Abstract
Actinobacillus actinomycetemcomitans is a gram-negative, facultatively anaerobic bacterium which is associated especially with aggressive forms of periodontitis. Contradictory results on the localization of the A. actinomycetemcomitans serotype-specific antigen have been reported. The aim of the present study was to characterize the A. actinomycetemcomitans serotype d-specific antigen. The antigen was isolated by affinity chromatography. The affinity column was prepared from immunoglobulin G isolated from rabbit antiserum raised against A. actinomycetemcomitans serotype d. The isolated antigen was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blotting, and silver staining, all of which revealed a ladder-like structure typical for the O antigen of lipopolysaccharide (LPS). In a displacement enzyme-linked immunosorbent assay (ELISA), the isolated antigen displaced in a concentration-dependent manner the binding of the polyclonal rabbit antiserum raised against A. actinomycetemcomitans serotype d to the competing whole-cell serotype d antigen. The isolated antigen contained LPS, and an equal concentration of LPS isolated from A. actinomycetemcomitans serotype d gave a similar displacement curve in the ELISA. In order to test the immunogenic properties of the isolated antigen, it was used to immunize a rabbit. The antiserum raised against the isolated antigen displayed specificity in Western blotting and ELISA similar to that of antibody raised against LPS isolated from A. actinomycetemcomitans serotype d. In conclusion, our results show that the A. actinomycetemcomitans serotype d-specific antigen contains the O-antigenic structure of LPS.
Actinobacillus actinomycetemcomitans is a gram-negative, facultatively anaerobic coccobacillus which is associated with aggressively progressing periodontitis. In addition to oral infections, A. actinomycetemcomitans causes serious nonoral infections, such as endocarditis (31). The species is divided into six serotypes (a to f) (8, 26, 39), and fewer than 10% of the strains are nonserotypeable with serological methods (19). Different clonal types exhibit intraspecies differences in virulence characteristics, such as leukotoxin production (5, 39). Antibiotic resistance patterns differ among the serotypes and even among the genotypes of the same serotype (20, 22). Although the serotype distribution of A. actinomycetemcomitans seems to differ geographically, evidence from Finland and the United States indicates that serotype b is associated with periodontitis and that serotype c is most frequently found in periodontally healthy individuals (2, 4, 39). The distribution pattern of serotype f is not yet known, whereas serotype e occurs both in periodontally diseased and healthy individuals (4, 6, 12). Among the A. actinomycetemcomitans serotypes, serotype d is infrequent in most populations studied. Thus, its role in the oral flora is even less well known than those of the other serotypes.
The serotype-specific antigen of A. actinomycetemcomitans is the immunodominant antigen of the species (27). The composition and location of the antigen have been studied extensively (1, 18, 23, 34). Researchers agree that the antigen is composed of polysaccharide, but whether it resides in the capsular polysaccharide distinct from lipopolysaccharide (LPS) (1, 3) or in the polysaccharide moiety of LPS (18, 34) is still unclear. Further attempts to characterize the serotype-specific antigens are needed to advance understanding of the role of A. actinomycetemcomitans as a human pathogen and to clarify why serotypes are distributed differently in geographically distant populations, in periodontal health and disease, or among various periodontal diseases.
The composition of the LPS of serotype d differs from that of the LPSs of serotypes a, b, c, and e. In contrast to these other serotypes, whose LPS O antigens are composed of repeating disaccharide or trisaccharide units, the O antigen of serotype d is composed of repeating tetrasaccharide units (23). Therefore, in Western blots, the characteristic ladder-like pattern of LPS O antigen is seen in serotype d strains only (14). Furthermore, it was recently shown that eight unique genes code for the serotype d-specific polysaccharide and that the biosynthesis and structure of this polysaccharide antigen are different from those of serotypes b, c, and e (14). However, despite the known structure of the O antigen of serotype d and the unique genes coding for the serotype-specific antigen, it is not known on which surface structure the particular antigen resides. Thus, in the present study we exploited an immunological approach to isolate the serotype d-specific antigen from the A. actinomycetemcomitans outer membrane complex (OMC) by using affinity chromatography. The isolated serotype d-specific antigen was characterized by silver staining, Western blotting, and displacement enzyme-linked immunosorbent assay (ELISA). Additionally, to test the immunogenic properties of the isolated antigen, we immunized rabbits with the antigen and characterized the antibodies.
MATERIALS AND METHODS
Bacterial strains.
The study used five clinical A. actinomycetemcomitans serotype d strains (20) and reference strains ATCC 29523, ATCC 43758, ATCC 33384, IDH781, and IDH1708, representing A. actinomycetemcomitans serotypes a, b, c, d, and e, respectively. The bacterial strains were stored in skim milk at −70°C.
Isolation of LPS.
LPSs of the A. actinomycetemcomitans strains were isolated as previously described (21). Briefly, the strains were grown on Trypticase soy agar (Difco Laboratories, Detroit, Mich.) plates in 5% CO2 in air at 37°C for 2 to 3 days. Bacterial colonies were suspended in phosphate-buffered saline (10 mM phosphate [pH 7.4], 150 mM NaCl) (PBS), and the cells were broken by sonication for 4.5 min on ice. The total bacterial cell membrane fraction was recovered by centrifugation at 100,000 × g for 1 h and treated with Sarkosyl (sodium lauryl sarcosinate) (Sigma Chemical Co., St. Louis, Mo.) at room temperature for 1 h, and the solution was centrifuged at 100,000 × g for 1 h. The pellet was suspended in distilled water, dispersed in lysing buffer (125 mM Tris-HCl [pH 6.8] containing 5% 2-mercaptoethanol, 2% sodium dodecyl sulfate [SDS], and 10% glycerol), and solubilized by incubation at 100°C for 10 min. The suspension was cooled to room temperature, 10 μg of proteinase K per ml was added, and the solution was incubated at 60°C for 60 min.
