyses were gated on CD3+ cells. Numbers at each time point were determined from the percentage of CD4+ or CD8+ T cells positive for IFN-γ or IL-10 multiplied by the total number of CD4+ or CD8+ T cells extracted per ear.
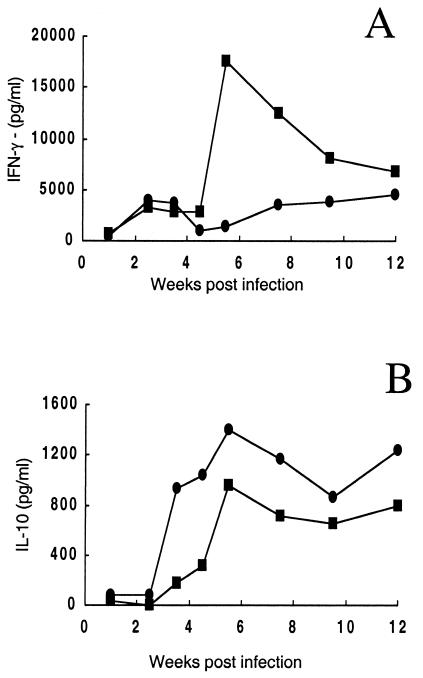
The amounts of IFN-γ and IL-10 were also determined in supernatants of cells isolated from draining lymph nodes after in vitro restimulation using L. major-infected BMDDC (Fig. 5). By 2.5 weeks, significant IFN-γ secretion (3 to 4 ng/ml) was detected in both groups of mice, and despite the increased accumulation of cytokine-producing cells in the dermis of mice vaccinated with parasites plus CpG ODNs, the levels of cytokine produced by draining lymph node cells was not greater in these mice than in mice infected with parasites alone during early time points. IFN-γ production peaked sharply at week 6 in the mice infected with parasites alone, whereas it maintained roughly the same levels in mice infected with parasites plus CpG ODNs. IL-10 was detectable in both groups by 3.5 weeks and was higher at every time point thereafter in the CpG ODN-treated mice.
FIG. 5.
IFN-γ (A) and IL-10 (B) cytokine production by draining lymph node cells at different time points following live vaccination of mice in the ear dermis with 104 metacyclic promastigotes alone (▪) or with 50 μg of CpG ODNs (•). Cytokines were measured by enzyme-linked immunosorbent assay in culture supernatants of pooled lymph node cells (three mice at each time point) 48 h following in vitro restimulation with L. major-infected BMDDC. Data shown are the mean concentrations of duplicate assays.
Reducing the pathogenicity of the live vaccine does not compromise its potency or durability.
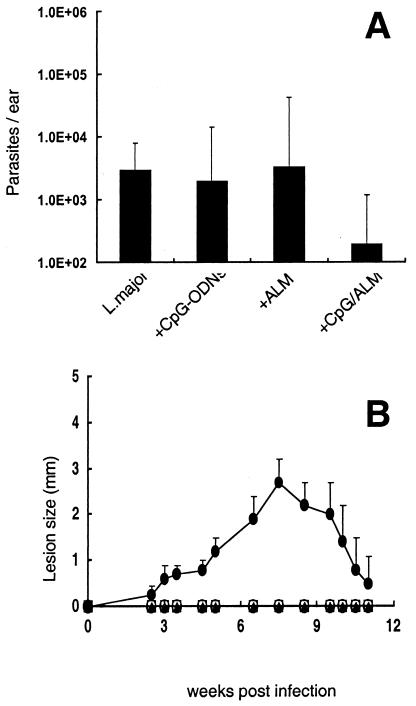
Since long-term protection in this model is thought to be associated with persistent infection following clinical cure (7), we were interested in knowing whether the moderation of lesion development and the early control of parasite growth following infection with L. major and CpG ODNs with or without ALM was accompanied by complete clearance of parasites from the site. Figure 6A shows parasite quantitation in the ears of vaccinated mice during the chronic phase, 15 weeks postinfection, when the lesions were already resolved. A low-level, persistent infection was detected within the ear dermis of every mouse in each of the groups (200 to 3,000 parasites per ear). The group infected with L. major and CpG ODNs plus ALM had the lowest parasite load, with 200 parasites per ear, although the differences between the groups were not statistically significant.
FIG. 6.
