Abstract
The effect of targeting strategies for improving the interaction of liposomal PorA with dendritic cells (DC) on the immunogenicity of PorA was investigated. PorA, a major antigen of Neisseria meningitidis, was purified and reconstituted in different types of (targeted) liposomes, i.e., by using mannose or phosphatidylserine as targeting moieties, or with positively charged liposomes. We studied the efficiency of liposome uptake and its effect on the maturation of and interleukin 12 (IL-12) production by murine DC. Moreover, mice were immunized subcutaneously to study the localization and immunogenicity of PorA liposomes. Uptake of liposomes by DC was significantly increased for targeted liposomes and resulted in the maturation of DC, but to various degrees. Maturation markers (i.e., CD80, CD86, major histocompatibility complex class II, and CD40) showed enhanced expression on DC incubated with targeted PorA liposomes relative to those incubated with nontargeted PorA liposomes. Moreover, only the uptake of targeted PorA liposomes induced production of IL-12 by DC, with levels similar to those produced by lipopolysaccharide (LPS)-pulsed DC. Mannose-targeted PorA liposomes administered subcutaneously had an increased localization in draining lymph nodes compared to nontargeted PorA liposomes. Liposomes in draining lymph nodes interacted preferentially with antigen-presenting cells, an effect that was enhanced with targeted PorA liposomes. Immunization studies showed an improvement of the bactericidal antibody response (i.e., increased number of responders) generated by targeted PorA liposomes compared to that generated by nontargeted ones or LPS-containing outer membrane vesicles. In conclusion, the use of targeted PorA liposomes results in an improved uptake by and activation of DC and an increased localization in draining lymph nodes. These effects correlate with an enhanced immune response toward the vaccine.
Infections caused by Neisseria meningitidis are a serious threat to children and young adults. Vaccines based on capsular polysaccharides are available against serogroups A, C, and W135 (18). A polysaccharide-based vaccine is not available for serogroup B meningococci, due to the low immunogenicity of their polysaccharides and the risk of induction of autoantibodies that cross-react with glycosylated host antigens. Still, it remains important to develop new vaccines for serogroup B meningococci using other antigenic epitopes in the outer membrane, as 63 and 32%, respectively, of the reported cases of meningococcal disease in Europe and the United States are attributable to serogroup B (6, 25).
Class 1 porin protein (PorA) is a good vaccine candidate because it is a major antigen and induces a strong bactericidal immune response (24). These features make PorA a good vaccine candidate. Serogroup B meningococcal vaccines are being developed by different groups, based on PorA formulated in outer membrane vesicles (OMVs) (9, 24, 32). However, despite purification, OMVs still contain small amounts of toxic lipopolysaccharides (LPS). Furthermore, the composition of OMVs is difficult to manipulate because it is governed by the strain from which the OMVs are derived. As an alternative for OMVs, liposomes are an attractive presentation form for purified membrane proteins like PorA, as the liposomal bilayer mimics the membrane environment; the liposomes, therefore, are excellently suited for incorporation of native PorA (2, 5, 21, 35). Moreover, liposomes are well-defined structures, allowing improvement of the formulation, e.g., through attachment of targeting ligands or variations of the (membrane) composition.
A rational approach for making better vaccines is to improve the delivery of (liposomal) antigens to dendritic cells (DC). DC are the most efficient antigen-presenting cells (APC), being able to initiate and modulate immune responses both in vitro and in vivo (33). Several strategies can be conceived to target liposomes to DC. On the surface of DC, various receptors of the C-type lectin family are expressed, including the macrophage mannose receptor and DEC-205 (12). Receptors of this family have in common the recognition of bacterial structures, and antigen capture by these receptors has been shown to result in efficient antigen presentation (17, 27). Another efficient uptake mechanism by DC and other APC is the endocytosis of apoptotic bodies. This is probably mediated by specific recognition of phosphatidylserine (PS) at the surface of apoptotic cells (13).
The aim of this study was to design well-defined PorA-based vaccines with improved immunogenicity compared to that of OMVs by formulating PorA into liposomes targeted to DC. For this purpose, P1.7-2,4 was used as a representative PorA serosubtype (2). Three strategies were used to improve the uptake of PorA liposomes by DC—use of liposomes with mannose ligands on the surface for targeting C-type lectin receptors, use of liposomes containing PS for targeting to the PS receptor, and use of positively charged liposomes to induce an efficient uptake mediated by nonspecific electrostatic interactions with (negatively charged) DC membranes. As controls, nontargeted negatively charged PorA liposomes and OMVs were used. In vitro, the uptake of targeted and nontargeted PorA liposomes by bone marrow-derived murine DC was studied, as was their ability to induce functional maturation of DC. In vivo, the localization of targeted liposomes (Man liposomes) and nontargeted liposomes was compared in draining lymph nodes after subcutaneous immunization of mice. Finally, the immune response of all liposomal PorA formulations was studied in mice and compared with the immune response induced by (standard) OMVs. Our data suggest that an increased antigen loading of DC through the use of targeted liposomes results in an improved immune response. Therefore, targeted liposomes are attractive presentation forms for the future development of type B meningococcal vaccines.
MATERIALS AND METHODS
Materials.
All phospholipids used were synthetic. Dimyristoyl phosphatidylcholine (PC) was purchased at Rhône-Poulenc Rorer (Köln, Germany). Dimyristoyl phosphatidylglycerol (PG) was a gift from Lipoïd GmbH (Ludwigshafen, Germany). Cholesterol (Chol) was obtained from Sigma (Zwijndrecht, The Netherlands). Dimyristoyl PS, dimyristoyl phosphatidylethanolamine (PE), and dimyristoyl trimethylammonium propane (TAP) were purchased from Avanti Polar Lipids (Alabaster, Ala.). Mannosylated PE (Man-PE) was prepared by ethyldi-isopropylamine-promoted coupling of PE and mannopyranosylphenyl isothiocyanate (Sigma) in chloroform-methanol (7:1 [vol/vol]). The compound was purified by preparative thin-layer chromatography, and its structure was confirmed by nuclear magnetic resonance and liquid chromatography-mass spectrometry.
