Abstract
Cryptococcus neoformans is a human-pathogenic fungus that has evolved into three distinct varieties that infect most prominently the central nervous system. A sexual cycle involving haploid cells of a and α mating types has been reported for two varieties (C. neoformans var. neoformans, serotype D, and C. neoformans var. gattii, serotypes B and C), yet the vast majority of infections involve a distinct variety (C. neoformans var. grubii, serotype A) that has been thought to be clonal and restricted to the α mating type. We recently identified the first serotype A isolate of the a mating type which had been thought to be extinct (strain 125.91). Here we report that this unusual strain can mate with a subset of pathogenic serotype A strains to produce a filamentous dikaryon with fused clamp connections, basidia, and viable recombinant basidiospores. One meiotic segregant mated poorly with the serotype A reference strain H99 but robustly with a crg1 mutant that lacks a regulator of G protein signaling and is hyperresponsive to mating pheromone. This meiotic segregant was used to create congenic a and α mating type serotype A strains. Virulence tests with rabbit and murine models of cryptococcal meningitis showed that the serotype A congenic a and α mating type strains had equivalent virulence in animal models, in contrast to previous studies linking the α mating type to increased virulence in congenic serotype D strains. Our studies highlight a role for sexual recombination in the evolution of a human fungal pathogen and provide a robust genetic platform to establish the molecular determinants of virulence.
Sexual reproduction involves the mixing of genetic material from two parents to form progeny that contain genes from both parents (4). While sex is the most prevalent mode of reproduction in eukaryotes, there is much debate as to why sexual reproduction predominates (5, 49, 61). Two hypotheses have been proposed. In the first, sex provides variation for natural selection to act upon and promotes adaptation by allowing beneficial mutations to spread and escape deleterious mutations in other genes (6, 37). A second model suggests that sexual reproduction facilitates DNA repair or dilutes deleterious mutations (7, 65). This model is consistent with the idea that organisms with low levels of detrimental DNA damage would not necessarily require sexual reproduction (3, 21, 58).
A few organisms, such as the bdelloid rotifers, are exclusively asexual (3, 7, 21, 58). Asexual reproduction involves copying of a parent to produce genetically identical progeny (65). While exclusively asexual organisms are thought to be rare, organisms with facultative parthenogenesis, the ability to produce offspring sexually or asexually, are more common. Many insect species, such as aphids and cockroaches, as well as nematodes have the ability to produce either females asexually or males and females sexually (2, 11, 43). Many lower eukaryotes reproduce asexually; in the cases of yeasts and filamentous fungi, 20% or more of species have no defined sexual cycle (19).
The role of the sexual cycle in human-pathogenic fungi is unclear. The yeast Candida albicans is the most common cause of human fungal infections yet has long been thought to be an obligate asexual diploid (8). The recent discovery of the mating type (MAT) locus of C. albicans led to the identification of mating and the characterization of a unique cell type specialized for mating (22, 23, 25, 38, 40). While mating has been identified in C. albicans, meiosis and ploidy reduction to complete the sexual cycle have not yet been detected, and population genetic studies reveal a largely clonal population (53, 64). Hence, when and where C. albicans employs its sexual cycle and the role the sexual cycle may play in virulence remain elusive.
Cryptococcus neoformans is an opportunistic human fungal pathogen that infects the central nervous system and causes meningoencephalitis (9, 24). C. neoformans occurs in three varieties: C. neoformans var. grubii (serotype A), C. neoformans var. gattii (serotypes B and C), and C. neoformans var. neoformans (serotype D). Although these varieties can be interfertile (29, 31, 46, 47), intervarietal mating results in hybrid strains (serotype AD) that produce largely inviable spores (34). C. neoformans occurs in the environment associated with pigeon guano and certain tree species (9). Humans are thought to be exposed by inhalation of basidiospores, which are small enough to lodge in the alveoli of the lung (52). Spores are produced via sexual reproduction, haploid fruiting, or diploid filamentation (27, 28, 48, 59). Importantly, virulence has been linked to mating type. Most clinical isolates are of the α mating type, and α strains have been reported to be more virulent than congenic a strains in serotype D (9, 30).
A sexual cycle has been reported for C. neoformans var. gattii and C. neoformans var. neoformans and involves fusion of a and α cells to produce heterokaryotic filaments (27, 28). These filaments ultimately produce basidia, where nuclear fusion and meiosis occur, and long chains of infectious spores are produced. However, mating has only recently been characterized for C. neoformans var. grubii serotype A strains, which are the predominant clinical isolates. Population studies of this variety indicate that it is predominantly clonal, and only strains of the α mating type had been identified (15, 32). Recently, however, two a mating type strains (125.91 and IUM 96-2828) were identified from a worldwide screen of ∼1,500 serotype A strains (36, 41, 55). While strain 125.91 was unable to mate with the serotype A reference strain H99, strain IUM96-2828 could mate with H99 to produce viable recombinant progeny (26). Thus, a sexual cycle exists for the most common clinical isolates of C. neoformans. However, the role that this sexual cycle plays in the population structure and virulence of C. neoformans var. grubii remains unclear.
Here we present additional evidence that strain 125.91 is a bona fide haploid a mating type serotype A strain and show that it is capable of undergoing a sexual cycle with a subset of C. neoformans var. grubii α mating type strains. We constructed congenic serotype A strains and used these strains to test whether mating type is linked to virulence in this variety. These congenic serotype A strains will allow characterization of the role that the sexual cycle plays in pathogenicity and will enable genetic and molecular analyses of the most prevalent variety of this ubiquitous human pathogen.
MATERIALS AND METHODS
Strains.