Live, attenuated vaccines establish low-level, persistent infection and confer complete protection against reinfection with L. major. (A) Parasite quantitation in the ear dermis at 15 weeks following live vaccination of B/6 mice with 104 metacyclic promastigotes alone or with 50 μg of ALM, 50 μg of CpG ODNs, or 50 μg each of ALM and CpG ODNs. Results are expressed in terms of geometric mean number of parasites per ear ± SD for three ears per group. (B) Development of ear lesions in mice challenged in the opposite ear by intradermal inoculation of 500 metacyclic promastigotes of L. major. Mice were unvaccinated (•) or vaccinated 15 weeks previously with 104 live parasites alone (□) or in combination with 50 μg of ALM (▴), 50 μg of CpG ODNs (○), or 50 μg each of ALM and CpG ODNs (X). Results are expressed as the mean induration ± SD for six mice per group.
The different vaccinated groups were challenged with 500 metacyclic promastigotes in the left ear 15 weeks after live vaccination in the right ear. This challenge model was designed to reproduce some of the main aspects of natural sandfly challenge: low dose and intradermal inoculation (6). All of the mice receiving live vaccine were completely protected against the development of dermal lesions (Fig. 6B), indicating that the attenuation of the live vaccine with respect to dermal pathology did not compromise its ability to elicit powerful immunity against reinfection. As mice injected with CpG ODNs or ALM alone are not protected against L. major (37), it is clear that the complete protection in mice immunized with parasites plus CpG ODNs or ALM was dependent on the live vaccine.
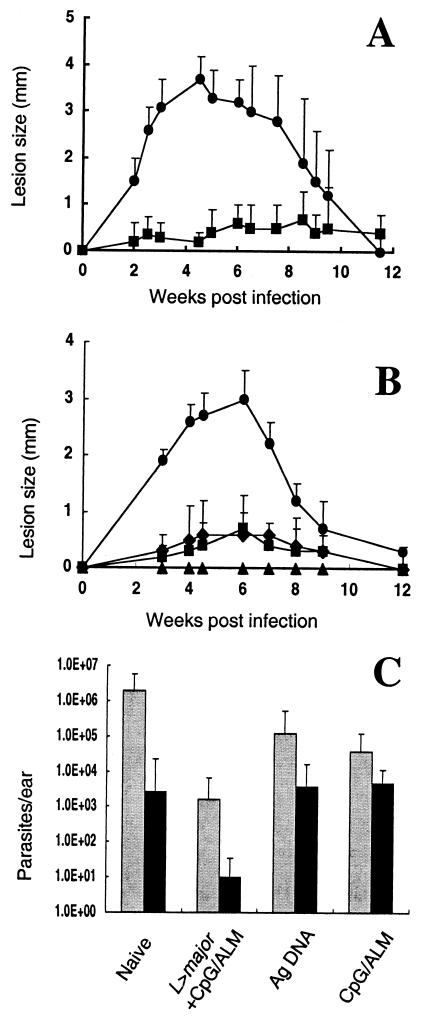
Since the combination of ALM and CpG ODNs can itself induce long-lasting protection (37), it was decided to rigorously compare after 6 months the durability of protection and immune responses elicited by live vaccination using L. major and CpG ODNs plus ALM with that elicited by the nonlive vaccine containing CpG ODNs plus ALM alone. Comparison was also made with mice vaccinated 6 months previously with a cocktail vaccine containing DNA encoding the antigens LACK, LmST1, and TSA, which have previously been reported to provide long-lived protection against L. major (28). Mice immunized with live parasites with CpG ODNs plus ALM again showed significantly reduced dermal pathology during their primary infection compared with mice immunized with live parasites alone (P < 0.001) (Fig. 7A). These mice were completely protected against cutaneous leishmaniasis when challenged with L. major in the opposite ear after 6 months (Fig. 7B). The mice vaccinated with antigen DNA and CpG ODN plus ALM were also strongly protected, although some of the mice in each group developed a small induration. When the parasite loads in the ears were determined at 4 weeks (acute phase) and 20 weeks (chronic phase) postchallenge (Fig. 7C), the live, attenuated vaccine provided an approximately 1,000-fold reduction in parasite number at each time point compared to what was seen with the unvaccinated animals (P < 0.001). In contrast, the number of dermal parasites was not significantly reduced at either time point in mice immunized with antigen DNA, while the group vaccinated with CpG ODNs plus ALM showed a small but significant reduction (P < 0.05) in parasite burden only at the early time point.
FIG. 7.