PorA was purified from OMVs obtained from N. meningitidis strain F91, a capsule-negative mutant of H44/76 (B:P1.7-2,4:L3; PorB−, RmpM−; low expression of Opa and Opc), yielding LPS-depleted PorA (LPS/protein ratio, <0.01 [wt/wt]), as previously described (2). Prior to incorporation of PorA into liposomes, the protein was precipitated with 80% (vol/vol) ethanol at −20°C and solubilized in 150 mM octylglucopyranoside (Sigma) in Tris-buffered saline (TBS; 50 mM Tris-HCl and 150 mM NaCl [pH 7.4]).
Monoclonal antibodies (mAb) against CD80 (clone 1G10), CD86 (clone GL1), fluorescein isothiocyanate-labeled mAb against CD11b (clone M1/70), phycoerythrin-labeled mAb against Gr-1 (clone RB6-8C5), and biotinylated mAb against CD11c (clone HL3) and CD45R/B220 (clone RA3-6B2) were obtained from BD PharMingen (San Diego, Calif.). Streptavidin-phycoerythrin was purchased from Becton Dickinson (San Jose, Calif.). Biotinylated mAb against F4/80 (clone Cl: A3-1) was obtained from Serotec (Oxford, United Kingdom). Phycoerythrin-labeled anti-CD40 mAb (clone 3.23) was purchased from Immunotech (Marseille, France), and mouse anti-rat immunoglobulin G (IgG) (H+L) F(ab′)2 fragment was purchased from Jackson ImmunoResearch (West Grace, Pa.). M5/114 anti-major histocompatibility (MHC) class II (3) was kindly provided by Georg Kraal (Free University, Amsterdam, The Netherlands).
Preparation and characterization of PorA liposomes.
Targeted liposomes were made as follows: PC, PG, Chol, and Man-PE in an 8/2/2/0.6 mol ratio (Man liposomes); PC, PS, and Chol in an 8/2/2 ratio (PS liposomes); or PC, TAP, and Chol in an 8/2/2 ratio (TAP liposomes). Control liposomes consisted of PC, PG, and Chol in an 8/2/2 mol ratio (PG liposomes). Liposomes were prepared by detergent dilution and characterized as previously described (2). The initial protein/lipid ratio used was 25 μg of protein/μmol of lipid. As fluorescent marker, 0.1 mol% 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt (DiD; Molecular Probes, Leiden, The Netherlands) was included in the bilayer when indicated. Liposomes were filtered through sterile 0.45-μm-pore-size filters.
Particle size of the liposomes was measured by dynamic light scattering (DLS) at 25°C with a Malvern 4700 system equipped with a 75-mW argon ion laser (488 nm; Uniphase, San José, Calif.), a remote interface controller, and PCS software. The zeta potential of the liposomes was measured with a Zetasizer 2000 with an aqueous dip-in cell and a computer with PCS software version 1.35 (Malvern Ltd., Malvern, United Kingdom). The phospholipid content was determined according to the method of Rouser et al. (26), with sodium phosphate (Merck, Darmstadt, Germany) used as the standard. The presence of mannose on the outside of Man liposomes was studied by monitoring liposome aggregation in time in the presence of 8 μg of concanavalin A (ConA; Sigma) per ml by DLS (see above). Protein content was determined according to the method of Peterson (23), with bovine serum albumin (Pierce, Rockford, Ill.) used as the standard.
The correct folding of PorA into liposomes was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in “native” gels as previously described (16). Antigen concentration was estimated by an inhibition enzyme-linked immunosorbent assay (ELISA) with the anti-P1.4 mAb MN20B9.34, as previously described (2). Antigenicity was calculated as the ratio of the antigen concentration (determined by ELISA) to the protein concentration (determined according to the method of Peterson). As a reference, the antigenicity of PorA in OMVs was arbitrarily set at 1.0.
Mice.
BALB/cOlaHsd mice were obtained from Harlan (Horst, The Netherlands) and maintained under conventional conditions at the Central Animal Laboratory (Utrecht University, Utrecht, The Netherlands) or at The Netherlands Vaccine Institute (Bilthoven, The Netherlands). All experiments were done with 8- to 12-week-old animals and were approved by the Animal Ethics Committee.
Generation of DC and characterization following incubation with PorA liposomes.
Bone marrow-derived DC were obtained as described by Inaba et al. (15). Briefly, bone marrow was flushed from mouse femora, erythrocytes were lysed, and cells were grown at a concentration of 106/ml in filtered culture medium consisting of RPMI 1640 medium with 10% fetal bovine serum, 50 IU of penicillin per ml, and 50 μg of streptomycin per ml in the presence of 10 ng of granulocyte-macrophage colony-stimulating factor (Immunex, Seattle, Wash.) per ml and 50 ng of interleukin-4 (IL-4; R&D Systems, Minneapolis, Minn.) per ml. Nonadherent cells were replated on day 1, and cells still nonadherent at days 2 and 4 were removed from the cultures, with concomitant refreshment of culture medium. On day 7, nonadherent and loosely adherent cells were harvested. These cells were MHCII+, CD11c+, and CD11b+. From these, 3 × 106 cells per well were plated with fresh culture medium together with PorA liposomes (80 nmol of phospholipid) and further incubated for 48 h at 37°C. As controls, cells were incubated in medium with nonlabeled liposomes or with LPS (1 μg/ml). After incubation, cells were harvested and analyzed. The supernatants were kept at −70°C until IL-12 quantitation. IL-12 was quantified in 48-h supernatants of DC cultures incubated with liposomes and controls using an OptEIA ELISA kit (BD PharMingen) according to the manufacturer's protocol.