The LPS of A. actinomycetemcomitans strain IDH781, a kind gift from M. Wilson, was isolated by the phenol-water method (33).
Preparation of the columns.
The immunoglobulin G (IgG) fraction was isolated from rabbit antiserum raised against whole cells of A. actinomycetemcomitans serotype d (IDH781) for the anti-serotype d column and from antiserum from a nonsuccessfully immunized rabbit for the control column. The sera were recycled in a Protein G-Sepharose 4B column (Amersham Pharmacia Biotech, Piscataway, N.J.) at a flow rate of 5 ml/h at 4°C overnight. The column was extensively washed with PBS and eluted with 0.1 M glycine-HCl [pH 2.5] into tubes containing 125 μl of 1 M Tris-HCl [pH 8.0]. The fraction size was 1.6 ml, and the elution was monitored spectrophotometrically at 280 nm. The peak fractions containing IgG were pooled, dialyzed against the coupling buffer, and coupled to CNBr-activated Sepharose 4B matrix (Amersham Pharmacia Biotech) according to the manufacturer's instructions. The coupling percentages were 76 and 72% for the anti-serotype d column and the control column, respectively.
Isolation of the serotype d-specific antigen.
A. actinomycetemcomitans serotype d strains were cultured on brucella agar plates (containing 5% horse blood, 5 μl of hemin per ml, 100 mg of vitamin K1 per ml, and brucella agar [BBL, Cockeysville, Md.]) for 2 days and subsequently in Todd-Hewitt broth (3% Todd-Hewitt medium [Gibco Laboratories], 1% yeast extract) in 5% CO2 at 37°C for 3 days. The purity of the culture was confirmed by typical colony morphology and Gram staining. To isolate the OMC, the bacterial cells were suspended in 5 ml of PBS and broken by sonication on ice for 10 min at 1-min intervals. Cell remnants were removed by centrifugation at 1,200 × g, and the supernatant was stored at −20°C. Before application to the column, the supernatant was boiled in 5 mM 2-mercaptoethanol for 5 min. Nonspecific binding was eliminated by recycling the supernatant in the control column at a flow rate of 5 ml/h at 4°C overnight. The column was washed and eluted as described above. In order to isolate the serotype d-specific antigen, the flowthrough fraction from the control column was recycled in the anti-serotype d column at 4°C overnight, and the column was washed and eluted as described for the control column. The peak elution fractions from the control column containing the nonspecific material (control fraction) and from the anti-serotype d column containing the serotype d-specific antigen (affinity-purified serotype d-specific fraction) were pooled and dialyzed against PBS. The control fraction was used as a control in all experiments.
Determination of LPS and protein concentrations.
The LPS concentration in the affinity-purified serotype d-specific fraction was determined by the malondealdehyde thiobarbituric acid reaction, which detects the 3-deoxy-d-mannooctulsonic acid (KDO) portion of LPS (32). Protein concentrations were determined as described previously (11).
Characterization of the antigen.
The isolated fractions were electrophoresed in an SDS-12.5% polyacrylamide gel (10), and the gels were silver stained (7) or immunoblotted (30). The Western blots were treated with rabbit antisera raised against strains of A. actinomycetemcomitans serotypes a through e (ATCC 29523, ATCC 43781, ATCC 33384, IDH781, and IDH1708, respectively) (26), and the antigens were detected with horseradish peroxidase-conjugated goat anti-rabbit IgG.
The specificity of the affinity-purified serotype d-specific fraction was examined by a displacement ELISA modified from the ELISA described previously (24). Briefly, the density of formalin-fixed A. actinomycetemcomitans cell suspensions was adjusted to give an absorbance of 0.15 at 580 nm, and the suspension was used to coat microtiter plates (Cliniplate Uni; Labsystems, Helsinki, Finland). The reference strains used for coating were the same as the immunization strains representing A. actinomycetemcomitans serotypes a, b, c, d, and e. Nonspecific binding was prevented by use of 5% bovine serum albumin in PBS. Antisera (dilution of 1:15,000) against different A. actinomycetemcomitans serotypes alone or mixed with increasing amounts of competitors (1:5, 1:10, 1:50, 1:100, 1:1,000, and 1:10,000) were added to the microtiter plate and incubated at room temperature for 2 h. The affinity-purified serotype d-specific fraction and isolated serotype d LPS preparations (21) were used as competitors. The control fraction and LPS isolated from Escherichia coli were used as control competitors for nonspecific binding. The bound antibodies were visualized with horseradish peroxidase-conjugated goat anti-rabbit IgG (Sigma Chemical Co.) diluted 1:20,000. Nonspecific binding was monitored with blank wells containing no antibody. The results were calculated as relative binding, with the maximum absorbances (the binding of antisera without any competitor) taken as 100%.
Immunization of rabbits.