The live, attenuated vaccine confers more durable immunity than the nonlive vaccines. (A) Development of ear lesions in B/6 mice following live vaccination with 104 metacyclic promastigotes alone (•) or with 50 μg each of ALM and CpG ODNs (▪). Data represent the mean induration ± SD for six mice per group. (B) Development of ear lesions in mice challenged in the opposite ear by intradermal inoculation of 500 metacyclic promastigotes of L. major. Mice were unvaccinated (•) or vaccinated 6 months previously with CpG ODN and ALM (⧫), with antigen DNA (▪), or with live parasites plus CpG ODN and ALM (▴). Data shown represent the mean induration ± SD for three to six mice per group. (C) Parasite quantitation in the ear dermis at 4 (gray bars) and 20 (black bars) weeks postchallenge. Results are expressed as geometric mean ± SD for three mice per group.
The difference in long-term protection conferred by live vaccine versus that conferred by nonlive vaccine was correlated with a significant difference in the number of IFN-γ-producing CD4+ and CD8+ T cells recruited to an intradermal challenge site 6 months after vaccination. Table 1 shows the total number of cells extracted from the ear dermis 48 h following injection of antigen in the nonvaccinated ear and the frequencies and total numbers of recruited CD4+ and CD8+ T cells able to produce IFN-γ following restimulation with infected BMDDC in vitro. Recruitment of T cells to the site of antigen challenge was negligible in naïve mice. By comparison, the number of recruited lymphocytes able to make IFN-γ was maintained at a high level in mice that had been vaccinated with live parasites and CpG ODNs plus ALM (4.7 × 105 and 1.8 × 105 per ear for CD4+ and CD8+ T cells, respectively). The number of CD4+ T cells able to produce IFN-γ was especially striking and was 10-fold greater than the number determined for either of the nonlive vaccines. The number of IFN-γ-producing CD8+ T cells was significantly increased relative to unvaccinated controls in mice vaccinated with ALM plus CpG ODNs (1.1 × 105/ear). The recall response in mice vaccinated with antigen DNA was relatively poor for either T-cell subset, though still greater than the response in unvaccinated mice.
TABLE 1.
CD4+ IFN-γ+ and CD8+ IFN-γ+ T cells recruited to the site of intradermal challenge in C57BL/6 mice 6 months postvaccination
| Vaccination group | Total no. of cells/eara | Total no. of CD4+ IFN-γ+ cells (%)b | Total no. of CD8+ IFN-γ+ cells (%)b |
|---|---|---|---|
| Unvaccinated | 0.9 × 106 | 0.3 × 104 (0.3) | 0.3 × 104 (0.3) |
| ALM plus CpG ODNs | 2.0 × 106 | 4.7 × 104 (2.3) | 11 × 104 (5.5) |
| Antigen DNA | 1.2 × 106 | 4.3 × 104 (3.5) | 3.2 × 104 (2.6) |
| L. major and ALM plus CpG ODNs | 2.8 × 106 | 47 × 104 (16.7) | 18 × 104 (6.4) |
Dermal cells were extracted from mouse ears 48 h after intradermal injection of 106 metacyclic promastigotes plus 12.5 μg of soluble leishmanial antigen, and the number of cells per ear was calculated from cell preparations pooled from three to four mice (six to eight ears) for each group.
Double-positive cells as a percentage of total number of extracted cells, determined by fluorescence-activated cell sorter analysis following 24-h in vitro restimulation using L. major-infected BMDDC.
DISCUSSION
The only successful immunization strategy in humans has been leishmanization, based on the development of durable immunity after recovery of infection in a chosen site, usually the arm, with viable, nonattenuated parasites (14, 21, 33). Because leishmanization typically produces cutaneous ulcers of relatively long duration, at-risk children have in most instances been excluded from live vaccination. Furthermore, due to concerns regarding the small number of cases where persistent lesions, secondary infection, or exacerbation of chronic dermatological conditions have been reported, leishmanization has been abandoned in virtually all countries where it was formerly practiced, including Israel, Iran, and many republics of the former Soviet Union. Nonetheless, given its proven efficacy, leishmanization might be readopted in certain regions where leishmaniasis is endemic and applied on a broader scale were the size and duration of the cutaneous lesions moderated without compromising its ability to confer strong and durable immunity. In the present study, we used C57BL/6 mice inoculated in the ear dermis with 10,000 metacyclic promastigotes of a virulent strain of L. major to reproduce the pathology and immunity associated with leishmanization in humans. We demonstrate that injection of CpG ODNs with or without heat-killed Leishmania promastigotes at the time and site of live vaccination resulted in early containment of parasite growth and a striking reduction in dermal pathology. Importantly, the vaccine still conferred complete, long-lasting protection against reinfection with L. major.