Flow cytometry.
Cells (105 in 50 μl of medium) were blocked with 5% heat-inactivated mouse serum in fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline [PBS], 0.1% azide, 1% bovine serum albumin) for 30 min at room temperature. Blocked cells were then washed with FACS buffer and incubated with relevant antibodies for 20 min at room temperature. After this time, cells were washed and, if required, incubated with a specific fluorescently labeled secondary antibody. Cells (104) were analyzed by flow cytometry using a FACS Calibur and Cell Quest software (BD Biosciences). Win MDI 2.8 software was kindly provided by Joseph Trotter and was used for further analysis. Negative controls included unstained cells, cells stained with isotype control antibody, and cells stained with only the secondary antibody.
Confocal laser scanning microscopy.
Cells (105) incubated with PorA liposomes under conditions similar to those for flow cytometry studies were washed with 2 ml of PBS and subsequently fixed in 4% formaldehyde in PBS for 20 min at room temperature. After fixation, cells were washed twice with PBS and mounted on glass slides with FluorSafe reagent (Calbiochem, San Diego, Calif.). Slides were examined by using a confocal laser scanning microscope equipped with a 488-nm argon, 568-nm krypton, and 633-nm HeNe laser (Leica TCS-SP; Leica Microsystems, Rijswijk, The Netherlands). Images were analyzed by using Leica TCS-SP Power Scan software.
In vivo studies.
For the localization study, mice (three animals per group) were immunized subcutaneously in the groin (0.25 ml; 1,000 nmol of phospholipid per mouse) with DiD-labeled PorA liposomes. After 48 h, popliteal and inguinal lymph nodes located near the injection site were removed and pooled for each mouse. Lymph nodes were treated with 160 U of collagenase type 3 (Worthington Biochemicals, Lakewood, N.J.) per ml and 180 U of DNase I (Sigma) per ml for 45 min at 37°C and forced through a 70-μm-pore-size filter. The resultant cells were washed with FACS buffer and analyzed by flow cytometry as described above.
For the immunization study, mice (eight animals per group) were immunized subcutaneously (0.25 ml per mouse) on day 0, 14, and 28 with 1.5 μg of liposomal PorA or PorA-containing OMVs. Sera were collected at day 42 and stored at −20°C until analysis.
Serum analysis.
The antibody titers (total IgG and individual isotypes) in mouse sera were determined in an ELISA as previously described (1). N. meningitidis strain H44/76 (B:15P1.7-2,4:L3,7,9) and the H44/76-derived mutant strain HI.5 (lacking PorA) were used. Isotypes were determined with goat anti-mouse Ig isotype-specific conjugates labeled with horseradish peroxidase (Southern Technology Associates; dilution, 1/5,000 [except for IgG1 dilution, which was 1/2,500]) using tetramethyl benzidine as the substrate. The titer is defined as the dilution of the serum for which 50% of the maximum optical density (λ = 450 nm) in the assay is reached. The maximum optical density is the absorbance obtained with twofold-diluted serum.
The serum bactericidal activity was measured as previously described (2, 14) against the N. meningitidis strains H44/76 (B:15P1.7-2,4:L3,7,9) and HI.5 (PorA−). Sera from mice were heat inactivated for 30 min at 56°C prior to use. Bacteria were incubated for 10 to 15 min at room temperature with the serum samples before the addition of complement. As the complement source, 80% (vol/vol) rabbit serum was used. As controls, the bactericidal anti-P1.4 (MN20B9.34) and anti-LPS (MN15A17F12) mAb were used. Also, test sera were incubated without complement as a negative control. The serum bactericidal titer was measured as the reciprocal serum dilution showing killing of more than 90% of bacteria used.
Statistical methods.
Antibody and bactericidal titers were log10 transformed in order to obtain a gaussian distribution. The analysis of variance test was used for statistical evaluation of the data. The significance of the differences between the mean values of the antibody titers was determined by the least significant difference (LSD) test at a confidence level of 95%. This test could not be used for bactericidal titers, as groups included both responders and nonresponders. Individual values are given together with the means for the indicated responders.
RESULTS
Liposomal PorA formulations.
All liposomes had particle sizes around 200 nm (Table 1). As expected, PG, PS, and Man liposomes were negatively charged, whereas TAP liposomes were positively charged, as indicated by their zeta potential. The initial protein/lipid ratio (25 μg of protein per μmol of lipid) was preserved in the resulting liposomes. The presence of accessible mannose residues in the surface of Man liposomes was confirmed by their aggregation in the presence of the carbohydrate binding lectin ConA (Fig. 1).
TABLE 1.
Characteristics of PorA liposomesa
| Liposome batch | Composition | Particle size (nm) | PDb | Zeta potentialc (mV) | Antigen- icityd |
|---|---|---|---|---|---|
| PG liposomes | PC:PG:Chol | 191 | 0.27 | −54 | 0.87 |
| Man liposomes | PC:PG:Chol:Man-PE | 201 | 0.34 | −37 | 0.51* |
| PS liposomes | PC:PS:Chol | 228 | 0.35 | −50 | 0.73 |
| TAP liposomes | PC:TAP:Chol | 175 | 0.40 | 44 | 0.57* |
Data in this table are representative for PorA liposomes (with or without DiD) used in the experiments described.