Reference strains used in this study were the serotype A strain H99 (mating type α, serotype A [αA]) (54) and the congenic serotype D strains JEC20 (aD) and JEC21 (αD) (20, 30). The isolates 125.91, 8-1, S25J, 4476B, ZG287, ZG290, and KW5 were from the Duke University strain collection (34, 36). Strains CBS132 and ATCC 48184 were from the American Type Culture Collection, and strains CDC94-383, CDC92-74, CDC228, and CDC304 were from the Centers for Disease Control and Prevention (CDC) (34). The H99 crg1 mutant was derived from strain H99 by gene disruption. A 1.3-kb CRG1 coding domain fragment was generated by PCR with primers 5′-GCAACCAATCACTATGAA and 5′-TGTGATCTGGTTCATGTA. PCR was performed for 30 cycles of 94°C for 40 s, 40°C for 1 min, and 72°C for 1 min. PCR products were gel excised, cloned, and verified by sequencing. A two-step PCR amplification procedure (13) was used to generate the crg1::URA5 disruption allele used to transform strain F99 (H99 ura5). Biolistic transformation and mutant isolation were as described previously (54, 57).
Media.
Strains were grown on yeast extract-peptone-dextrose (YPD) medium. Mating assays were on V8 medium (29) (5% [vol/vol] V8 juice, 3 mM KH2PO4, 4% [wt/vol] agar) at pH 5 or 7. Mating assays with strain 125.91 used V8 medium at pH 7, V8 medium at pH 5, carrot medium (5% [vol/vol] carrot juice, 3 mM KH2PO4, 4% agar), or SLAD medium (1). Confrontation assays were on SLAD medium.
FACS analysis.
Fluorescence-activated cell sorter (FACS) analysis was performed as described previously (34). Approximately 50 μl of vegetative cells was washed with normal saline (0.9%), fixed in 70% ethanol, washed with 10 mM Tris-HCl (pH 7.2)-0.25 M sucrose-1 mM EDTA-1 mM MgCl2-0.1 mM CaCl2-0.1 mM ZnCl2, and incubated with 10 μg of propidium iodide per ml and 1 mg of RNase A per ml. Cells were diluted to ∼108 cells/ml, sonicated for 10 s, and subjected to FACS analysis. More than 10,000 cells of each strain were analyzed for DNA content, determined by relative fluorescence of stained genomic DNA.
NCP1a gene analysis.
The NCP1a gene was amplified by touchdown PCR (44) with primers 5′-TTGAGCGTCATATTGGTCATGAC and 5′-GAAGACCGTCATCACACACAAG. PCR mixtures contained 50 to 80 ng of genomic DNA and 50 pmol of each primer. PCR was performed for 20 cycles of 94°C for 30 s, 60°C with a temperature increment of −1°C at every cycle for 1 min, and 72°C for 45 s. After 20 cycles, an additional 20 cycles were performed at an annealing temperature of 40°C. The ACT1 gene was amplified with primers 5′-ATGGAAGAAGAAGGTACG and 5′-TTAGAAACACTTTCGGTG. PCR mixtures contained 50 ng of genomic DNA and 50 pmol of each primer, and PCR was performed for 30 cycles of 94°C for 15 s, 55°C for 30 s, and 72°C for 45 s.
Southern blot analysis.
Genomic DNA was prepared by using the Camgen (Cambridge, United Kingdom) yeast genomic DNA extraction kit. DNA (10 μg) was digested to completion with PstI (New England Biolabs) and fractionated by electrophoresis in a 0.8% agarose gel. The gel was ethidium bromide stained to verify equal DNA loading and digestion, transferred to a nylon membrane (Hybond-N+; Amersham), and fixed by UV cross-linking. The gel was also ethidium bromide stained after transfer to verify complete DNA transfer to the membrane. The membrane was hybridized overnight with a gel-purified DNA probe labeled with 32P by random priming (Amersham) and then washed, dried, and exposed to film for 24 hr. The transposon T1 and T2 probes were generated by PCR with genomic DNA (αD strain JEC21) and primers 5′-CACTGTGGGAAAGGGTCGCC and 5′-CACTGTGGGAATTCCTCGCC for T1 and 5′-GGGGTACAAAATGCA for T2. The T1 and T2 primers were also used to PCR amplify these transposons from serotype A and D strains, with PCR performed for 30 cycles of 94°C for 15 s, 55°C for 30 s, and 72°C for 45 s.
Serotyping.
PCR-based genotyping for the identification of serotype was as described previously (34, 36). PCR was performed for 30 cycles of 94°C for 15 s, 60°C (for PAK1, GPA1, and CNA1) or 66°C (for STE20) for 30 s, and 72°C for 30 s. PCR products were cloned and sequenced. Classic serotyping was done with the Crypto Check serotyping kit from Iatron Laboratories (Tokyo, Japan) as described previously (34).
Mating type analysis.
Mating to reference strains was used to determine mating type. Strains were pregrown on YPD agar for 2 days, and a small amount of cells was removed and patched onto V8 solid medium (pH 5.0) either alone or mixed with a reference strain. Plates were incubated at room temperature in the dark and assessed by light microscopy for filament and basidiospore formation. PCR-based mating type determination was as described previously (34, 36), using primers to the a or α allele of the STE20 gene for serotypes A and D.
Cell wall and nuclear staining.
Strains were grown on V8 medium (pH 5) as described above for mating reactions. Areas with filamentation were excised, washed with deionized water, and stained with 0.1% calcofluor white (Difco) or 1 μg of DAPI (4′,6′-diamidino-2-phenylindole-2HCl) (Sigma) per ml. Calcofluor white-stained filaments were analyzed for fused clamps. DAPI-stained cells were analyzed to establish whether cells were uni- or binucleate.
Confrontation assays.
Two strains were streaked onto SLAD medium in parallel lines 5 mm apart without touching. Conjugation tube formation by α cells and swelling of a cells were assessed by light microscopy after 24, 48, and 72 h at room temperature.
Single-basidiospore isolation.
