Abstract
The gene for the nicotinamide riboside (NR) transporter (pnuC) was identified in Haemophilus influenzae. A pnuC mutant had only residual NR uptake and could survive in vitro with high concentrations of NR, but could not survive in vivo. PnuC may represent a target for the development of inhibitors for preventing H. influenzae disease.
Haemophilus influenzae does not have the enzymes necessary for the de novo synthesis of NAD+ (5, 10) and therefore has an absolute requirement for an exogenous source of factor V (6). Most of the factor V uptake pathway in H. influenzae has been characterized (11, 14, 16). The organism in vivo can utilize NAD (NAD+), nicotinamide (NAm) mononucleotide (NMN), and NAm riboside (NR) as factor V sources, but not the precursor of these, NAm (7). The e(P4) outer membrane protein and the NadN periplasmic enzyme convert NAD+ to NMN and NR (11, 14), and only NR is able to cross the inner membrane to the cytoplasm (5, 11), where NadR recycles it back to NAD+ (13, 17). Through BLAST analysis, we identified the hypothetical gene HI1077.1 as a paralog of the E. coli pnuC gene (NT01EC0901), an NMN transporter in E. coli but a putative NR transporter in H. influenzae. PCR amplification and resequencing of the HI1077.1 gene region (coordinates 1144355 to 1145045) identified two errors with respect to the original genome annotation and restored a complete open reading frame. We reannotated HI1077.1 as pnuC, defined by coordinates 1144355 to 1145035, with nucleotides G and A missing at positions 1144511 and 1144972, respectively. The H. influenzae pnuC gene encodes a 226-amino-acid protein with 81.1% similarity to the Pasteurella multocida putative PnuC protein (PM1838).
H. influenzae pnuC was disrupted. Two DNA fragments flanking the gene were PCR amplified with primers pnuc-F1 (GGTTCTGCAATAAGTGCG), pnuc-R1 (CAAGGATCCATGATTTTGCCGTTATCG), pnuC-F2 (CTTGGATCCTGCTAACCAAGAATCAGG) (underlining indicates restriction enzyme sites used in subsequent cloning of the PCR products), and pnuC-R2 (AGATCCTGAATTGGTGGG); BamHI digested; ligated together with T4 DNA ligase; amplified by PCR for 15 cycles with primers pnuC-F1 and pnuC-R2; and cloned into the pCR4-TOPO cloning vector (Invitrogen). The resulting plasmid was digested with BamHI, dephosphorylated with shrimp alkaline phosphatase, and ligated with the BamHI-cut kanamycin resistance gene of pUC4k (11, 19). The construct was excised from this plasmid with EcoRI and transformed (9) into H. influenzae strain Rd-b+ (21). A pnuC mutant was isolated on brain heart infusion (BHI) agar containing Levinthals medium (90 μM NAD+).
To complement the pnuC mutant, the H. influenzae pnuC gene and its promoter region, including a partial Shine-Dalgarno site (genome coordinates 1144090 to1145083), were amplified by PCR with primers pnuC-E and pnuC-KB (sequences AAAGATATCCAATGCGAAAATGGTCACCTC and AAAGGTACCGGATCCCCTTGGTTTGTCGCTTGTCA, respectively). The pnuC gene was cloned as an EcoRV-BamHI fragmentinto plasmid pACYC184 (15), and the construct, designated “pSEpnuC,” was transformed into the pnuC mutant. Growth of the pnuC mutant was compared with that of Rd-b+ on BHI medium supplemented with various NR concentrations. In the presence of 0.05 μM NR, the pnuC mutant had reduced growth compared with Rd-b+, but with 0.5 μM NR, it had growth similar to that of Rd-b+ (Fig. 1). That a pnuC mutant could be created and was viable in vitro indicated the possibility of alternative routes by which NR gains access to the cytoplasm, albeit only in the presence of elevated NR concentrations. The pnuC mutant complemented with pSEpnuC had growth similar to that of Rd-b+, even in the presence of low NR concentrations.
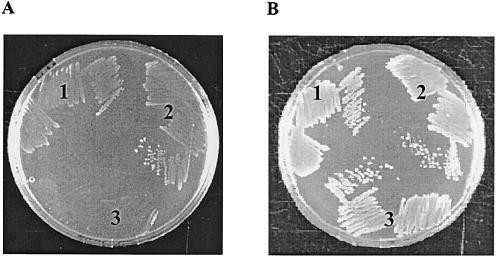
FIG. 1.
Growth analysis. Shown is an overnight culture of the following strains on BHI agar plates: 1, Rd-b+; 2, the pnuC mutant complemented with pSEpnuC; 3, the pnuC mutant. (A) BHI agar supplemented with 0.05 μM NR. (B) BHI agar supplemented with 0.5 μM NR.