Rabbits (n = 3) were immunized with three antigens (one rabbit per antigen): the control fraction, the affinity-purified serotype d-specific fraction, and LPS isolated from A. actinomycetemcomitans serotype d (IDH781). The antigens, containing 1.3 μg of LPS, were emulsified in Freund's complete adjuvant. The LPS concentration was determined with malondealdehyde thiobarbituric acid reaction (32). As a booster, the rabbits received a similar dose of the antigen in Freund's incomplete adjuvant twice at 3-week intervals, and the antisera against the control fraction, the affinity-purified serotype d-specific fraction, and the LPS isolated from IDH781 were collected 3 weeks after each injection. The specificities of the antisera were determined by ELISA and Western blot analysis.
RESULTS
Western blotting of A. actinomycetemcomitans LPS.
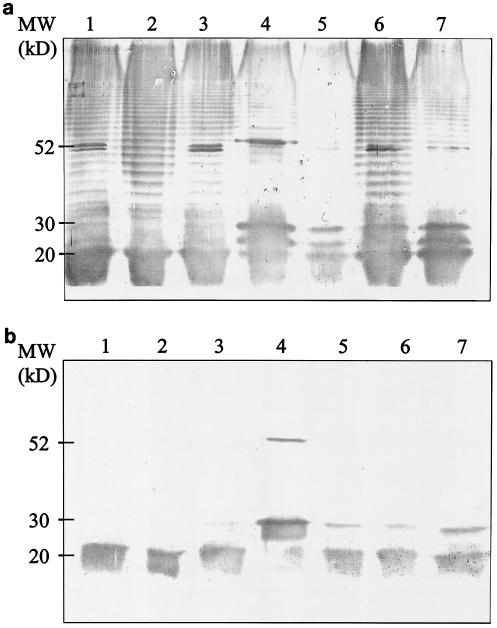
LPS was isolated by Sarkosyl extraction from five different clinical A. actinomycetemcomitans serotype d strains and one reference strain (IDH781) representing A. actinomycetemcomitans serotype d. For strain IDH781, two LPS preparations isolated with two different protocols were used. To compare the antigenic composition of A. actinomycetemcomitans serotype d, the isolated LPS preparations from six strains were electrophoresed and immunoblotted with rabbit antisera raised against A. actinomycetemcomitans strains of the five serotypes. The blot treated with the antiserum raised against serotype d displayed a 30- to 70-kDa ladder-like structure (Fig. 1a), which was not seen in the blots treated with antisera raised against A. actinomycetemcomitans serotype a, b, c, or e (data for serotype c are shown in Fig. 1b). The protocol used to isolate the LPSs did not have an effect on the results of the immunoblots. LPSs isolated by two different procedures from strain IDH781 both revealed ladder-like structures in the Western blot that were identical to each other and to those of the five LPS preparations isolated from the clinical strains (Fig. 1a). The antisera raised against serotypes, a, b, c, and e each detected a broad band with a molecular size of 15 to 20 kDa. The blot detected with antiserum raised against serotype c is shown in Fig. 1b.
FIG. 1.
Western blot analysis of A. actinomycetemcomitans serotype d LPS. LPS was isolated by hot phenol-water extraction from five clinical strains representing A. actinomycetemcomitans serotype d (lanes 1 to 5) and by hot phenol-water extraction and Sarkosyl extraction from one reference strain (lanes 6 and 7). Lane 1, strain 328; lane 2, strain 685; lane 3, strain 3033; lane 4, strain T-2222; lane 5, strain 508; lanes 6 and 7, reference strain IDH781. Isolated LPS preparations were subjected to SDS-12.5% PAGE, transferred on a nitrocellulose membrane, and treated with polyclonal antisera raised against A. actinomycetemcomitans serotypes a through e. The antigens were detected with rabbit antiserum raised against serotype d (a) or against serotype c (b).
Isolation of the serotype d-specific antigen.
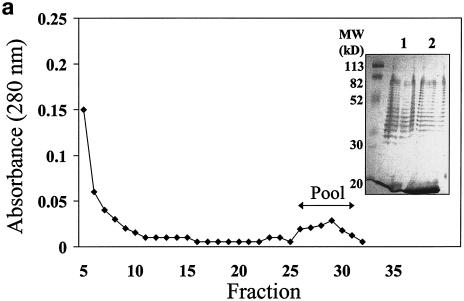
To isolate the serotype d-specific antigen by affinity chromatography, the OMC extract of A. actinomycetemcomitans serotype d (IDH781) was applied to the control column (Fig. 2a) and its flowthrough fraction was applied to the anti-serotype d column (Fig. 2b). Silver staining of the OMC extract as well as the flowthrough fraction of the control column (Fig. 2a, inset, lanes 1 and 2, respectively) revealed a ladder-like banding pattern similar to that seen in the Western blots of the OMC extract (data not shown) or of IDH781 LPS (Fig. 1a) when treated with antiserum against serotype d. As detected at 280 nm, only a small proportion of protein was bound to the anti-serotype d column. This suggests that the serotype d elution fraction contained mainly antigenic material other than protein. The protein recovery percentages of the control column and anti-serotype d column were 103 and 90%, respectively.
FIG. 2.