To isolate basidiospores, strains were grown on V8 medium (pH 5) as described above for mating reactions. Areas showing basidiospore formation upon microscopic examination were excised, and spores were transferred to a fresh YPD plate and dissected by micromanipulation.
RFLP analysis.
PAK1 and GPA1 gene sequences from strain KNA14 were PCR amplified, cloned, and sequenced as described above. The 125.91, 8-1, KNA14, and H99 sequences were compared to identify restriction fragment length polymorphisms (RFLPs). The restriction enzyme PvuI (CGATCG) (New England Biolabs) was used to characterize the GPA1 gene, and the restriction enzyme BstXI (CCANNNNNNTGG) (New England Biolabs) was used to characterize the PAK1 gene. Briefly, gene fragments were PCR amplified as described above, and the DNA was incubated with 1 to 1.25 U of the appropriate restriction enzyme and buffer for 4 h. The PvuI digests were at 37°C, and the BstXI digests were at 55°C.
PFGE.
Cells were grown in yeast nitrogen base minimal medium supplemented with 1 M NaCl at 30°C with shaking at 225 rpm to an optical density at 600 nm of 0.5 to 0.6. Cells were harvested and plugs were prepared as described previously (35). Samples were run in a 1% pulsed-field gel electrophoresis (PFGE)-grade agarose (Bio-Rad) gel with 0.5× Tris-borate-EDTA running buffer in a Bio-Rad contour-clamped homogeneous electric field DRII apparatus with the following settings: initial A time, 75 s; final A time, 150 s; start ratio, 1.0; run time, 30 h; mode, 10; initial B time, 200 s; final B time, 400 s; start ratio, 1.0; run time, 60 h; mode, 11. The voltage was set to 125 V and the buffer temperature was set to 12°C. The running buffer was changed every 24 h.
Congenic strain construction.
Strain 125.91 (aA) was crossed with strain 8-1 (αA), and single basidiospores were isolated. One of the a progeny from the mating, KNA14, was crossed with strain H99 crg1 (αA), and single basidiospores of a and α strains were isolated. An a offspring from this mating, KNB-5, was crossed to H99 to yield first-generation progeny. The process of isolating a single-basidiospore cultures and backcrossing to H99 was repeated a total of 10 times to produce KN99a, an a strain that is congenic with the α strain H99. Strain KN99-5a is the product of the fifth backcross.
Virulence studies.
Virulence studies were performed with established rabbit and murine cryptococcal meningitis models (1, 39, 56). Groups of 4- to 6-week-old female A/Jcr mice (10 mice per strain) were anesthetized by phenobarbital injection and infected with 1 × 105, 5 × 104, or 1 × 103 fungal cells in 50 μl of normal saline pipetted into the nostrils. Mice were monitored twice daily, and those in pain or sick were sacrificed by CO2 inhalation. The percentage of surviving mice was plotted against time, and P values were calculated by the Mann-Whitney test. Male New Zealand White rabbits weighing 2.5 to 3 kg (three per group) were administered cortisone acetate at 5 mg/kg intramuscularly 1 day prior to inoculation and then daily for the entire test period. One day after the initial steroid treatment, the rabbits were anesthetized with xylazine and ketamine intramuscularly and inoculated intracisternally with 108 cells in 0.3 ml of normal saline. Rabbits were anesthetized on days 4 and 7 following infection, and 0.5 ml of cerebrospinal fluid (CSF) was withdrawn, serially diluted, and plated onto YPD medium. The colonies were counted, and the mean number of CFU was plotted, with the standard error of the mean indicated. A standard Student t test was performed to establish P values for the differences between groups.
Nucleotide sequence accession numbers.
The sequences from strains 125.91 and 8-1 have been deposited in GenBank under accession numbers AY219893 to -96.
RESULTS
Strain 125.91 is a serotype A MATa haploid strain.
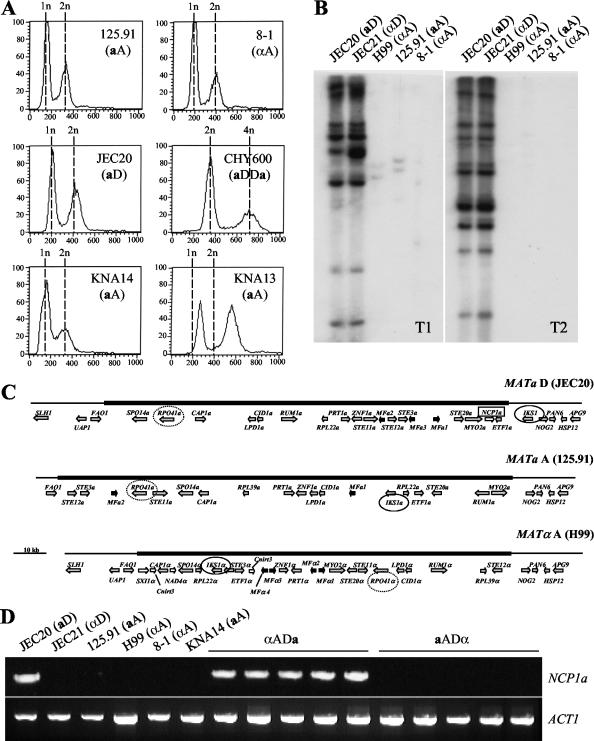
We recently identified the first a mating type isolate of the pathogenic serotype A C. neoformans var. grubii (strain 125.91) (36). Identification of this strain as serotype A and a mating type was based on serotyping, PCR-based genotyping for serotype, and the sequence of the MATa-encoded STE20a gene. Because strain 125.91 would not mate with the serotype A reference strain H99, we established by additional stringent criteria that strain 125.91 is a bona fide haploid a mating type serotype A (aA) strain and not an unusual aneuploid or hybrid isolate. First, by FACS analysis strain 125.91 is haploid (1n/2n) and not aneuploid or diploid (2n/4n) (Fig. 1A). Second, by Southern blotting we confirmed that strain 125.91 lacks two transposable elements (T1 and T2) that are common in serotype D strains and absent or rare in serotype A strains (Fig. 1B) (12, 36). PCR with primers specific to the T1 and T2 transposons also showed that only the serotype D strains produced PCR products of the appropriate size; this is consistent with an analysis of the 2× genomic sequence coverage of the serotype A reference strain (H99), which shows no T1 or T2 transposons (not shown). Third, by phylogenetic analysis based on amplified fragment length polymorphism analysis, strain 125.91 is evolutionarily more related to serotype A strains than to serotype D or AD hybrid strains (T. Boekhout, personal communication). Fourth, mitochondrial DNA sequences of strain 125.91 were characteristic of serotype A and not serotype D strains (F. Dietrich, personal communication). Finally, in contrast to serotype AD strains, which typically contain both serotype A- and D-specific gene alleles at multiple loci (34), strain 125.91 was previously found to contain only serotype A alleles by PCR-based genotyping (36). To verify this PCR analysis, the PAK1, GPA1, and CNA1 genes from strain 125.91 were sequenced and found to be more similar to serotype A gene sequences (96 to 99%) than to serotype D gene sequences (91 to 94%). In summary, strain 125.91 is a haploid serotype A strain.