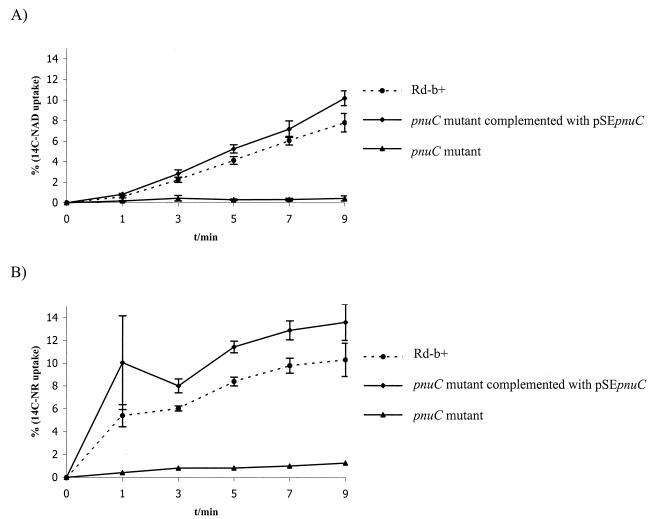
The uptake of [14C]NAD+ and [14C]NR was determined in Rd-b+, in the pnuC mutant, and in the pnuC mutant complemented with pSEpnuC. The uptake procedure has been reported previously (11). In brief, cells were cultured to an optical density at 490 nm (OD490) of 1, washed, and resuspended in BHI medium to an OD of 2. Samples were incubated with [14C]NAD+ or [14C]NR (1 μM each) (Amersham Pharmacia, Freiburg, Germany), and aliquots were removed at time intervals. Samples were then filtered and washed with an excess volume of NaCl (10 ml [0.1 M]). The 14C uptake was measured in an SL 6000SC scintillation counter (Beckman, Munich, Germany). For both [14C]NAD+ and [14C]NR, the pnuC mutant showed a marked decrease in label accumulation compared with that in Rd-b+, an effect that was reversed in the pnuC mutant complemented with pSEpnuC (Fig. 2). A small increase in label accumulation (up to 1%) could be observed in the pnuC mutant over the range of 0 to 9 min, indicating residual uptake ability. Uptake of label derived from [14C]NAD+ was delayed compared with the uptake of [14C]NR, reflecting the dynamics of NAD+ transfer and processing across the outer membrane (1) and degradation to NR (11). Other, possibly low-affinity, NR uptake systems presumably coexist, which may explain the growth of the mutant at high NR or NAD+ concentrations.
FIG. 2.
[14C]NR and [NAD+] uptake by H. influenzae. Transport kinetics for 1 μM [14C]NAD+ (A) or [14C]NR (B) in H. influenzae strains Rd-b+, the pnuC mutant complemented with pSEpnuC, and the pnuC mutant. All experiments were performed in triplicate. Bars represent the standard deviation.
To ascertain that the PnuC transporter is required for H. influenzae to cause disease in humans, the ability of the mutant to survive in the 5-day old infant rat model was ascertained by competitive index (CI) assay (8). Rats were inoculated with a dual infection of 105 CFU of Rd-b+ in combination with either the pnuC mutant or the pnuC mutant complemented with pSEpnuC. Rd-b+ established a bacteremia of ∼2 × 106 CFU/ml in each infant rat (n = 4), whereas the pnuC mutant did not survive at all (CI < 0.001). The pnuC mutant complemented with pSEpnuC was partially virulent and produced a bacteremia of ∼2 × 105 CFU/ml (CI = 0.1), indicating that the complemented plasmid-borne pnuC partly corrected the deficit produced by disrupting the chromosomal copy of pnuC and supporting our contention that the phenotype of the mutant was due to the pnuC disruption. Similar results were obtained with the standard infant rat bacteremia model (18; data not shown). The residual uptake ability of the pnuC mutant is insufficient to permit survival in vivo, implying that pnuC is a potential target for the development of inhibitors that prevent H. influenzae disease.