Polydispersity: indication of the size distribution of the liposomes, which ranges from 0.0 for a monodisperse to 1.0 for an entirely heterodisperse suspension.
Surface charge of liposomes.
Ratio between antigen content (determined by ELISA) and protein concentration (determined by protein assay), relative to that of OMVs; *P < 0.01.
FIG. 1.
ConA-mediated aggregation of Man liposomes measured by DLS. Squares, particle size of Man liposomes (▪) and (control) PG liposomes (□). Diamonds, polydispersity index of Man liposomes (♦) and (control) PG liposomes (⋄).
A correct PorA conformation is necessary in order to induce an optimal B-cell response that will result in the production of protective, i.e., bactericidal, antibodies. In order to investigate the PorA conformation, SDS-PAGE under mild conditions was performed. In all formulations, PorA was present, functioning as trimers (Fig. 2). Even in samples incubated for 5 min at 100°C, some PorA trimers were still visible, suggesting that the incorporation of PorA into a lipid bilayer partially protects the protein from heat denaturation.
FIG. 2.
SDS-PAGE of PorA liposomes. Each sample was dissolved in sample buffer containing 0.05% SDS and incubated either at room temperature (lanes a) or at 100°C (lanes b). The numbers on the right indicate the molecular masses of PorA monomers (43 kDa) and trimers (130 kDa).
The ability of PorA liposomes to interact with specific bactericidal mAb (i.e., antigenicity) was measured by ELISA. The antigenicity of PorA in PG and PS liposomes was only slightly lower than the antigenicity of the same PorA in OMVs (Table 1), thus indicating that PorA is preferentially embedded in the liposomal bilayer, with relevant epitopes directed to the outside. In Man and TAP liposomes, the antigenicity of PorA was significantly reduced (P < 0.01), possibly caused by steric hindrance due to mannose molecules located on the surface and the positive charge, respectively.
Interaction and uptake of PorA liposomes by bone marrow-derived DC.
We studied the interaction of DiD-labeled PorA liposomes with DC. After 4 h, only the incubation of DC with TAP liposomes resulted in high fluorescence levels of the cells (data not shown). After 48 h of incubation with all PorA liposome types, almost all cells showed fluorescence, regardless of the targeting moiety (Fig. 3, inset). However, differences in the mean fluorescence intensities (MFI) of cells were found. The use of targeted PorA liposomes resulted in an increased fluorescence of DC compared to the use of nontargeted liposomes. The highest MFI was observed for DC incubated with TAP liposomes. Incubation with PS and Man liposomes induced intermediate MFI, and cells incubated with (nontargeted) PG liposomes showed the lowest MFI (Fig. 3).
FIG. 3.
Interaction of DiD-labeled liposomes with DC after 48 h of incubation. The results are indicated as MFI, and error bars represent standard error of the mean (SEM) of 10-fold measurements. Inset, typical example of the shift observed in the fluorescence of DC cultured for 48 h in the presence of fluorescent PorA liposomes (PG liposomes shown).
Cells were also visualized by confocal microscopy (Fig. 4). After incubation for 48 h with any of the PorA liposome types, bright punctate fluorescence could be observed intracellularly, which indicated internalization of the liposomes. In agreement with the flow cytometry data, the fluorescence intensity was increased in cells incubated with targeted liposomes (Man, PS, and TAP liposomes). Moreover, when incubated with positively charged TAP liposomes, all cells showed fluorescence. That was not the case with other liposome types, thereby indicating that the positive charge on the surface of PorA liposomes induced an (unspecific) electrostatic interaction with the negative surface of cultured cells.
FIG. 4.
Internalization of PorA liposomes by DC. Typical examples of confocal laser scanning micrographs (CLSM) and light microscopy images (LM) of DC after 48 h of incubation with PG liposomes, Man liposomes, PS liposomes, and TAP liposomes as indicated. Bars, 10 μm.
Maturation and IL-12 production by liposome-pulsed DC.
Bone marrow-derived DC were characterized with specific monoclonal antibodies (MHC class II, CD80, CD86, and CD40) by flow cytometry (Fig. 5A). Day 7 DC (t = 0) showed moderate levels of MHC class II expression and low levels of CD80, CD86, and CD40 expression. After 48 h of incubation (day 9 DC), the levels of these markers were increased. LPS-pulsed DC showed a clear maturation, with increased expression of MHC class II, CD80, CD86, and CD40.
FIG. 5.
(A) Characterization of cultured DC. Histograms illustrate the expression of specific cell surface markers on DC at day 7 (t = 0 h, filled histograms) and day 9 (t = 48 h, gray lines) and on DC pulsed for 48 h with LPS (black lines). Data are representative of results from four independent experiments. (B) Effect of uptake of PorA liposomes on DC maturation. Histograms illustrate the expression of specific cell surface markers on DC cultured with medium only for 48 h (filled histograms) or DC pulsed with PorA liposomes (as indicated) for 48 h (black lines). Data are representative of results from at least two experiments.
All liposomal formulations induced increased levels of maturation markers on DC after 48 h of incubation (Fig. 5B). A substantial increase in MHC class II (comparable to MHC class II expression in LPS-pulsed DC), CD80, CD86, and CD40 expression was observed with all targeted PorA liposomes (Man, PS, and TAP liposomes). Much less pronounced effects were observed for nontargeted (i.e., PG) liposomes, which suggested that enhanced uptake of PorA liposomes through targeting leads to increased maturation of DC.