FIG. 1.
Identification of a and α haploid serotype A C. neoformans strains. (A) FACS analysis of the aA strains 125.91, KNA13, and KNA14; the αA strain 8-1; and the control aD haploid strain JEC20 and aDDa diploid strain CHY600. (B) Southern blots of 125.91 (aA), 8-1 (αA), H99 (αA), JEC20 (aD), and JEC21 (αD) PstI-digested genomic DNAs probed with transposon T1 or transposon T2. (C) Structures of the MAT locus alleles from the aD strain JEC20, the aA strain 125.91, and the αA strain H99. The mating type-specific regions are shown as thick lines, and the flanking regions are shown as thin lines. Genes are represented as arrows in the direction of transcription. Genes encoding pheromones are shown as black arrows, locus-specific genes are shown as white arrows, and all other genes are in grey. The IKS1 genes are circled with a black line, the RPO41 genes are circled with a dotted line, and the NCP1a gene is boxed. (D) PCR assays were conducted with primers specific to NCP1a. Genomic DNAs from serotype D, serotype A, and AD hybrid strains were assayed. aADα hybrid strains were ZG290, ATCC 48184, CDC92-74 (ADα), CDC228, and CDC304. αADa hybrid strains were ZG287, MMRL774, KW5, CBS132, and CDC94-383. PCR with primers specific to the actin gene served as a positive control for the presence of genomic DNA.
In a related study by Lengeler et al. (35) the MAT loci of strain 125.91 and reference serotype A and D strains were sequenced. Comparison of these MAT loci supports the conclusion that strain 125.91 is a serotype A, a mating type strain (Fig. 1C). The MAT locus of strain 125.91 contains only mating factor a genes and no mating factor α pheromone genes. Sequence homology was highest between strains 125.91 and JEC20 (aD) for most genes in the MAT locus, with the notable exception of the serotype A RPO41a gene, which was more similar to the serotype A RPO41α allele of strain H99. The IKS1 gene flanks the MAT locus in serotype D but is part of the MAT locus in both serotype A strains (H99 and 125.91). Importantly, the NCP1a gene was present only in the MAT locus of the serotype D mating type a strain. Here the NCP1a gene was found to be present in all aD strains tested and to be absent in all α strains of either serotype tested (Fig. 1D and data not shown). In AD hybrid strains heterozygous for the MAT locus, the NCP1a gene was present only in hybrids that inherited the MATa allele from the serotype D parent (αADa) and not in those that inherited the serotype A MATa allele (aADα) (Fig. 1D). Based on these findings, we conclude that strain 125.91 contains a serotype A MATa allele at the MAT locus.
Strain 125.91 mates with a subset of serotype A, α mating type strains.
By molecular analysis strain 125.91 is an aA strain, yet it fails to mate with the α reference strains H99 and JEC21 (36). Comparison of the entire MAT locus sequences of the sterile aA strain 125.91 and the fertile aD strain JEC20 reveals that all of the genes in the fertile MATa allele are present in the 125.91 MAT locus except the NCP1a gene, which shares homology with a Neurospora crassa gene of unknown function (35). ncp1a mutants are fertile (22), and aADα hybrids that result from intervarietal mating lack NCP1a (Fig. 1C); thus, the absence of this gene cannot account for the apparent sterility of strain 125.91. Alterations in temperature, medium, or supplementation with cyclic AMP did not stimulate mating of strain 125.91.
Because strain 125.91 (aA) cannot mate directly with the αA reference strain H99, we tested the ability of strain 125.91 to mate with other αA strains. A total of 150 strains were tested, and mating was examined on four different media that support mating of serotype D strains (V8 [pH 7], carrot, or SLAD medium) or serotype B strains (V8 [pH 5]). Three strains mated with strain 125.91. Strain S25J is an αA clinical isolate from Asia that mated with strain 125.91 only on V8 (pH 5). Strain 4476B, a clinical isolate from Uganda, mated with strain 125.91 only on carrot medium and was found to be an αAD hybrid strain. Strain 8-1 is an αA clinical isolate from a U.S. organ transplant center, and it mated with strain 125.91 on all media tested and to the greatest extent on V8 (pH 5) (Fig. 2). We focused on strain 8-1.