Elution curves from affinity chromatography. The OMC of A. actinomycetemcomitans IDH781 was isolated as described in Materials and Methods and applied to a control column (a) to eliminate the nonspecific binding. The flowthrough fraction was applied to the anti-serotype d column (b), and the elution fractions from the columns were pooled as indicated by the arrows. The inset in panel a shows silver staining of the OMC applied to the control column (lane 1) and of the flowthrough fraction from the control column applied to the anti-serotype d column (lane 2).
Characterization of the affinity-purified serotype d-specific fraction.
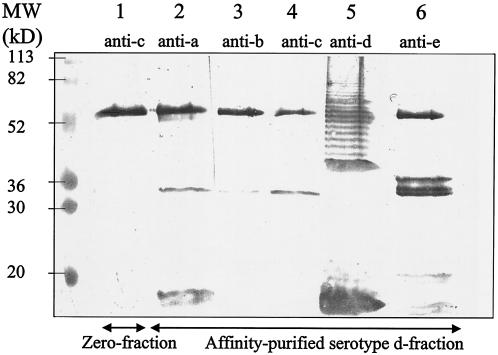
The affinity-purified serotype d-specific fraction was analyzed by Western blotting in which the antigens were detected with polyclonal antisera against the five A. actinomycetemcomitans serotypes. Only when treated with the antiserum raised against serotype d did the blot containing the affinity-purified serotype d-specific fraction (Fig. 3, lane 5) reveal a ladder-like structure similar to that displayed by LPS isolated from IDH781 (Fig. 1, lanes 6 to 7). In the affinity-purified serotype d-specific fraction, antisera against the five serotypes (a to e) detected bands with molecular masses of 60, 34, and 15 to 20 kDa. In the control fraction, each antiserum also detected a 60-kDa band (data for the antiserum against serotype c are shown in Fig. 3, lane 1) without any additional bands.
FIG. 3.
Western blot of the affinity-purified serotype d-specific antigen. The serotype d-specific antigen was isolated as described in Materials and Methods. The affinity-purified serotype d fraction and the control fraction were subjected to SDS-12.5% PAGE, transferred to a nitrocellulose membrane, and treated with polyclonal rabbit antisera raised against A. actinomycetemcomitans serotypes a through e. Lane 1, control fraction detected with rabbit antiserum raised against serotype c. Lanes 2 to 6, affinity-purified serotype d-specific antigen detected with rabbit antisera raised against serotypes a through e, respectively.
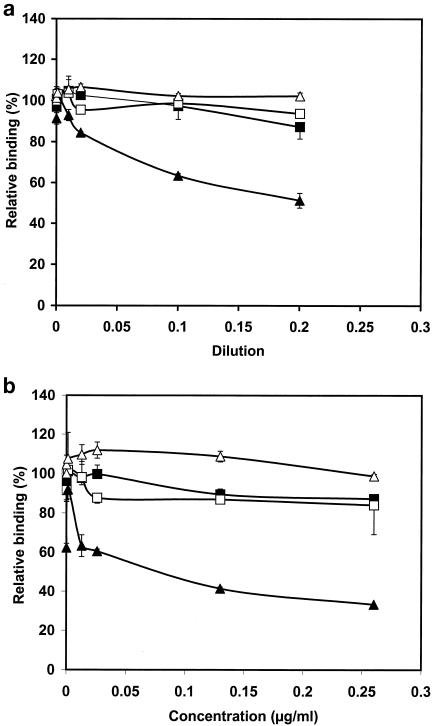
To further analyze the specificity of the affinity-purified serotype d-specific fraction, we used a displacement ELISA (Fig. 4). In the assay, the affinity-purified serotype d-specific fraction competed with the immobilized whole-cell antigens of A. actinomycetemcomitans for binding to the corresponding antiserum. As tested independently with five A. actinomycetemcomitans serotypes, the affinity-purified serotype d-specific antigen displaced binding of the antiserum raised against IDH781 to serotype d whole cells (IDH781) up to 51% in a concentration-dependent manner (Fig. 4a). The affinity-purified serotype d-specific fraction did not act as a competitor in the displacement ELISA of other serotypes (data for serotype c are shown in Fig. 4a). The control fraction did not displace binding of any antiserum to the immobilized antigen (Fig. 4a).
FIG. 4.
Characterization of the specificity of the affinity-purified serotype d-specific antigen. ELISA plates were coated with formalin-killed whole cells of A. actinomycetemcomitans strains representing serotypes a to e as described in Materials and Methods. The isolated fractions, the affinity-purified serotype d fraction and the control fraction, competed with the immobilized whole-cell antigen for binding to the corresponding antiserum. The binding of the antiserum was detected with peroxidase-conjugated anti-rabbit IgG. (a) Microtiter plates were coated with serotype d whole cells, and the affinity-purified serotype d-specific antigen (▴) and the control fraction (▵) competed for binding to the rabbit antiserum raised against serotype d. Microtiter plates were coated with serotype c whole cells, and affinity-purified serotype d-specific antigen (▪) and the control fraction (□) competed for the binding to the rabbit antiserum raised against serotype c. (b) As measured with the malondealdehyde-thiobarbituric acid reaction, the affinity-purified serotype d-specific antigen contained 0.13 μg of LPS per ml. Displacement ELISA was repeated with known LPS concentrations, and the affinity-purified serotype d-specific fraction and LPS isolated from E. coli (0.13 μg/ml) were used as competitors. ELISA plates were coated with A. actinomycetemcomitans serotype d whole cells, and affinity-purified serotype d-specific antigen (▴) and LPS isolated from E. coli (▵) competed for the binding to the rabbit antiserum raised against serotype d. The microtiter plates were coated with serotype c whole cells, and affinity-purified serotype d-specific antigen (▪) and LPS isolated from E. coli (□) competed for the binding to the rabbit antiserum raised against serotype c. Error bars indicate standard deviations.