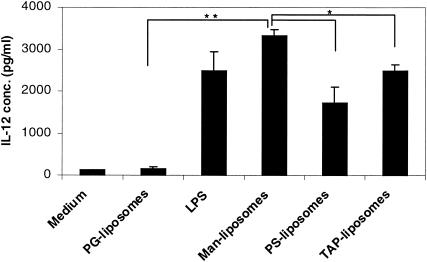
The IL-12 concentration was measured in supernatants of DC pulsed with liposomes and LPS (Fig. 6). After 48 h of incubation, only targeted PorA liposomes (Man, PS, and TAP liposomes) had induced IL-12 production to the same extent as did LPS. Nontargeted liposomes failed to induce IL-12 production by cultured DC. Differences were also found in the concentration of IL-12 in supernatants of the different types of targeted liposomes. For DC that had been incubated with Man liposomes, the IL-12 production was significantly higher than that of DC incubated with PS or TAP liposomes (P < 0.01). These findings suggest that besides increased maturation, targeted PorA liposomes induce functional maturation of DC.
FIG. 6.
IL-12 quantitation in supernatants of DC cultured for 48 h. The results are shown as means (± SEM) of at least four measurements. *, P < 0.01; **, P < 0.001.
In vivo localization of PorA liposomes.
The localization of fluorescently labeled PG and Man liposomes after immunization was studied in draining (popliteal and inguinal) lymph nodes. The choice of these formulations was based on the results obtained with bone marrow-derived DC, for which targeted liposomes showed an improved binding, uptake, and subsequent maturation with respect to control PG liposomes. Of the three types of targeted liposomes, Man liposomes induced the highest IL-12 production and were therefore chosen for the localization study.
The number of cells isolated from draining lymph nodes increased by 50% in mice immunized with both PG liposomes and Man liposomes compared to that in control mice (which were injected with PBS). No differences were found in the number of cells isolated from animals immunized with the two types of PorA liposomes (PG and Man liposomes). The percentage of fluorescent cells found in animals immunized with Man liposomes was significantly higher than that in animals immunized with PG liposomes (P = 0.031; Fig. 7A). This indicates that Man liposomes effectively target the draining lymph nodes in vivo. PorA liposomes were preferentially associated in draining lymph nodes with APC. Around 30% of CD11c+ cells, 15 to 25% of MHC class II+ cells, 10 to 15% of B220+ cells, and 50 to 70% of F4/80+ cells showed DiD fluorescence, whereas only 5% of CD4+ cells were fluorescent. Interestingly, targeted liposomes (i.e., Man liposomes) showed a significantly increased interaction with MHC class II+, B220+, and F4/80+ cells compared with nontargeted liposomes (P < 0.05; Fig. 7B), thus indicating that the use of mannosylated liposomes results in preferential uptake by APC.
FIG. 7.
Localization of PorA liposomes in draining lymph nodes. (A) Percentage of fluorescent cells isolated from draining lymph nodes of control mice and mice immunized with PG liposomes and Man liposomes. (B) Percentage of fluorescent cells found in various cell populations. White bars, mice immunized with PG liposomes; black bars, mice immunized with Man liposomes. The results are shown as means ± SEM of results for three mice. *, P < 0.05.
Immune responses generated by PorA liposomes.
The immune response generated by PorA liposomes and OMVs was studied in vivo. All targeted liposomes (Man, PS, and TAP liposomes) were tested, together with nontargeted liposomes and OMVs as controls. This choice was based on in vitro data, in which the antigenicity of PorA in PorA liposomes was variable (lower for Man and TAP liposomes than for PS and PG liposomes; Table 1). On the other hand, all targeted PorA liposomes were able to improve the interaction with DC and induce their functional maturation (i.e., IL-12 production).
Sera were analyzed not only for the presence of IgG and the IgG isotype distribution but also for their functionality, i.e., complement-mediated bactericidal serum activity. Despite the variable antigenicity, all formulations induced similarly high IgG titers (Table 2). No differences were found in the isotype distribution of IgG in sera of mice immunized with the different liposomal PorA formulations and OMVs. In contrast with what was expected from the in vitro data, the mean bactericidal titers in the responding mice were similar in all groups (Table 2). However, the percentage of responding mice increased in the groups immunized with targeted liposomes with respect to that in the groups immunized with PG liposomes and OMVs (both nontargeted; Table 2).
TABLE 2.
Humoral immune response induced by PorA liposomes and OMV
| Antigen | Whole-cell ELISA titera
|
Bactericidal assay
|
|||||
|---|---|---|---|---|---|---|---|
| IgG | IgG1 | IgG2a | IgG2b | IgG3 | % Responders | Titerb | |
| OMV | 3.40 | 3.16 | 3.21 | 3.52 | 2.87 | 62.5 | 2.38 ± 0.29 |
| PG liposomes | 3.34 | 3.19 | 3.15 | 3.49 | 2.77 | 50 | 2.25 ± 0.52 |
| Man liposomes | 3.26 | 2.98 | 3.15 | 3.30 | 2.84 | 87.5 | 2.30 ± 0.49 |
| PS liposomes | 3.40 | 3.01 | 3.20 | 3.33 | 2.78 | 100 | 1.99 ± 0.47 |
| TAP liposomes | 3.34 | 3.09 | 3.51 | 3.56 | 2.71 | 87.5 | 1.88 ± 0.54 |
The titer of each anti-PorA P1.7-2,4 IgG isotype was determined by whole-cell ELISA and is expressed as the mean log10 titer. The means were compared by the LSD test with a confidence level of 95%: LSD0.05 (IgG) = 0.23; LSD0.05 (IgG1) = 0.35; LSD0.05 (IgG2a) = 0.29; LSD0.05 (IgG2b) = 0.3; LSD0.05 (IgG3) = 0.35.
The bactericidal titer was determined as the reciprocal value of the serum dilution that effectuates killing of >90% of strain H44/76 7-24 and is expressed as the averaged mean log10 titer in the responders ± standard deviation.