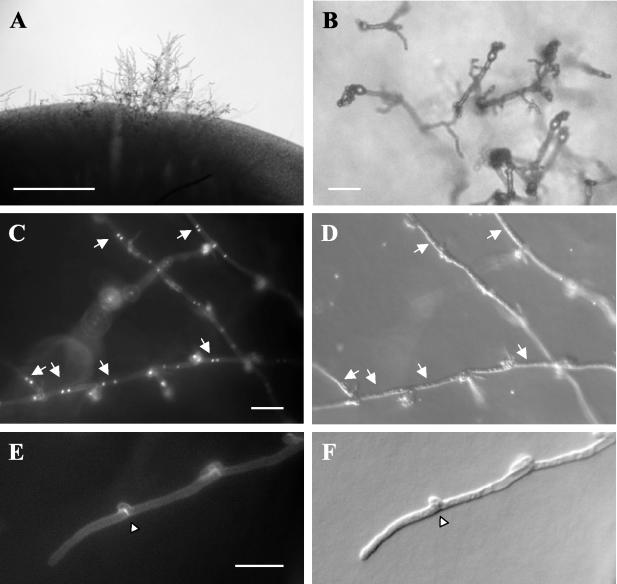
FIG. 2.
Morphological features of C. neoformans var. grubii mating. The a mating type, serotype A strain 125.91 was mixed with the α mating type, serotype A strain 8-1. (A) Example of filaments observed after 10 days of growth on V8 (pH 5.0) at 25°C in the dark. Bar, 1 mm. (B) Example of filaments, basidia, and spores produced after 10 days of growth on V8 (pH 5.0) at 25°C in the dark. Bar, 25 μm. (C) DAPI staining of nuclei in filaments produced in a 125.91 (aA)-to-8-1 (αA) cross. Arrows designate paired nuclei in the dikaryons. Bar, 25 μm. (D) Differential contrast image of the filaments seen in panel C. (E) Calcofluor White staining of the septa of filaments produced in a 125.91 (aA)-to-8-1 (αA) cross. The arrowhead designates a fused clamp connection. Bar, 25 μm. (F) DIC image of the filament seen in panel E.
We first verified that strain 8-1 is an authentic αA strain. Strain 8-1 typed as serotype A in an antibody agglutination test (reacted with antigens 1 and 7) and was haploid by FACS analysis (Fig. 1A). By PCR, strain 8-1 contains only the serotype A alleles of the STE20α, PAK1, GPA1, and CNA1 genes, and the sequences of these genes are more similar to serotype A than to serotype D reference sequences. Finally, by transposon fingerprinting, strain 8-1 lacks the T1 and T2 elements characteristic of serotype D strains (Fig. 1B). Thus, strain 8-1 is a haploid serotype A strain.
Characterization of strain 125.91 mating.
Mating between the aA strain 125.91 and the αA strain 8-1 produced filaments characteristic of mating. Filament cells contained two nuclei (dikaryons), were linked by fused clamp cells whose septa stained with calcofluor white, and produced basidium fruiting bodies decorated with four long chains of basidiospores (Fig. 2). These morphological features are all hallmarks of mating of serotype D C. neoformans var. neoformans strains (28) and are distinct from those of haploid fruiting filaments (monokaryotic, unfused clamps, and short spore chains) (59).
Basidiospores isolated from the 125.91 (aA)-to-8-1 (αA) mating germinated (∼25%) to produce viable meiotic progeny. Of 15 progeny tested, all were serotype A by antibody agglutination tests and PCR analysis of the STE20, PAK1, GPA1, and CNA1 genes (not shown). Seven meiotic segregants were α, six were a, and two were indeterminate, based on mating to the parental strains and PCR analysis with STE20 primers specific for the α and a mating types (not shown). These results are consistent with an approximate 1:1 ratio of α to a progeny.
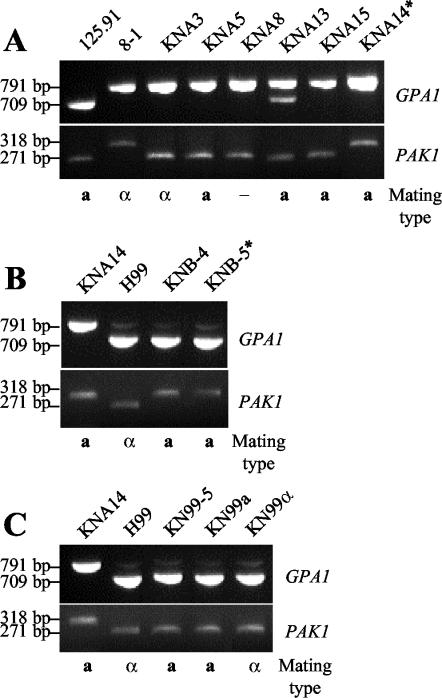
RFLP analysis of the GPA1 and PAK1 genes combined with mating type analysis established that recombinant progeny were produced by the 125.91 (aA)-to-8-1 (αA) cross. Strain 125.91 contains a PvuI restriction site near the 3′ end of the GPA1 gene that is not present in strain 8-1. Digestion of a PCR product spanning the GPA1 gene from strain 125.91 resulted in cleavage of the gene fragment to produce 709- and 82-bp products, compared to the 791-bp product from strain 8-1 (Fig. 3A). Similarly, the PAK1 gene in strain 125.91 contains a BstXI restriction site at the 3′ end of the gene that is absent in strain 8-1. Digestion of the PCR-amplified PAK1 gene from strain 125.91 resulted in cleavage of the gene fragment to produce 271- and 47-bp products, compared to the 318-bp product from strain 8-1 (Fig. 3A). Of the 15 progeny tested for recombination, 9 progeny exhibited the RFLP and mating type of the parental strains 125.91 (aA) and 8-1 (αA), whereas ∼4 segregants with either parental genotype would be expected based on random segregation of three markers and 16 progeny analyzed. Other progeny were clearly products of meiotic recombination. Four progeny (two a, one α, and one indeterminate mating type [Fig. 3A]) inherited the strain 8-1 GPA1 allele and the strain 125.91 PAK1 allele, and one progeny (KNA13, a mating type [Fig. 3A]) contained both the 8-1 and 125.91 GPA1 alleles and was aneuploid by FACS analysis (Fig. 1A). One of the meiotic progeny, designated strain KNA14, inherited the GPA1 and PAK1 strain 8-1 alleles and the a mating type from strain 125.91 (Fig. 3A).