The members of the family Pasteurellaceae can be classified into two subgroups (12): the NR-dependent Pasteurellaceae, including H. influenzae, Haemophilus parainfluenzae, Haemophilus parasuis, and Actinobacillus pleuropneumoniae; and the NR-independent, or NAm-utilizing, Pasteurellaceae, including Pasteurella multocida, Mannheimia haemolytica, Haemophilus haemoglobulinophilus, and Actinobacillus actinomycetemcomitans. This division depends on whether there is a second mechanism for generating NAD+, other than NR scavenging. A few NR-independent Haemophilus species also exist—for instance, Haemophilus paragallinarum, H. parainfluenzae, and Haemophilus ducreyi—that have been shown to harbor nadV (encoding NAm phosphoribosyltransferase) on a plasmid (3, 20). These species can synthesize NMN from NAm and can thus utilize exogenous NAm, which freely diffuses across the membrane. Acquisition of a nadV-harboring plasmid could thus transform H. influenzae into an NR-independent species. To our knowledge, there are no reports of H. influenzae isolates containing nadV, but the potential for this phenomenon could undermine a strategy for targeted PnuC inhibition. To examine whether H. influenzae could utilize NAm if the uptake of NR were impeded, we complemented the pnuC mutant with nadV and determined its survival in vivo. A 2-kb nadV-containing DNA fragment was PCR amplified from a plasmid preparation derived from H. ducreyi strain ATCC 27722 (4, 12) by using primers nadV5EcoRV (TAGATATCAGACTTATGTCTCGGAGTATAACG) and nadV3EcoRV (TTGATATCTCATAGCGTAGTGCGACTAAC). The product was digested with EcoRV and ligated into EcoRV-cut pACYC184 (15) to yield pSEnadV. An EcoRV fragment of pSEnadV was subcloned into a SwaI restriction site of plasmid pSEhel (14), to create pSEhelnadV. The SwaI site is located 7 bp downstream of the stop codon of the hel gene (HI0693) so that in pSEhelnadV, nadV is subcloned immediately adjacent to hel and is flanked by H. influenzae DNA preceding HI0694. A hel-nadV DNA fragment was amplified from pSEhelnadV with primers hel5′PstI (AAAACTGCAGCAGAAAGACTTACTATACCCTG) and helEcoRV3′ (TCGATATCACAAATGCGCTATTCTGACGG), and this was transformed into the pnuC mutant. Transformants were selected on BHI agar containing only NAm as the factor V source. The pnuC mutant complemented with chromosomally integrated nadV+ exhibited NR independence when grown on MIc minimal medium (2) (Fig. 3) and was confirmed to have a chromosomal copy of nadV (data not shown). We compared the virulence of Rd-b+, the pnuC mutant, and the pnuC mutant complemented with nadV by competitive index (CI) experiments in infant rats. The pnuC mutant complemented with nadV was as virulent as Rd-b+ (CI = 1), indicating that acquisition of nadV permits H. influenzae to utilize NAm from host sources during invasive disease.
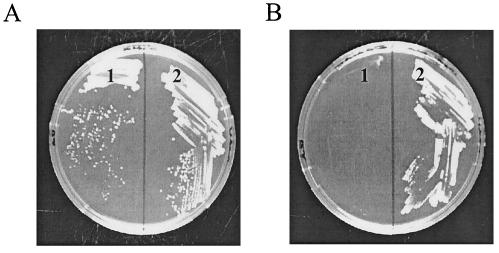
FIG. 3.
nadV+ growth phenotype of H. influenzae. H. influenzae mutants were grown for 2 days on a MIc-minimal medium agarose plate supplemented with 0.5 μM NR (A) or 60 μM NAm (B). Section 1, pnuC mutant; section 2, pnuC mutant complemented with nadV+.
H. influenzae is defined by its requirement for exogenous NAD+ or, in absolute terms, its requirement for NR (8, 12, 14). We have demonstrated an NR transport role for PnuC and have supported this by in vitro and in vivo growth analyses and factor V uptake studies. PnuC is therefore a potential target for development of novel methods to prevent disease caused by H. influenzae. Acquisition of nadV could potentially allow H. influenzae to escape the therapeutic effect of a PnuC inhibitor, but, reassuringly, natural nadV acquisition has not been described in H. influenzae.
Acknowledgments
J.R. was supported by the “Deutsche Forschungsgemeinschaft” grant RE1561/1-1 and “Nachwuchsgruppenförderprogramm, des Landes Bayern.” The research was also supported by Oxfordshire Health Services Research grant G72093.
M.H and E.S. contributed equally to this work.