CpG ODNs act on innate immunity and rapidly trigger immune activation, including NO production. Thus, the CpG ODN may have acted locally on skin macrophages to facilitate Leishmania clearance, as has been described recently (47). Alternatively, the earlier killing may have been due to the ability of the CpG ODN to promote Leishmania-specific immunity, including the recruitment of effector T cells to the site. Coadministration of live parasites and CpG ODNs provoked a transient inflammation in the skin that was dominated by an early accumulation of IFN-γ-producing CD4+ lymphocytes. The mechanism by which CD4+ T cells specifically migrated to the dermis of CpG ODN-treated mice is under investigation. Stan et al. (42) have suggested that CpG ODNs can induce transfected myocytes to upregulate major histocompatibility complex class II molecules and chemokines with subsequent recruitment of inflammatory cells secreting IFN-γ. Takeshita et al. (44) showed that murine BALB/c macrophages upregulate the expression of chemokines such as MIP1B, MIP2, RANTES, JE/MCP-1, and IP-10 after exposure to CpG ODNs. These chemokines are associated with Th1 responses (34) and therefore contribute to preferential generation of Th1-driven responses after treatment with CpG ODNs. It is interesting that CD8+ T cells comprised a relatively minor component of the early response to L. major in the mice treated with CpG ODN and that their numbers in the site remained low relative to the mice infected with L. major alone. As it is known that both CD8+ and CD4+ T cells are necessary to efficiently kill the high number of parasites that accumulate during the normal “silent” phase of L. major expansion in the skin (8), these results suggest that the earlier activation and recruitment of effector CD4+ T cells to the site is sufficient to control the initial stage of parasite growth, resulting in a significantly reduced inflammatory response, including CD8+-T-cell recruitment, that would normally accompany the immune clearance of high antigen loads from the skin. It is interesting that compared with the mice immunized with L. major alone, the mice immunized with L. major plus CpG ODNs also had an earlier recruitment and/or expansion of CD4+ IL-10+ cells in the site and a higher level of IL-10 produced by draining lymph node cells at early time points following infection. Their numbers were still approximately fivefold less than the numbers of IFN-γ−producing effector cells in the site, which is consistent with the rapid control of infection that was achieved. Nonetheless, it is likely that the IL-10 produced by these cells contributed to parasite persistence and to the moderation of tissue pathology associated with the early control of infection in the skin (4, 7). Whether or not these cells were CD4+ CD25+ regulatory T cells and were driven by CpG ODNs directly or as a homeostatic response to the inflammation elicited by the vaccine are important questions to be addressed in future studies.
The addition of ALM without CpG ODNs did not seem to substantially reduce the pathogenicity of the live vaccine, and its application with CpG ODNs produced only a slightly better outcome compared to that with CpG ODNs alone. Nonetheless, as there is evidence from a preliminary clinical study in Uzbekistan that killed promastigotes delivered with live vaccine reduced the size and duration of active lesions (12), and as there is by now extensive experience regarding the safety of ALM in human trials (3, 22, 39), there may be a rationale for pursuing clinical studies using CpG ODNs in conjunction with ALM for attenuation of live vaccine.
Previous studies have shown that maintenance of acquired immunity in resistant mice is due to the persistence of low numbers of parasites in the inoculation site and draining lymph node after challenge and that complete elimination of parasites results in loss of immunity (7, 46). Thus, it is to be emphasized that despite the early containment of parasite growth and the absence of dermal lesions, the attenuated live vaccines still established a latent infection in which a small number of parasites persisted in the dermis and local draining lymph nodes. When long-term protection conferred by the live parasites plus CpG ODNs and ALM was carefully compared with that conferred by two powerful nonlive vaccines that have been described previously, i.e., the same CpG plus ALM used alone (37) or a cocktail antigen DNA vaccine (29), we found that the protection achieved by the nonlive vaccines, while still substantial, began to wane by 6 months, as revealed by development of small lesions and only slightly reduced parasite burdens compared to unvaccinated controls. By comparison, live vaccination with CpG ODNs and ALM provided complete protection against rechallenge infection. The live vaccine prevented the development of dermal lesions and significantly reduced dermal parasite numbers while maintaining greater numbers of antigen-specific CD4+ and CD8+ T cells that were tissue seeking and able to produce IFN-γ. It is likely that the difference between live and nonlive vaccination protocols in terms of the durability of protection would become even more pronounced as the interval between vaccination and challenge is prolonged.