As measured by detection of KDO by the malondealdehyde thiobarbituric acid reaction, the affinity-purified serotype d-specific fraction contained 0.13 μg of LPS per ml. The displacement ELISA was repeated with known LPS concentrations (Fig. 4b). LPSs isolated from E. coli and from IDH781 were used as control competitors at the same concentrations (0.13 μg/ml) as the affinity-purified serotype d-specific fraction. Similarly to the affinity-purified serotype d-specific fraction, LPS isolated from A. actinomycetemcomitans IDH781 displaced only the binding to serotype d whole cells by antiserum raised against A. actinomycetemcomitans serotype d (IDH781) (Fig. 4b). The displacement was concentration dependent and reached its maximum of 67% at an LPS concentration of 0.26 μg/ml. E. coli LPS did not interfere with the binding in any of the displacement ELISA experiments.
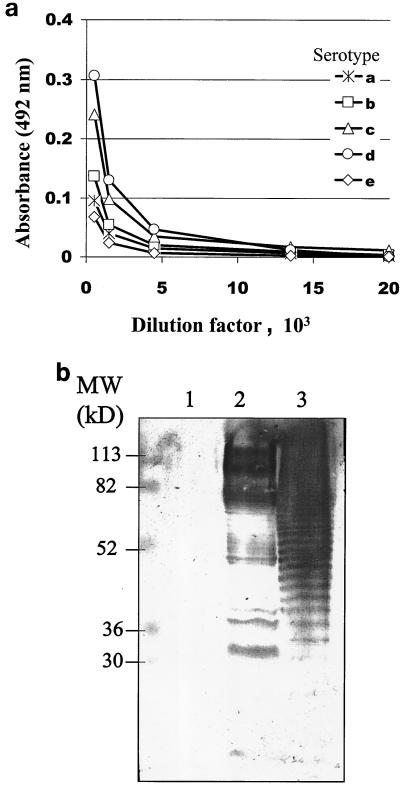
Immunization.
To test the immunogenic properties of the isolated antigen, the affinity-purified serotype d-specific fraction was used to immunize a rabbit. Rabbits were also immunized with LPS isolated from A. actinomycetemcomitans serotype d (IDH781) as a positive control and with the control fraction as a negative control. Both the affinity-purified serotype d-specific fraction and LPS isolated from A. actinomycetemcomitans serotype d were immunogenic (Fig. 5), but the avidity of the antibodies was lower than that of the antibody raised against the whole-cell antigen. The antiserum raised against the affinity-purified serotype d-specific fraction was characterized in an ELISA where whole cells representing all A. actinomycetemcomitans serotypes (a to e) were used as immobilized antigens (Fig. 5a). The affinity of the antiserum raised against the affinity-purified serotype d-specific fraction was the highest to serotype d, with an area under the curve of 4.36 mm2. The corresponding areas under the curves for serotypes a, b, c, and e were 1.78, 1.94, 3.50, and 0.83 mm2, respectively. The antisera raised against the affinity-purified serotype d-specific fraction, the LPS isolated from IDH781, and the control fraction were further characterized by Western blotting. The corresponding antigens (the affinity-purified serotype d-specific fraction, the LPS isolated from IDH781, and the control fraction) were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted, and the immunoblots were treated with the antisera mentioned above. The antisera against the affinity-purified serotype d-specific fraction (Fig. 5b) and against the LPS isolated from IDH781 (data not shown) detected essentially similar banding patterns and demonstrated a ladder-like structure in both the affinity-purified serotype d-specific fraction (lane 2) and the LPS isolated from IDH781 (lane 3). These antisera did not detect any bands in the control fraction (Fig. 5b).
FIG. 5.
Characterization of the rabbit antiserum raised against A. actinomycetemcomitans affinity-purified serotype d-specific antigen. (a) ELISA plates were coated with whole-cell preparations of A. actinomycetemcomitans strains representing serotypes a to e, serial dilutions of antiserum raised against affinity-purified serotype d-specific antigen were added, and the binding of the antiserum was detected with peroxidase-conjugated rabbit IgG. (b) The control fraction (lane 1), A. actinomycetemcomitans affinity-purified serotype d-specific antigen (lane 2), and LPS isolated from A. actinomycetemcomitans serotype d (IDH781) (lane 3) were subjected to SDS-12.5% PAGE, transferred to a nitrocellulose membrane, treated with rabbit antiserum raised against affinity-purified serotype d-specific antigen, and detected with peroxidase-conjugated goat anti-rabbit IgG.
DISCUSSION
In the present study we analyzed the location and composition of A. actinomycetemcomitans affinity-purified serotype d-specific antigen. This study is the first one to use affinity chromatography in order to isolate the serotype-specific antigen of A. actinomycetemcomitans. As a starting material we used supernatant from bacterial cell sonication in order to allow all of the potential antigenic material to bind to the immunoaffinity column. To ascertain that the binding of the antigen to the column was specific, the nonspecific binding to rabbit IgG was first eliminated with the control column. Previous studies reporting isolation of the serotype-specific antigens of this species have utilized gel filtration (1, 35, 36). Immunoaffinity chromatography is especially useful for the purposes of the present study, since the binding is based on biospecific interactions between the antibody and the antigen regardless of the molecular size, charge, or composition of the ligand.