DISCUSSION
In this study, we designed well-defined liposomal PorA formulations that aimed to improve the immunogenicity of PorA. DC in the periphery capture and process antigens, express lymphocyte costimulatory molecules, migrate to lymphoid organs, and secrete cytokines to initiate immune responses (33). The question is, then, does an enhancement of antigen loading of DC result in an improved immune response? In order to address this question, diverse strategies were chosen to increase the uptake of PorA liposomes by DC: first, receptor-mediated targeting toward receptors with specificity for mannose (Man liposomes) (17, 27) or the PS receptor (PS liposomes) (13); second, increasing the uptake of PorA liposomes by nonspecific electrostatic interaction of positively charged liposomes (TAP liposomes) with (negatively charged) DC.
The formulation of PorA in both Man liposomes and TAP liposomes resulted in a decreased ability of PorA to interact with specific mAb (Table 1), despite the preservation of the trimeric protein conformation (Fig. 2). This was probably due to steric hindrance caused by mannose molecules at the outside of the liposomes and the positive charge of TAP liposomes, respectively, which hindered the interaction of PorA and its specific mAb.
Targeted PorA liposomes showed an increased uptake by DC in vitro, compared to nontargeted liposomes (Fig. 3). Increased uptake has been ascribed to the multivalent character of liposomal antigens compared with soluble antigens (28). More efficient antigen presentation by DC was obtained by targeting liposomes toward Fc receptor or class I and class II molecules (28). Also, mannosylated protein antigen and peptides showed enhanced potency to stimulate specific T-cell clones compared to nonmannosylated peptides (31). More recently, the same has been proven with mannosylated liposomes (8). However, these systems have been tested only in vitro and with model antigens. In our study, experiments in vivo were included, as promising in vitro results are not always predictive for improved vaccine efficacy in vivo.
Our results confirmed that PorA liposomes are efficiently internalized by cultured DC (Fig. 4). The uptake of PorA liposomes induced maturation of DC (Fig. 5). However, only targeted liposomes induced IL-12 production by DC (Fig. 6). The improved uptake of Man liposomes can be explained by the interaction of mannose moieties with receptors of the C-type lectin family (macrophage mannose receptor; DEC-205). For PS liposomes, their increased internalization with respect to nontargeted liposomes can be explained by an uptake mechanism similar to that of apoptotic cells. Phagocytosis of apoptotic cells is related to the recognition of PS exposed externally due to the loss of asymmetry of plasma membrane phospholipids by the PS receptor (10) during apoptosis (11, 19). On the other hand, the uptake of positively charged TAP liposomes is probably not receptor mediated but caused by nonspecific electrostatic interactions with negatively charged DC. This mechanism is just as efficiently mediating the uptake of PorA liposomes as receptor-mediated endocytosis. Moreover, the confocal micrographs indicate that electrostatic interaction of TAP liposomes with the DC surface resulted in punctate fluorescence inside the cells similar to that observed with other types of PorA liposomes. This suggests internalization of all types of PorA liposomes via an endocytotic pathway.
The in vivo localization of targeted (i.e., Man liposomes) and nontargeted liposomes was studied in afferent lymph nodes after subcutaneous administration. The findings of these experiments confirm some of the results obtained in vitro. Targeted liposomes (Man liposomes) had an increased localization in draining lymph nodes with respect to nontargeted PG liposomes (Fig. 7A). Both types of liposomes interacted preferentially with APC. However, targeted liposomes (i.e., Man liposomes) showed a significantly increased interaction with MHC class II+, B220+, and F4/80+ cells compared with nontargeted liposomes. (Fig. 7B). These results indicate a good correlation between the in vitro and in vivo results, as the use of mannosylated liposomes resulted in both cases in an improved loading of APC compared to the use of nontargeted liposomes.
The cytokines generated by DC are critically important for subsequent T-cell differentiation. IL-12 produced by DC is pivotal for the development of Th1 responses (4). A Th1-type immune response can switch B cells to IgG2a production (20). This is preferable, as IgG of the isotypes 2a and 2b is able to interact with complement and induce complement-mediated killing of bacteria (14). Bactericidal antibody titers are considered one of the correlates of protection in type B meningococcal vaccination (34). It has been demonstrated that IL-12 is required for development of an effective cellular and/or humoral antimicrobial defense against bacteria (7), parasites (30), and viruses (22). Targeting of PorA liposomes resulted in induction of IL-12 in vitro, in contrast to nontargeted PorA liposomes. Surprisingly, all types of liposomes induced similarly high IgG titers in vivo. These IgG titers were comparable to those induced by (LPS-containing) OMVs. Moreover, the production of IL-12 in vitro induced by targeted PorA liposomes was not translated in vivo into an IgG isotype switching (Table 2). Also, the bactericidal titers found in the sera of mice that had been immunized with targeted liposomes were not improved with respect to those in mice that were immunized with nontargeted PorA liposomes and OMVs. However, the number of responding mice per group increased from 50 to 60% with nontargeted PorA liposomes and OMVs to almost 100% (Table 2) of mice immunized with targeted PorA liposomes. We can thus conclude that targeting of PorA liposomes to DC resulted in a more homogeneous immune response in vivo than other vaccine formulations such as OMVs, despite the absence of a measurable isotype switching.
Previous studies have shown that targeting of DC results in a better in vitro immune response (i.e., T-cell activation) (8, 28, 31). The very different conditions in vivo—with complex interactions between various cell types and tissues—complicate the extrapolation of in vitro data to apply to the in vivo situation. This is confirmed by our observation that the clear improvement of the liposomal antigen uptake and subsequent DC maturation in vitro was translated as only a marginal improvement of the immune response in vivo (i.e., increase of percentage of responders).