FIG. 3.
RFLP analysis of progeny from C. neoformans var. grubii crosses shows recombination. The presence or absence of the PvuI restriction site in the GPA1 gene and the BstXI restriction site in the PAK1 gene was examined by digestion of the PCR-amplified gene with the appropriate enzyme. An asterisk indicates the strain used to generate the serotype A congenic strains. (A) RFLP analysis of the parental strains 125.91 (aA) and 8-1 (αA) and progeny from a cross of the parental strains. Strain KNA14 was used to generate the serotype A congenic strains. (B) RFLP analysis of the parental strains KNA14 (aA) and H99 crg1 (αA) and progeny from a cross of the parental strains. KNB-5 was used to generate the serotype A congenic strains. (C) RFLP analysis of the parental strains KNA14 (aA) and H99 (αA), the 5th-backcross strain KN99-5a, and the 10th-backcross strains KN99a and KN99α.
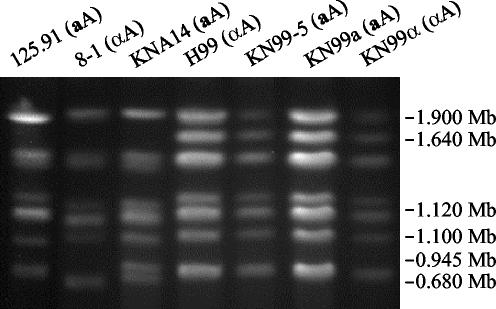
Progeny from the 125.91 (aA)-to-8-1 (αA) cross exhibited differences in ability to mate with the parental strains and the reference serotype A and D strains (Table 1). Strain KNA14 mated with the αA parental strain 8-1 and the αA and αD reference strains H99 and JEC21 to produce viable spores (Fig. 4A and B). PFGE was used to compare the karyotypes of strains 125.91, 8-1, KNA14, and H99 to determine whether differences in chromosome number or size might prevent successful meiosis and account for mating compatibility differences (Fig. 5). The chromosomal banding patterns of strains 125.91 (aA) and 8-1 (αA) were similar, and both strains lacked a high-molecular-weight band present in H99 (αA). The karyotype of strain KNA14 (aA) was similar to those of the 8-1 (αA) and 125.91 (aA) parental strains but contained two lower-molecular-weight bands, one from each parent. We note that strain KNA14 (aA) mates normally (Table 1) and is haploid by FACS analysis (Fig. 1A).
TABLE 1.
Mating abilities of serotype A strains of C. neoformans var. grubii
| Strain | Mating with strain:
|
||||
|---|---|---|---|---|---|
| 125.91 (aA) | 8-1 (αA) | H99 (αA) | JEC20 (aD) | JEC21 (αD) | |
| 125.91 (aA) | − | + | − | − | − |
| 8-1 (αA) | + | − | − | + | − |
| KNA1 (αA) | + | − | − | + | − |
| KNA2 (αA) | − | − | − | + | − |
| KNA3 (αA) | + | − | − | + | − |
| KNA4 (aA) | − | + | − | − | − |
| KNA5 (aA) | − | + | − | − | + |
| KNA6 (aA) | − | − | − | − | + |
| KNA7 (αA) | − | − | − | + | − |
| KNA8 (−A) | − | − | − | − | − |
| KNA9 (αA) | − | − | − | + | − |
| KNA10 (αA) | − | − | − | + | − |
| KNA11 (αA) | − | − | − | + | − |
| KNA13 (aA) | − | + | − | − | + |
| KNA14 (aA) | − | + | + | − | + |
| KNA15 (aA) | − | + | − | − | + |
| KNA16 (−A) | − | − | − | − | − |
FIG. 4.
The crg1 mutation enhances filamentation during mating of serotype A strains. The aA strain KNA14 was mated with the αA strain H99 or an isogenic crg1 αA mutant strain on V8 (pH 5.0) medium at 25°C in the dark for 10 days. (A) Example of aA (KNA14)-to-αA (H99) mating. Bar, 500 μm. (B) Filaments, basidia, and spores produced during an aA (KNA14)-to-αA (H99) mating. Bar, 50 μm. (C) Example of an aA (KNA14)-to-αA crg1 (H99 crg1) mutant mating. Bar, 100 μm. (D) Filaments, basidia, and spores produced during an aA (KNA14)-to-αA crg1 (H99 crg1) mutant mating. Bar, 50 μm.
FIG. 5.
Karyotype analysis of C. neoformans var. neoformans strains. Chromosomes from the aA strains 125.91, KNA14, KN99-5a, and KN99a and the αA strains 8-1, H99, and KN99α were separated by PFGE, and the gel was stained with ethidium bromide. Size markers are based on S. cerevisiae chromosomes.
Production of C. neoformans var. grubii congenic strains.
Congenic strains of serotype D have been invaluable in classical genetic studies of the physiology and virulence of C. neoformans var. neoformans (20, 30). However, the majority of clinical isolates of C. neoformans are serotype A (C. neoformans var. grubii). Therefore, it would be of great experimental value to have congenic strains in the more common serotype A genetic background. The mating observed between the serotype A strains 125.91 (aA) and 8-1 (αA) suggested that congenic strains could be generated in serotype A. However, strain H99 (αA) is the sequence reference strain with 11× genome-wide sequence coverage and a BAC fingerprint map (45). Thus, it would be preferable to generate congenic strains in this strain background.