The more conventional approach to the development of a nonpathogenic, live L. major vaccine has involved the use of parasites attenuated by irradiation, serial culture, or targeted gene deletion. Partial protection of BALB/c mice could be induced by inoculation of high doses of nonpathogenic clones or irradiated parasites (20, 23) or parasites bearing auxotrophic gene deletions (45). In these studies, however, only short-term protection was evaluated and was achieved only if a high-dose inoculum was administered by the intraperitoneal or intravenous route. Since in the BALB/c model short-term protection can also be achieved by intravenous, but not by subcutaneous, immunization using killed parasites (20), it seems unlikely in this study that the protection was dependent on parasite viability and infection. In the study by Rivier et al. (38) in which radioattenuated parasites were shown to protect CBA mice following subcutaneous inoculation, the protection required viable parasites. Those authors also demonstrated that a small number of parasites remained viable in the inoculation site at the time of the challenge and could be propagated in culture, suggesting that the protection may have been dependent on subclinical infection established by the few parasites that were not inactivated by irradiation. Again, the long-term persistence of the parasites or the durability of protection was not further addressed. The only other studies, so far as we are aware, to show that subcutaneous inoculation of live parasites can confer protection without producing a lesion involved the use of low doses (100 to 1,000) of nonattenuated promastigotes to establish subclinical infection (9, 27, 35). In a follow-up study by Uzonna et al. (46), the relationship between parasite persistence and immunity was established when it was shown that transfer of immune cells from subclinical mice could protect naïve BALB/c mice against L. major challenge and could completely clear the parasite, leaving the mice susceptible to a rechallenge infection. Thus, there is accumulating evidence suggesting that the key to long-term immunity is parasite persistence and there is no clear indication that the live, attenuated parasites used to date retain this ability.
Our approach to vaccine attenuation has been to employ live, virulent organisms inoculated with an appropriate adjuvant in order to induce an immune response similar to natural infection, only more rapid and robust so as to promote earlier control of parasite growth and healing of cutaneous lesions. Modifications of this same approach might include using the ALM plus CpG ODNs prophylactically to protect against the pathogenic effects of the live vaccine delivered within weeks or months of the killed vaccine or therapeutically to promote healing of vaccine lesions as they appear. The inoculation of CpG ODNs with or without ALM at the time and site of live vaccination seems to be the most practical approach to improving the safety while maintaining the immunogenicity of a highly effective vaccine that has already been widely applied.
Editor: J. M. Mansfield
REFERENCES
- 1.Abdelhak, S., H. Louzir, J. Timm, L. Blel, Z. Benlasfar, M. Lagranderie, M. Gheorghiu, K. Dellagi, and B. Gicquel. 1995. Recombinant BCG expressing the leishmania surface antigen Gp63 induces protective immunity against Leishmania major infection in BALB/c mice. Microbiology 141:1585-1592. [DOI] [PubMed] [Google Scholar]
- 2.Afonso, L. C., T. M. Scharton, L. Q. Vieira, M. Wysocka, G. Trinchieri, and P. Scott. 1994. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science 263:235-237. [DOI] [PubMed] [Google Scholar]
- 3.Bahar, K., Y. Dowlati, B. Shidani, M. H. Alimohammadian, A. Khamesipour, S. Ehsasi, R. Hashemi-Fesharki, S. Ale-Agha, and F. Modabber. 1996. Comparative safety and immunogenicity trial of two killed Leishmania major vaccines with or without BCG in human volunteers. Clin. Dermatol. 14:489-495. [DOI] [PubMed] [Google Scholar]