Liposomes are well-defined systems composed of purified materials. In our experiments, liposomes contained LPS-depleted PorA and therefore offer an important safety advantage over LPS-containing OMVs, which are the only PorA vaccines tested in the clinic so far (9, 24, 32). LPS is a potent activator of DC (25), as confirmed in this study (Fig. 5 and 6), but is also a toxic compound, which should preferably be absent in vaccine formulations (29).
In summary, targeted PorA liposomes showed efficient loading and activation of cultured DC compared to nontargeted PorA liposomes. In line with these findings, improved uptake in draining lymph nodes as well as preferential uptake by APC was observed in vivo. Importantly, immunization with targeted PorA liposomes resulted in an improvement of the immune response in comparison with that with nontargeted liposomes or OMVs. However, the improvement in vivo is less pronounced than the strong effects seen in vitro. Our results encourage the development of meningococcal immunization strategies that use antigens encapsulated in liposomes targeted toward APC.
Acknowledgments
We thank Jan-Hein van Steenis for the synthesis and purification of mannosylated PE, Marjolein van Winden for the formulation work, and Liana Steeghs for helping with the IL-12 quantitation.
The work of Lisette Bevaart is sponsored by the Dutch Cancer Society (K.W.F. project UU 2001-2496).
Editor: J. N. Weiser
REFERENCES
- 1.Abdillahi, H., and J. T. Poolman. 1987. Whole-cell ELISA for typing Neisseria meningitidis with monoclonal antibodies. FEMS Microbiol. Lett. 48:367-371. [PubMed] [Google Scholar]
- 2.Arigita, C., G. F. A. Kersten, A. Hazendonk, W. E. Hennink, D. J. A. Crommelin, and W. Jiskoot. 2003. Restored functional immunogenicity of purified meningococcal PorA by incorporation into liposomes. Vaccine 21:950-960. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharya, A., M. E. Dorf, and T. A. Springer. 1981. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J. Immunol. 127:2488-2495. [PubMed] [Google Scholar]
- 4.Cella, M., D. Scheidegger, L. K. Palmer, P. Lane, A. Lanzavecchia, and G. Alber. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin 12 and enhances T-cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184:747-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christodoulides, M., J. L. Brooks, E. Rattue, and J. E. Heckels. 1998. Immunization with recombinant class 1 outer-membrane protein from Neisseria meningitidis: influence of liposomes and adjuvants on antibody avidity, recognition of native protein and the induction of a bactericidal immune response against meningococci. Microbiology 144:3027-3037. [DOI] [PubMed] [Google Scholar]
- 6.Connolly, M., N. Noah, et al. 1999. Is group C meningococcal disease increasing in Europe? A report of surveillance of meningococcal infection in Europe 1993-6. Epidemiol. Infect. 122:41-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper, A. M., J. Magram, J. Ferrante, and I. M. Orme. 1997. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J. Exp. Med. 186:39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Copland, M. J., M. A. Baird, T. Rades, J. L. McKenie, B. Becker, F. Reck, P. C. Tyler, and N. M. Davies. 2003. Liposomal delivery of antigens to human dendritic cells. Vaccine 21:883-890. [DOI] [PubMed] [Google Scholar]
- 9.de Kleijn, E. D., R. de Groot, J. Labadie, A. B. Lafeber, G. van den Dobbelsteen, L. van Alphen, B. Kuipers, G. W. van Omme, M. Wala, R. Juttmann, and H. C. Rümke. 2000. Immunogenicity and safety of hexavalent meningococcal outer membrane vesicle vaccine in children of 2-3 and 7-8 years of age. Vaccine 18:1456-1466. [DOI] [PubMed] [Google Scholar]
- 10.Fadok, V. A., D. L. Bratton, D. M. Rose, A. Pearson, R. A. B. Ezekewitz, and P. M. Henson. 2000. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 405:85-90. [DOI] [PubMed] [Google Scholar]
- 11.Fadok, V. A., D. R. Voelker, P. A. Campbell, J. J. Cohen, D. L. Bratton, and P. M. Henson. 1992. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148:2207-2216. [PubMed] [Google Scholar]
- 12.Guermonprez, P., J. Valladeau, L. Zitvogel, C. Thery, and S. Amigorena. 2002. Antigen presentation and T-cell stimulation by dendritic cells. Annu. Rev. Immunol. 20:621-667. [DOI] [PubMed] [Google Scholar]
- 13.Henson, P. M., D. L. Bratton, and V. A. Fadok. 2001. The phosphatidylserine receptor: a crucial molecular switch? Nat. Rev. Mol. Cell Biol. 2:627-633. [DOI] [PubMed] [Google Scholar]
- 14.Hoogerhout, P., E. M. L. M. Donders, J. A. M. van Gaans-van den Brink, B. Kuipers, H. F. Brugghe., L. M. van Unen, H. A. M. Timmermans, G. J. ten Hove, A. P. J. M. de Jong, C. C. A. M. Peeters, E. J. H. J. Wiertz, and J. T. Poolman. 1995. Conjugates of synthetic cyclic peptides elicit bactericidal antibodies against a conformational epitope on a class 1 outer membrane protein of Neisseria meningitidis. Infect. Immun. 63:3473-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R. M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte-macrophage colony-stimulating factor. J. Exp. Med. 176:1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen, C., A. Wiese, L. Reubsaet, N. Dekker, H. de Cock, U. Seydel, and J. Tommassen. 2000. Biochemical and biophysical characterization of in vitro folded outer membrane porin PorA of Neisseria meningitidis. Biochim. Biophys. Acta 1464:284-298. [DOI] [PubMed] [Google Scholar]