Since the KNA14 (aA) F1 progeny of the 125.91 (aA)-to-8-1 (αA) cross mated with H99 (αA), we used this strain to generate congenic serotype A strains. However, strain KNA14 (aA) mated with either the αA strain 8-1 or the αD strain JEC21 to produce long hyphal filaments with basidia and spores (Fig. 2A and B), whereas basidia and spores were produced on very short hyphae closely associated with the yeast colony when KNA14 was crossed with the αA strain H99 (Fig. 4A and B). Thus, isolation of spores from the KNA14 (aA)-to-H99 (αA) cross was technically challenging due to the atypical short-hypha mating morphology. We sought to increase filamentation in this mating to spatially separate spores from vegetative yeast cells and allow microdissection. We used confrontation assays to examine filamentation of these strains. In confrontation assays, αD strains filamented and aD strains enlarged in response to mating pheromones prior to any direct cell-cell contact (Fig. 6, left panel) (41). However, the αA strain H99 failed to filament when confronted with aA or aD strains (Fig. 6, middle panel, and data not shown). Likewise, the aA strains 125.91 and KNA14 failed to filament or produce enlarged cells when confronted with αA or αD strains. Based on these results, we deduced that pheromone insensitivity might impair filamentation in the KNA14 (aA)-to-H99 (αA) cross.
FIG. 6.
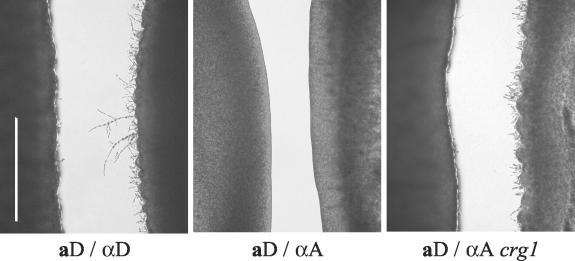
The crg1 mutation enhances pheromone response. The a and α strains were grown in confronting lines ∼1 mm apart on SLAD medium and incubated at 25°C in the dark for 72 h. Strains were examined microscopically for the presence of conjugation tubes. aD, JEC20; αD, JEC21; αA, H99; αA crg1, H99 crg1. Bar, 1 mm.
We addressed this apparent lack of pheromone response by creating a pheromone-supersensitive strain. In Saccharomyces cerevisiae, mutation of a regulator of G-protein signaling (SST2) confers dramatic hypersensitivity to mating pheromone (10, 14). The C. neoformans gene CRG1 (for Cryptococcus regulator of G proteins) encodes an SST2 homolog (P. Wang and J. Heitman, unpublished data), and an H99 crg1 mutant strain (αA) forms robust conjugation tubes in confrontation with the aD strain JEC20 (Fig. 6, right panel). Most importantly, the aA strain KNA14 mated with the H99 crg1 mutant (αA) to produce increased filamentation, abundant basidia, and spores (Fig. 4C and D).
When spores from the KNA14 (aA)-to-H99 crg1 (αA) cross were microdissected, a and α meiotic progeny segregated in a ratio of 1:1 based on mating assays. All of the a progeny mated with wild-type H99 (αA), producing long hyphae and abundant viable spores. Comparison of the H99 and KNA14 GPA1 and PAK1 sequences showed that H99 lacks the PvuI and BstXI restriction sites, respectively. RFLP analysis of progeny from the KNA14-to-H99 crg1 cross showed that recombinant progeny were produced (Fig. 3B).
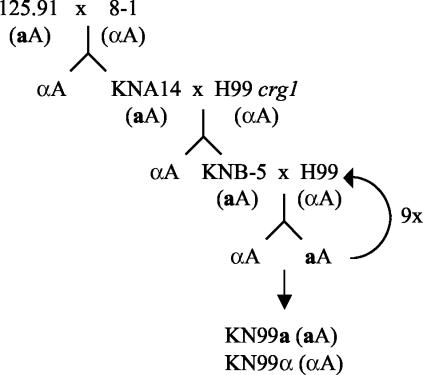
Figure 7 depicts the mating scheme used to generate congenic aA and αA strains. An aA progeny (KNB-5) from the KNA14 (aA)-to-H99 crg1 (αA) cross was used to initiate nine backcrosses to wild-type H99 (αA). The aA strain generated by the fifth backcross, designated KN99-5a, and its descendents all contained the wild-type CRG1 gene yet still produced long hyphae and abundant viable spores. The aA and αA strains generated by the 10th backcross were designated KN99a and KN99α, respectively. The 5th and 10th backcross strains were examined by RFLP analysis and PFGE karyotyping for similarity to H99. KN99-5a (aA), KN99a (aA), and KN99α (αA) all contained the H99 GPA1 and PAK1 alleles as determined by RFLP analysis (Fig. 3C). All three strains also had karyotypes identical to that of H99 (Fig. 5). Additionally, the 5th and 10th backcross strains were equivalent to H99 in melanin production, capsule production, and growth at 30 and 37°C (not shown). We have currently analyzed a limited number of genetic markers, but if we assume a 50% recombination frequency, the 5th backcross strain KN99-5a (aA) and the 10th backcross strains KN99a (aA) and KN99α (αA) are expected to be 96.9 and 99.9% similar to H99 (αA), respectively.
FIG. 7.
Schematic diagram of the mating scheme used to produce a serotype A congenic strain pair. KN99-5a is the product of the 5th backcross and KN99a and KN99α are products of the 10th backcross to H99.
Virulence of congenic serotype A strains in animal models.