- 4.Belkaid, Y., K. F. Hoffmann, S. Mendez, S. Kamhawi, M. C. Udey, T. A. Wynn, and D. L. Sacks. 2001. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 194:1497-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belkaid, Y., S. Kamhawi, G. Modi, J. Valenzuela, N. Noben-Trauth, E. Rowton, J. Ribeiro, and D. L. Sacks. 1998. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J. Exp. Med. 188:1941-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belkaid, Y., S. Mendez, R. Lira, N. Kadambi, G. Milon, and D. Sacks. 2000. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 165:969-977. [DOI] [PubMed] [Google Scholar]
- 7.Belkaid, Y., C. A. Piccirillo, S. Mendez, E. M. Shevach, and D. L. Sacks. 2002. CD4+ CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502-507. [DOI] [PubMed] [Google Scholar]
- 8.Belkaid, Y., E. Von Stebut, S. Mendez, R. Lira, E. Caler, S. Bertholet, M. C. Udey, and D. Sacks. 2002. CD8+ T cells are required for primary immunity in C57BL/6 mice following low-dose, intradermal challenge with Leishmania major. J. Immunol. 168:3992-4000. [DOI] [PubMed] [Google Scholar]
- 9.Bretscher, P. A., G. Wei, J. N. Menon, and H. Bielefeldt-Ohmann. 1992. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science 257:539-542. [DOI] [PubMed] [Google Scholar]
- 10.Campos-Neto, A., J. R. Webb, K. Greeson, R. N. Coler, Y. A. Skeiky, and S. G. Reed. 2002. Vaccination with plasmid DNA encoding TSA/LmSTI1 leishmanial fusion proteins confers protection against Leishmania major infection in susceptible BALB/c mice. Infect. Immun. 70:2828-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Convit, J., P. L. Castellanos, A. Rondon, M. E. Pinardi, M. Ulrich, M. Castes, B. Bloom, and L. Garcia. 1987. Immunotherapy versus chemotherapy in localised cutaneous leishmaniasis. Lancet 1:401-405. [DOI] [PubMed] [Google Scholar]
- 12.Gafurov, I. M. 1999. Experience in controlling and preventing zoonotic cutaneous leishmaniasis in Uzbekistan. Med. Parazitol. (Mosk) 1:58-59. (In Russian.) [PubMed]
- 13.Gonzalo, R. M., G. del Real, J. R. Rodriguez, D. Rodriguez, R. Heljasvaara, P. Lucas, V. Larraga, and M. Esteban. 2002. A heterologous prime-boost regime using DNA and recombinant vaccinia virus expressing the Leishmania infantum P36/LACK antigen protects BALB/c mice from cutaneous leishmaniasis. Vaccine 20:1226-1231. [DOI] [PubMed] [Google Scholar]
- 14.Greenblatt, C. L. 1980. The present and future of vaccination for cutaneous leishmaniasis. Prog. Clin. Biol. Res. 47:259-285. [PubMed] [Google Scholar]
- 15.Gurunathan, S., C. Prussin, D. L. Sacks, and R. A. Seder. 1998. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat. Med. 4:1409-1415. [DOI] [PubMed] [Google Scholar]
- 16.Gurunathan, S., D. L. Sacks, D. R. Brown, S. L. Reiner, H. Charest, N. Glaichenhaus, and R. A. Seder. 1997. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J. Exp. Med. 186:1137-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurunathan, S., L. Stobie, C. Prussin, D. L. Sacks, N. Glaichenhaus, A. Iwasaki, D. J. Fowell, R. M. Locksley, J. T. Chang, C. Y. Wu, and R. A. Seder. 2000. Requirements for the maintenance of Th1 immunity in vivo following DNA vaccination: a potential immunoregulatory role for CD8+ T cells. J. Immunol. 165:915-924. [DOI] [PubMed] [Google Scholar]
- 18.Handman, E., F. M. Symons, T. M. Baldwin, J. M. Curtis, and J. P. Scheerlinck. 1995. Protective vaccination with promastigote surface antigen 2 from Leishmania major is mediated by a TH1 type of immune response. Infect. Immun. 63:4261-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashemi-Fesharki, R., S. Alle-Agha, P. Ahourai, E. Djavadian, A. Taghavi, A. Golabi, M. Kamail, K. Esmail-Nia, H. Ghafari, F. Mofasali, and R. Kheirol-Omour. 1992. Vaccine preparation and quality control of killed Leishmania major. Arch. Inst. Razi 43:39-50. [Google Scholar]
- 20.Howard, J. G., F. Y. Liew, C. Hale, and S. Nicklin. 1984. Prophylactic immunization against experimental leishmaniasis. II. Further characterization of the protective immunity against fatal Leishmania tropica infection induced by irradiated promastigotes. J. Immunol. 132:450-455. [PubMed] [Google Scholar]
- 21.Kellina, O. I. 1981. Problem and current lines in investigations on the epidemiology of leishmaniasis and its control in the U.S.S.R. Bull. Soc. Pathol. Exot. Fil. 74:306-318. [PubMed] [Google Scholar]