Editor: J. T. Barbieri
REFERENCES
- 1.Andersen, C. M., E. Kemmer, G. Blass, J. Hilpert, A. K. Benz, and J. R. Reidl. 2003. Porin OmpP2 of Haemophilus influenzae shows substrate specificity towards nicotinamide-derived nucleotide substrates. J. Biol. Chem. 278:24269-24276. [DOI] [PubMed] [Google Scholar]
- 2.Barcak, G. J., M. S. Chandler, R. J. Redfield, and J. F. Tomb. 1991. Genetic systems in Haemophilus influenzae. Methods Enzymol. 204:321-342. [DOI] [PubMed] [Google Scholar]
- 3.Bragg, R. R., L. Coetzee, and J. A. Verschoor. 1993. Plasmid-encoded NAD independence in some South African isolates of Haemophilus paragallinarum. J. Vet. Res. 60:147-152. [PubMed] [Google Scholar]
- 4.Brentjens, R. J., M. Ketterer, M. A. Apicella, and S. M. Spinola. 1996. Fine tangled pili expressed by Haemophilus ducreyi are a novel class of pili. J. Bacteriol. 178:808-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cynamon, M. H., T. B. Sorg, and A. Patapow. 1988. Utilization and metabolism of NAD by Haemophilus parainfluenzae. J. Gen. Microbiol. 134:2789-2799. [DOI] [PubMed] [Google Scholar]
- 6.Evans, N. M., D. D. Smith, and A. J. Wicken. 1974. Hemin and nicotinamide adenine dinucleotide requirements of Haemophilus influenzae. J. Med. Microbiol. 7:359-365. [DOI] [PubMed] [Google Scholar]
- 7.Godek, C. P., and M. H. Cynamon. 1990. In vitro evaluation of nicotinamide riboside analogs against Haemophilus influenzae. Antimicrob. Agents Chemother. 34:1473-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbert, M. A., S. Hayes, M. E. Deadman, C. M. Tang, D. W. Hood, and E. R. Moxon. 2002. Signature tagged mutagenesis of Haemophilus influenzae. Microb. Pathog. 33:1-13. [DOI] [PubMed] [Google Scholar]
- 9.Herriot, R. M., E. M. Meyer, and M. Vogt. 1979. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J. Bacteriol. 101:517-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn, D. W., and B. M. Anderson. 1986. Characterization of Haemophilus influenzae nucleotide pyrophosphatase. J. Biol. Chem. 261:6016-6025. [PubMed] [Google Scholar]
- 11.Kemmer, G., T. Reilly, J. Schmidt-Brauns, G. W. Zlotnik, B. A. Green, M. J. Fiske, M. Herbert, A. Kraiβ, S. Schlör, A. Smith, and J. Reidl. 2001. NadN and e (P4) are essential for the utilization of NAD and nicotinamide mononucleotide but not nicotinamide riboside in Haemophilus influenzae. J. Bacteriol. 183:3974-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin, P. R., R. J. Shea, and M. H. Mulks. 2001. Identification of a plasmid-encoded gene from Haemophilus ducreyi which confers NAD independence. J. Bacteriol. 183:1168-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mushegian, A. 1999. The purloined letter: bacterial orthologs of archael NMN adenylyltransferase are domains within multifunctional transcriptional regulator NadR. J. Mol. Microbiol. Biotechnol. 1:127-128. [PubMed] [Google Scholar]
- 14.Reidl, J., S. Schlör, A. Kraiss, J. Schmidt-Brauns, G. Kemmer, and E. Soleva. 2000. NADP and NAD utilization in Haemophilus influenzae. Mol. Microbiol. 35:1573-1581. [DOI] [PubMed] [Google Scholar]
- 15.Rose, R. E. 1988. The nucleotide sequence of pACYC184.Nucleic Acids Res. 16:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt-Brauns, J., M. Herbert, G. Kemmer, A. Kraiβ, S. Schlör, and J. Reidl. 2001. Is a NAD-pyrophosphatase activity needed by Haemophilus influenzae type b for multiplication in the bloodstream? Int. J. Med. Microbiol. 291:219-225. [DOI] [PubMed] [Google Scholar]
- 17.Singh, S. K., O. V. Kurnasov, B. Chen, H. Robinson, N. V. Grishin, A. L. Osterman, and H. Zhang. 2002. Crystal structure of Haemophilus influenzae NadR protein: a bifunctional enzyme endowed with NMN adenylyltransferase and ribosylnicotinamide kinase activities. J. Biol. Chem. 277:33291-33299. [DOI] [PubMed] [Google Scholar]
- 18.Smith, A. L., D. H. Smith, D. R. Averill, Jr., J. Marino, and E. R. Moxon. 1973. Production of Haemophilus influenzae b meningitis in infant rats by intraperitoneal inoculation. Infect. Immun. 8:278-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor, L. A., and R. E. Rose. 1988. A correction in the nucleotide sequence of the Tn903 kanamycin resistance determinant in pUC4K.Nucleic Acids Res. 16:7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Windsor, H. M., R. C. Gromkova, and H. J. Koornhof. 1991. Plasmid-mediated NAD independence in Haemophilus parainfluenzae. J. Gen. Microbiol. 137:2415-2421. [DOI] [PubMed] [Google Scholar]
- 21.Zwahlen, A., L. G. Rubin, and E. R. Moxon. 1986. Contribution of lipopolysaccharide to pathogenicity of Haemophilus influenzae: comparative virulence of genetically related strains in rats. Microb. Pathog. 1:465-473. [DOI] [PubMed] [Google Scholar]