Both the affinity-purified serotype d-specific antigen and the LPS preparations isolated from clinical A. actinomycetemcomitans serotype d strains produced a ladder-like banding pattern in Western blotting as detected by antiserum raised against an A. actinomycetemcomitans serotype d strain (IDH781). This similarity between the affinity-purified serotype d-specific fraction and the LPS preparations of the same serotype suggests similar compositions of these two preparations. Furthermore, the ladder-like banding pattern was not seen when LPSs from IDH781 and the affinity-purified serotype d-specific antigen were immunoblotted with antisera raised against other A. actinomycetemcomitans serotypes (a, b, c, and e). This indicates that the pattern is specific for serotype d. The O antigen is characteristic of and unique for a given LPS and its bacterial origin and serotype, and comparison of different gram-negative bacteria reveals enormous structural variability of the O-specific chain region (15). In any case, the ladder-like banding pattern of the affinity-purified serotype d-specific fraction resembled those typical for the LPS O antigens of other gram-negative bacterial species, such as E. coli (25), which indicates that the serotype-specific part of serotype d contains the O antigen of the LPS. The ladder-like banding pattern of the A. actinomycetemcomitans serotype d strain (IDH781) has been seen previously in cell lysates treated with proteinase K, but those authors did not report the serotype specificity of the preparation (14).
To characterize the specificity of the affinity-purified serotype d-specific antigen, we used displacement ELISA, which is commonly used to enhance the specificity of the ELISA method (16, 17). In order to ensure that all potential antigenic material of the outer membrane of the bacterium was available in the experiment, we used A. actinomycetemcomitans whole cells for coating ELISA plates. The displacement ELISA results suggest that the affinity-purified serotype d-specific antigen resides in the LPS or contains mainly LPS. Both the affinity-purified serotype d-specific antigen and LPS isolated from A. actinomycetemcomitans serotype d (IDH781) blocked the binding of the serotype d antiserum only to the corresponding bacterial cells in the same range. The displacement was specific, since the controls (the control fraction and the LPS isolated from E. coli) did not block the binding of the antiserum raised against A. actinomycetemcomitans serotype d (IDH781) to the corresponding whole cells.
The serotype d specificity of the affinity-purified serotype d-specific fraction was further controlled by repeating the Western blotting and displacement ELISA with the other four serotypes (a, b, c, and e) and antibodies against them. In these experiments we did not include antiserum against serotype f. Kaplan and collaborators recently pointed out that some strains of A. actinomycetemcomitans serotype b and f may share some surface antigens (8). It is most likely that including serotype f strains in our experiments would not have changed the present results. Several researchers have shown that the synthesis of the serotype-specific antigens of serotypes b, c, e, and f is different from the synthesis of the serotype-specific antigens of serotypes a and d (8, 13, 14, 28, 37, 38). The gene clusters responsible for the synthesis of the serotype a- and d-specific antigen are related but are different from the corresponding gene clusters of the other serotypes (14, 28). Additionally, the serotype f strains from Finland and Turkey tested to date have been serologically nonserotypeable with rabbit antiserum against A. actinomycetemcomitans serotype b (strain ATCC 43758) (S. Asikainen, unpublished data).
All of the bands detected in this study were not serotype specific. The Western blot of the affinity-purified serotype d-specific fraction revealed two bands with the sizes of 24 and 55 kDa (Fig. 3) which were detectable by antisera raised against each of the five A. actinomycetemcomitans serotypes (a to e). Of these bands, only the 24-kDa band was present in the control fraction. A. actinomycetemcomitans has outer membrane proteins with various functions (9), and it is possible that the 55-kDa band in our study represents a species-specific epitope common to all serotypes. However, characterization of these bands requires further experiments.
To determine whether the antigen isolated in the present study was able to induce antibody production similar to that induced by the whole-cell antigen, we immunized a rabbit with the affinity-purified serotype d-specific antigen. The specificity of the antibody against our antigen as well as against LPS isolated from IDH781 and the control fraction was characterized. First, the results showed that the affinity-purified serotype d-specific antigen was immunogenic as such. Furthermore, the antibodies produced by the rabbits were specific for A. actinomycetemcomitans serotype d as detected by the ELISA method and Western blotting. Earlier results on A. actinomycetemcomitans serotype b indicated that the serotype-specific polysaccharide antigen is poorly immunogenic without a conjugate (29). The observation that the present affinity-purified serotype d-specific antigen is immunogenic in rabbits without a conjugate suggests that it contains all of the antigenic components necessary to induce an immunogenic reaction already in the first bleeding. However, the avidity of the antibody produced was lower than that of an antibody raised against the whole-cell antigen.
The results from the Western blotting, displacement ELISA, and KDO determination showed that the A. actinomycetemcomitans affinity-purified serotype d-specific antigen contained LPS, that it is immunogenic, and that the antibody produced against affinity-purified serotype d-specific antigen is specific for serotype d whole cells and against LPS isolated from serotype d. As a conclusion from these results, we suggest that the serotype-specific antigen of A. actinomycetemcomitans serotype d contains the O polysaccharide of LPS.