- 22.Khalil, E. A., A. M. El Hassan, E. E. Zijlstra, M. M. Mukhtar, H. W. Ghalib, B. Musa, M. E. Ibrahim, A. A. Kamil, M. Elsheikh, A. Babiker, and F. Modabber. 2000. Autoclaved Leishmania major vaccine for prevention of visceral leishmaniasis: a randomised, double-blind, BCG-controlled trial in Sudan. Lancet 356:1565-1569. [DOI] [PubMed] [Google Scholar]
- 23.Kimsey, P. B., C. M. Theodos, T. K. Mitchen, S. J. Turco, and R. G. Titus. 1993. An avirulent lipophosphoglycan-deficient Leishmania major clone induces CD4+ T cells which protect susceptible BALB/c mice against infection with virulent L. major. Infect. Immun. 61:5205-5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klinman, D. M., S. Kamstrup, D. Verthelyi, I. Gursel, K. J. Ishii, F. Takeshita, and M. Gursel. 2000. Activation of the innate immune system by CpG oligodeoxynucleotides: immunoprotective activity and safety. Springer Semin. Immunopathol. 22:173-183. [DOI] [PubMed] [Google Scholar]
- 25.Klinman, D. M., A. K. Yi, S. L. Beaucage, J. Conover, and A. M. Krieg. 1996. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc. Natl. Acad. Sci. USA 93:2879-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krieg, A. M. 2000. The role of CpG motifs in innate immunity. Curr. Opin. Immunol. 12:35-43. [DOI] [PubMed] [Google Scholar]
- 27.Li, J., T. J. Nolan, and J. P. Farrell. 1997. Leishmania major: a clone with low virulence for BALB/c mice elicits a Th1 type response and protects against infection with a highly virulent clone. Exp. Parasitol. 87:47-57. [DOI] [PubMed] [Google Scholar]
- 28.Mendez, S., Y. Belkaid, R. A. Seder, and D. Sacks. 2002. Optimization of DNA vaccination against cutaneous leishmaniasis. Vaccine 20:3702-3708. [DOI] [PubMed] [Google Scholar]
- 29.Mendez, S., S. Gurunathan, S. Kamhawi, Y. Belkaid, M. A. Moga, Y. A. Skeiky, A. Campos-Neto, S. Reed, R. A. Seder, and D. Sacks. 2001. The potency and durability of DNA- and protein-based vaccines against Leishmania major evaluated using low-dose, intradermal challenge. J. Immunol. 166:5122-5128. [DOI] [PubMed] [Google Scholar]
- 30.Modabber, F. 1995. Vaccines against leishmaniasis. Ann. Trop. Med. Parasitol. 89(Suppl. 1):83-88. [DOI] [PubMed] [Google Scholar]
- 31.Momeni, A. Z., T. Jalayer, M. Emamjomeh, A. Khamesipour, F. Zicker, R. L. Ghassemi, Y. Dowlati, I. Sharifi, M. Aminjavaheri, A. Shafiei, M. H. Alimohammadian, R. Hashemi-Fesharki, K. Nasseri, T. Godal, P. G. Smith, and F. Modabber. 1999. A randomised, double-blind, controlled trial of a killed L. major vaccine plus BCG against zoonotic cutaneous leishmaniasis in Iran. Vaccine 17:466-472. [DOI] [PubMed] [Google Scholar]
- 32.Mougneau, E., F. Altare, A. E. Wakil, S. Zheng, T. Coppola, Z. E. Wang, R. Waldmann, R. M. Locksley, and N. Glaichenhaus. 1995. Expression cloning of a protective Leishmania antigen. Science 268:563-566. [DOI] [PubMed] [Google Scholar]
- 33.Nadim, A., E. Javadian, G. Tahvildar-Bidruni, and M. Ghorbani. 1983. Effectiveness of leishmanization in the control of cutaneous leishmaniasis. Bull. Soc. Pathol. Exot. Fil. 76:377-383. [PubMed] [Google Scholar]
- 34.Olson, T. S., and K. Ley. 2002. Chemokines and chemokine receptors in leukocyte trafficking. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283:R7-R28. [DOI] [PubMed] [Google Scholar]
- 35.Preston, P. M., and D. C. Dumonde. 1976. Experimental cutaneous leishmaniasis. V. Protective immunity in subclinical and self-healing infection in the mouse. Clin. Exp. Immunol. 23:126-138. [PMC free article] [PubMed] [Google Scholar]
- 36.Rafati, S., A. H. Salmanian, T. Taheri, M. Vafa, and N. Fasel. 2001. A protective cocktail vaccine against murine cutaneous leishmaniasis with DNA encoding cysteine proteinases of Leishmania major. Vaccine 19:3369-3375. [DOI] [PubMed] [Google Scholar]
- 37.Rhee, E. G., S. Mendez, J. A. Shah, C. Y. Wu, J. R. Kirman, T. N. Turon, D. F. Davey, H. Davis, D. M. Klinman, R. N. Coler, D. L. Sacks, and R. A. Seder. 2002. Vaccination with heat-killed leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T cell responses and protection against Leishmania major infection. J. Exp. Med. 195:1565-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rivier, D., R. Shah, P. Bovay, and J. Mauel. 1993. Vaccine development against cutaneous leishmaniasis. Subcutaneous administration of radioattenuated parasites protects CBA mice against virulent Leishmania major challenge. Parasite Immunol. 15:75-84. [DOI] [PubMed] [Google Scholar]