- 17.Jiang, W., W. J. Swiggard, C. Heufler, M. Peng, A. Mirza, R. M. Steinman, and M. C. Nussenzweig. 1995. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature 375:151-155. [DOI] [PubMed] [Google Scholar]
- 18.Jodar, L., I. M. Feavers, D. Salisbury, and D. M. Granoff. 2002. Development of vaccines against meningococcal disease. Lancet 359:1499-1508. [DOI] [PubMed] [Google Scholar]
- 19.Martin, S. J., C. P. Reutelingsperger, A. J. McGahon, J. A. Rader, R. C. A. A. van Schie, D. M. LaFace, and D. R. Green. 1995. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 82:1545-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosmann, T. R., and R. L. Coffman. 1989. Heterogeneity of cytokine secretion patterns and functions of T helper cells. Adv. Immunol. 46:111-147. [DOI] [PubMed] [Google Scholar]
- 21.Muttilainen, S., I. Idänpään-Heikkilä, E. Wahlström, M. Nurminen, P. H. Mäkelä, and M. Sarvas. 1995. The Neisseria meningitidis outer membrane protein P1 produced in Bacillus subtilis and reconstituted into phospholipid vesicles elicits antibodies to native P1 epitopes. Microb. Pathog. 18:423-436. [DOI] [PubMed] [Google Scholar]
- 22.Orange, J. S., S. F. Wolf, and C. A. Biron. 1994. Effects of IL-12 on the response and susceptibility to experimental viral infections. J. Immunol. 152:1253-1264. [PubMed] [Google Scholar]
- 23.Peterson, G. L. 1977. A simplification of the protein assay of Lowry et al., which is more generally applicable. Anal. Biochem. 83:346-356. [DOI] [PubMed] [Google Scholar]
- 24.Rosenqvist, E., E. A. Hoiby, E. Wedege, K. Bryn, J. Kolberg, A. Klem, E. Ronnild, G. Bjune, and H. Nokleby. 1995. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect. Immun. 63:4642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenstein, N. E., B. A. Perkins, D. S. Stephens, L. Lefkowitz, M. L. Cartter, R. Danila, P. Cieslak, K. A. Shutt, T. Popovic, A. Schuchat, L. H. Harrison, and A. L. Reingold. 1999. The changing epidemiology of meningococcal disease in the United States. J. Infect. Dis. 180:1894-1901. [DOI] [PubMed] [Google Scholar]
- 26.Rouser, G., S. Fleisner, and A. Yamamoto. 1970. Two-dimensional thin-layer chromatographic separation of polar lipids and determination of phospholipids by phosphorous analysis of spots. Lipids 5:494-496. [DOI] [PubMed] [Google Scholar]
- 27.Sallusto, F., M. Cella, C. Danielli, and A. Lanzavecchia. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serre, K., P. Machy, J.-C. Grivel, G. Jolly, N. Brun, J. Barbet, and L. Leserman. 1998. Efficient presentation of multivalent antigens targeted to various cell surface molecules of dendritic cells and surface Ig of antigen-specific B cells. J. Immunol. 161:6059-6067. [PubMed] [Google Scholar]
- 29.Steeghs, L., B. Kuipers, H. J. Hamstra, G. Kersten, L. van Alphen, and P. van der Ley. 1999. Immunogenicity of outer membrane proteins in lipopolysaccharide-deficient mutant of Neisseria meningitidis: influence of adjuvants on the immune response. Infect. Immun. 67:4988-4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su, A., and M. M. Stevenson. 2002. IL-12 is required for antibody-mediated protective immunity against blood-stage Plasmodium chabaudi AS malaria infection in mice. J. Immunol. 168:1348-1355. [DOI] [PubMed] [Google Scholar]
- 31.Tan, M. C. C. A., A. M. Momaas, J. W. Drijfhout, R. Jordens, J. J. M. Onderwater, D. Verwoerd, A. A. Mulder, A. N. van der Heiden, D. Scheidegger, L. C. J. M. Oomen, T. H. M. Ottenhoff, A. Tulp, J. J. Neefjes, and F. Koning. 1997. Mannose receptor-mediated uptake of antigens strongly enhances HLS class II restricted antigen presentation by cultured dendritic cells. Eur. J. Immunol. 27:2426-2435. [DOI] [PubMed] [Google Scholar]
- 32.Tappero, J. W., R. Lagos, A. M. Ballesteros, B. Plikaytis, D. Williams, J. Dykes, L. L. Gheesling, G. M. Carlone, E. A. Hoiby, J. Holst, H. Nokleby, E. Rosenqvist, G. Sierra, C. Campa, F. Sotolongo, J. Vega, J. Garcia, P. Herrera, J. T. Poolman, and B. A. Perkins. 1999. Immunogenicity of 2 serogroup B outer membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 281:1520-1527. [DOI] [PubMed] [Google Scholar]
- 33.Thery, C., and S. Amigorena. 2001. The cell biology of antigen presentation in dendritic cells. Curr. Opin. Immunol. 13:45-51. [DOI] [PubMed] [Google Scholar]
- 34.Vermont, C. L., H. H. van Dijken, C. J. P. van Limpt, R. de Groot, L. van Alphen, and G. P. J. M. van den Dobbelsteen. 2002. Antibody avidity and immunoglobulin G isotype distribution following immunization with a monovalent meningococcal B outer membrane vesicle vaccine. Infect. Immun. 70:584-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward, S. J., D. Scopes, M. Christodoulides, I. N. Clarke, and J. E. Heckels. 1996. Expression of Neisseria meningitidis class 1 porin as a fusion protein in Escherichia coli: the influence of liposomes and adjuvants on the production of a bactericidal immune response. Microb. Pathog. 21:499-512. [DOI] [PubMed] [Google Scholar]