Acknowledgments
This study was supported by grants 45921, 44626, 48725, and 75953 from the Academy of Finland; by the Finnish Dental Society; and by the Finnish Cultural Foundation.
Editor: J. D. Clements
REFERENCES
- 1.Amano, K., T. Nishihara, N. Shibuya, T. Noguchi, and T. Koga. 1989. Immunochemical and structural characterization of a serotype-specific polysaccharide antigen from Actinobacillus actinomycetemcomitans Y4 (serotype b). Infect. Immun. 57:2942-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asikainen, S., C. H. Lai, S. Alaluusua, and J. Slots. 1991. Distribution of Actinobacillus actinomycetemcomitans serotypes in periodontal health and disease. Oral Microbiol. Immunol. 6:15-18. [DOI] [PubMed] [Google Scholar]
- 3.Califano, J. V., H. A. Schenken, and J. G. Tew. 1989. Immunodominant antigen of Actinobacillus actinomycetemcomitans Y4 in high-responder patients. Infect. Immun. 57:1582-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dogan, B., M. H. Saarela, H. Jousimies-Somer, S. Alaluusua, and S. Asikainen. 1999. Actinobacillus actinomycetemcomitans serotype e-biotypes, genetic diversity and distribution in relation to periodontal status. Oral Microbiol. Immunol. 14:98-103. [DOI] [PubMed] [Google Scholar]
- 5.Haubek, D., K. Poulsen, J. Weztergaard, G. Dahlen, and M. Kilian. 1996. Highly toxic clone of Actinobacillus actinomycetemcomitans in geographically widespread cases of juvenile periodontitis in adolescents of African origin. J. Clin. Microbiol. 34:1576-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He, T., J. Hayashi, M. Yamamoto, and I. Ishikawa. 1998. Genotypic characterization of Actinobacillus actinomycetemcomitans isolated from periodontitis patients by arbitrarily primed polymerase chain reaction. J. Periodontol. 69:69-75. [DOI] [PubMed] [Google Scholar]
- 7.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan, J. B., M. B. Perry, L. L. MacLean, D. Fyrgang, M. E. Wilson, and D. H. Fine. 2001. Structural and genetic analyses of O polysaccharide from Actinobacillus actinomycetemcomitans serotype f. Infect. Immun. 69:5375-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komatsuzawa, H., R. Asakawa, T. Kawai, K. Ochiai, T. Fujiwara, M. A. Taubman, M. Ohara, H. Kurihara, and M. Sugai. 2002. Identification of six major outer membrane proteins from Actinobacillus actinomycetemcomitans. Gene 17:195-201. [DOI] [PubMed] [Google Scholar]
- 10.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 11.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 12.Mombelli, A., R. Gmür, R. Frey, J. Meyer, K. Y. Zee, J. O. W. Tam, E. C. M. Lo, J. DiRienzo, N. P. Lang, and E. F. Corbet. 1998. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in young Chinese adults. Oral Microbiol. Immunol. 13:231-237. [DOI] [PubMed] [Google Scholar]
- 13.Nakano, Y., Y. Yoshida, Y. Yamashita, and T. Koga. 1998. A gene cluster for 6-deoxy-l-talan synthesis in Actinobacillus actinomycetemcomitans. Biochim. Biophys. Acta 1442:409-414. [DOI] [PubMed] [Google Scholar]
- 14.Nakano, Y., Y. Yoshida, N. Suzuki, Y. Yamashita, and T. Koga. 2000. A gene cluster for the synthesis of serotype d-specific polysaccharide antigen in Actinobacillus actinomycetemcomitans. Biochim. Biophys. Acta 1493:259-263. [DOI] [PubMed] [Google Scholar]
- 15.Nikaido, H. 1970. Lipopolysaccharide in the taxonomy of gram-negative bacteria. Int. J. Syst. Bacteriol. 20:383-406. [Google Scholar]
- 16.Orosz, F., and J. Ovadi. 2002. A simple method for the determination of dissociation constants by displacement ELISA. J. Immunol. Methods 270:155-162. [DOI] [PubMed] [Google Scholar]
- 17.Özyorük, T., W. P. Cheevers, G. A. Hullinger, T. C. McGuire, M. Hutton, and D. P. Knowles. 2001. Monoclonal antibodies to conformational epitopes of the surface glycoprotein of caprine arthritis-encephalitis virus: potential application to competitive-inhibition enzyme-linked immunosorbent assay for detecting antibodies in goat sera. Clin. Diagn. Lab. Immunol. 8:44-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page, R. C., T. J. Sims, L. D. Engel, B. J. Moncla, B. Bainbridge, J. Stray, and R. P. Darveau. 1991. The immunodominant outer membrane antigen of Actinobacillus actinomycetemcomitans is located in the serotype-specific high-molecular-mass carbohydrate moiety of lipopolysaccharide. Infect. Immun. 59:3451-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paju, S., M. Saarela, S. Alaluusua, P. Fives-Taylor, and S. Asikainen. 1998. Characterization of serologically nontypeable Actinobacillus actinomycetemcomitans isolates. J. Clin. Microbiol. 36:2019-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paju, S., P. Carlson, H. Jousimies-Somer, and S. Asikainen. 2000. Heterogeneity of Actinobacillus actinomycetemcomitans strains in various human infections and relationships between serotype, genotype, and antimicrobial susceptibility. J. Clin. Microbiol. 38:79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paju, S., M. Saarela, C. Chen, H. Jousimies-Somer, V.-J. Uitto, and S. Asikainen. 2000. Altered antigenicity is seen in the lipopolysaccharide profile of non-serotypeable Actinobacillus actinomycetemcomitans strains. FEMS Immunol. Med. Microbiol. 1168:1-7. [DOI] [PubMed] [Google Scholar]