- 39.Sharifi, I., A. R. FeKri, M. R. Aflatonian, A. Khamesipour, A. Nadim, M. R. Mousavi, A. Z. Momeni, Y. Dowlati, T. Godal, F. Zicker, P. G. Smith, and F. Modabber. 1998. Randomised vaccine trial of single dose of killed Leishmania major plus BCG against anthroponotic cutaneous leishmaniasis in Bam, Iran. Lancet 351:1540-1543. [DOI] [PubMed] [Google Scholar]
- 40.Sjolander, A., T. M. Baldwin, J. M. Curtis, and E. Handman. 1998. Induction of a Th1 immune response and simultaneous lack of activation of a Th2 response are required for generation of immunity to leishmaniasis. J. Immunol. 160:3949-3957. [PubMed] [Google Scholar]
- 41.Stacey, K. J., and J. M. Blackwell. 1999. Immunostimulatory DNA as an adjuvant in vaccination against Leishmania major. Infect. Immun. 67:3719-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stan, A. C., S. Casares, T. D. Brumeanu, D. M. Klinman, and C. A. Bona. 2001. CpG motifs of DNA vaccines induce the expression of chemokines and MHC class II molecules on myocytes. Eur. J. Immunol. 31:301-310. [DOI] [PubMed] [Google Scholar]
- 43.Stobie, L., S. Gurunathan, C. Prussin, D. L. Sacks, N. Glaichenhaus, C. Y. Wu, and R. A. Seder. 2000. The role of antigen and IL-12 in sustaining Th1 memory cells in vivo: IL-12 is required to maintain memory/effector Th1 cells sufficient to mediate protection to an infectious parasite challenge. Proc. Natl. Acad. Sci. USA 97:8427-8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takeshita, S., F. Takeshita, D. E. Haddad, K. J. Ishii, and D. M. Klinman. 2000. CpG oligodeoxynucleotides induce murine macrophages to up-regulate chemokine mRNA expression. Cell Immunol. 206:101-106. [DOI] [PubMed] [Google Scholar]
- 45.Titus, R. G., F. J. Gueiros-Filho, L. A. de Freitas, and S. M. Beverley. 1995. Development of a safe live Leishmania vaccine line by gene replacement. Proc. Natl. Acad. Sci. USA 92:10267-10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uzonna, J. E., G. Wei, D. Yurkowski, and P. Bretscher. 2001. Immune elimination of Leishmania major in mice: implications for immune memory, vaccination, and reactivation disease. J. Immunol. 167:6967-6974. [DOI] [PubMed] [Google Scholar]
- 47.von Stebut, E., Y. Belkaid, B. Nguyen, M. Wilson, D. L. Sacks, and M. C. Udey. 2002. Skin-derived macrophages from Leishmania major-susceptible mice exhibit interleukin-12- and interferon-gamma-independent nitric oxide production and parasite killing after treatment with immunostimulatory DNA. J. Investig. Dermatol. 119:621-628. [DOI] [PubMed] [Google Scholar]
- 48.Walker, P. S., T. Scharton-Kersten, A. M. Krieg, L. Love-Homan, E. D. Rowton, M. C. Udey, and J. C. Vogel. 1999. Immunostimulatory oligodeoxynucleotides promote protective immunity and provide systemic therapy for leishmaniasis via IL-12- and IFN-γ-dependent mechanisms. Proc. Natl. Acad. Sci. USA 96:6970-6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Webb, J. R., A. Campos-Neto, P. J. Ovendale, T. I. Martin, E. J. Stromberg, R. Badaro, and S. G. Reed. 1998. Human and murine immune responses to a novel Leishmania major recombinant protein encoded by members of a multicopy gene family. Infect. Immun. 66:3279-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu, D., and F. Y. Liew. 1994. Genetic vaccination against leishmaniasis. Vaccine 12:1534-1536. [DOI] [PubMed] [Google Scholar]
- 51.Xu, D., S. J. McSorley, S. N. Chatfield, G. Dougan, and F. Y. Liew. 1995. Protection against Leishmania major infection in genetically susceptible BALB/c mice by gp63 delivered orally in attenuated Salmonella typhimurium (AroA− AroD−). Immunology 85:1-7. [PMC free article] [PubMed] [Google Scholar]
- 52.Zimmermann, S., O. Egeter, S. Hausmann, G. B. Lipford, M. Rocken, H. Wagner, and K. Heeg. 1998. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J. Immunol. 160:3627-3630. [PubMed] [Google Scholar]