- 22.Pajukanta, R., S. Asikainen, M. Saarela, S. Alaluusua, and H. Jousimies-Somer. 1993. In vitro antimicrobial susceptibility of different serotypes of Actinobacillus actinomycetemcomitans. Scand. J. Dent. Res. 101:299-303. [DOI] [PubMed] [Google Scholar]
- 23.Perry, M. B., L. L. MacLean, R. Gmür, and M. E. Wilson. 1996. Characterization of the O-polysaccharide structure of lipopolysaccharide from Actinobacillus actinomycetemcomitans serotype b. Infect. Immun. 64:1215-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pussinen, P. J., T. Vilkuna-Rautiainen, G. Alfthan, K. Mattila, and S. Asikainen. 2000. Multiserotype enzyme-linked immunosorbent assay as a diagnostic aid for periodontitis in large-scale studies. J. Clin. Microbiol. 40:512-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raetz, C. R. H., and C. Whitfield. 2002. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71:635-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saarela, M., S. Asikainen, S. Alaluusua, L. Pyhälä, C.-H. Lai, and H. Jousimies-Somer. 1992. Frequency and stability of mono- or poly-infection by Actinobacillus actinomycetemcomitans serotypes a, b, c, d or e. Oral Microbiol. Immunol. 7:277-279. [DOI] [PubMed] [Google Scholar]
- 27.Sims, T. J., B. J. Moncla, R. P. Darveau, and R. C. Page. 1991. Antigens of Actinobacillus actinomycetemcomitans recognized by patients with juvenile periodontitis and periodontally normal subjects. Infect. Immun. 59:913-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki, N., Y. Nakano, Y. Yoshida, H. Nakao, Y. Yamahita, and T. Koga. 2000. Genetic analysis of the gene cluster for the synthesis of serotype a-specific polysaccharide antigen in Actinobacillus actinomycetemcomitans. Biochim. Biophys. Acta 15:135-138. [DOI] [PubMed] [Google Scholar]
- 29.Takamatsu-Matsushita, N., N. Yamaguchi, M. Kawasaki, Y. Yamashita, T. Takehara, and T. Koga. 1996. Immunogenicity of Actinobacillus actinomycetemcomitans serotype b-specific polysaccharide-protein conjugate. Oral Microbiol. Immunol. 11:220-225. [DOI] [PubMed] [Google Scholar]
- 30.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of protein from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Winkelhoff, A. J., and J. Slots. 1999. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in nonoral infections. Periodontology 20:122-135. [DOI] [PubMed] [Google Scholar]
- 32.Waravdekar, V. S., and L. D. Saslaw. 1959. A sensitive colorimetric method for the estimation of 2-deoxy sugars with the use of the malonaldehyde-thiobarbituric acid reaction. J. Biol. Chem. 8:1945-1950. [PubMed] [Google Scholar]
- 33.Westphal, O., and K. Jann. 1965. Bacterial lipopolysaccharides. Extraction with phenol-water and further application of the procedure, p. 83-91. In E. R. Whistler (ed.), Methods in carbohydrate chemistry. Academic Press, New York, N.Y.
- 34.Wilson, M. E., and R. E. Schifferle. 1991. Evidence that the serotype b antigenic determinant of Actinobacillus actinomycetemcomitans Y4 resides in the polysaccharide moiety of lipopolysaccharide. Infect. Immun. 59:1544-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaguchi, N., Y. Yamashita, D. Ikeda, and T. Koga. 1996. Actinobacillus actinomycetemcomitans serotype b-specific polysaccharide antigen stimulates production of chemotactic factors and inflammatory cytokines by human monocytes. Infect. Immun. 64:2563-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaguchi, N., M. Kawasaki, Y. Yamashita, K. Nakashima, and T. Koga. 1995. Role of the capsular polysaccharide-like serotype-specific antigen in resistance of Actinobacillus actinomycetemcomitans to phagocytosis by human polymorphonuclear leukocytes. Infect. Immun. 63:4589-4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida, Y., Y. Nakano, Y. Yamashita, and T. Koga. 1998. Identification of a genetic locus essential for serotype b-specific antigen synthesis in Actinobacillus actinomycetemcomitans. Infect. Immun. 66:107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida, Y., Y. Nakano, N. Suzuki, H. Nakao, Y. Yamashita, and T. Koga. 1999. Genetic analysis of the gene cluster responsible for synthesis of serotype e-specific polysaccharide antigen in Actinobacillus actinomycetemcomitans. Biochim. Biophys. Acta 23:457-461. [DOI] [PubMed] [Google Scholar]
- 39.Zambon, J. J., J. Slots, and R. J. Genco. 1983. Serology of oral Actinobacillus actinomycetemcomitans and serotype distribution in human periodontal disease. Infect. Immun. 41:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]