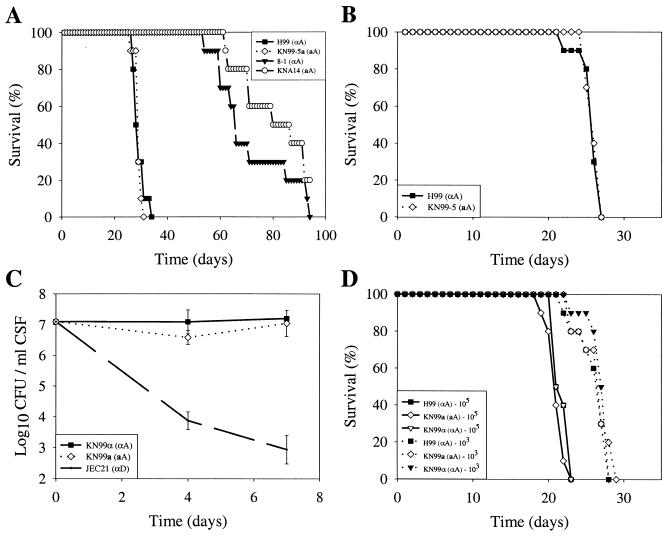
We showed previously that the aA strain 125.91 is less virulent than the αA reference strain H99 in a murine model of cryptococcosis (36). The relative pathogenicities of the parental strains 8-1 (αA) and KNA14 (aA) were compared to that of H99 (αA) in a murine inhalation model (Fig. 8A). Both parental strains had attenuated virulence compared to H99 (Fig. 8A) (P = 0.001). In contrast, no difference in virulence was observed between H99 and the fifth-backcross strain KN99-5a (aA) when mice were inoculated with either 1 × 105 (Fig. 8A) (P = 0.57) or 5 × 104 (Fig. 8B) (P = 0.85) fungal cells. In addition, we tested the virulence of the congenic 10th-backcross strains in both the mouse and rabbit models of cryptococcal meningitis. In the rabbit model there was no difference in the viability of the serotype A congenic strains KN99a and KN99α in the CSF as determined by comparing quantitative yeast counts over the first week of infection (P > 0.05), and these strains were more virulent in comparison to the reduced yeast counts of the serotype D strain JEC21 (P < 0.05) (Fig. 8C). While the rabbit model is not intended to measure host survival, we observed that both serotype A congenic strains caused 100% mortality in this model over a 10-day observation period, whereas rabbits infected with the serotype D strain were alive and appeared well. Furthermore, there was no difference in virulence between the congenic strains H99 (αA), KN99a (aA), and KN99α (αA) in the murine model when animals were infected with either 105 (P = 0.11 for KN99a and P = 1.0 for KN99α) or 103 (P = 0.68 for KN99a and P = 0.31 for KN99α) fungal cells (Fig. 8D). In summary, the serotype A congenic strains were fully virulent, and no difference in virulence was observed between the congenic a and α mating type strains in either a murine inhalation model or the rabbit immunosuppressed infection.
FIG. 8.
Virulence of serotype A strains. (A) Groups of 10 A/Jcr mice were infected with 105 cells of the αA strains H99 and 8-1, the aA strain KNA14, and the fifth-backcross progeny KN99-5a (aA), and survival was monitored. (B) Groups of 10 A/Jcr mice were infected with 5 × 104 cells of H99 (αA) and KN99-5a (aA), and survival was monitored. (C) Groups of three immunosuppressed rabbits were inoculated with 108 cells of strains KN99a (aA), KN99α (αA), and JEC21 (αD). CSF was withdrawn on days 4 and 7 postinfection, and the number of surviving yeast cells was determined by plating serially diluted CSF on YPD medium. (D) Groups of 10 A/Jcr mice were infected with either 105 or 103 cells of strains H99 (αA), KN99a (aA), and KN99α (αA), and survival was monitored. Error bars indicate standard errors of the means.
DISCUSSION
C. neoformans var. grubii serotype A strains cause ∼95% of all C. neoformans infections and >99% of the infections in AIDS patients (9). The predominance of serotype A in clinical isolates underscores the importance of understanding the mechanisms of virulence in this serotype. Basidiospores are thought to be the infectious propagule for C. neoformans, and yet spores are typically produced by mating, which had not been observed in serotype A. Moreover, serotype A strains were thought to be clonal and only of the α mating type. The recent identification of diploid AD hybrid strains with a MATa mating type allele derived from serotype A first suggested that fertile a mating type, serotype A strains might exist (34).
Subsequently, two aA strains were identified: one was a Tanzanian clinical isolate, strain 125.91, and the other was an Italian environmental isolate, strain IUM96-2828 (36, 41, 55). Strain IUM96-2828 (aA) has recently been shown to mate with the αA reference strain H99, and karyotypic analysis of progeny revealed evidence of recombination (26), thus defining a sexual cycle in C. neoformans var. grubii. While the aA strain IUM96-2828 mates with the reference strain H99 (αA), the other aA strain, 125.91, appeared to have a mating defect (36). Here we examined mating of strain 125.91 (aA) and discovered that this strain mates with only a subset of αA strains. Mating produced dikaryotic filaments with fused clamp connections and basidial fruiting structures with long chains of spores. By RFLP analysis and mating type tests, we document that mating between the aA strain 125.91 and the αA clinical isolate 8-1 produced recombinant progeny with variable mating ability. The meiotic progeny strain KNA14 (aA) exhibited the widest mating repertoire and was used here with the serotype A reference strain H99 (αA) to generate a congenic pair of a and α mating type serotype A strains (KN99a and KN99α). The production of congenic strains was facilitated by the identification of a crg1 pheromone-supersensitive mutant that lacks a regulator of G protein signaling for pheromone response. The observation that the 125.91 MAT locus could be used to produce congenic serotype A strains with apparently normal mating ability suggests that this locus is fully functional.
Virulence of C. neoformans var. neoformans strains has been linked to the MAT locus. The effect of mating type is unclear in a heterogeneous population, but comparison of the virulence of a and α congenic strains of serotype D showed that α strains were more virulent than a strains (30, 33, 60). We examined the role of mating type in virulence in C. neoformans var. grubii strains. The aA strain 125.91 is less virulent that the αA reference strain H99 in animal models (36) and is less virulent than its mating partner strain 8-1 (αA). Interestingly, the virulence of congenic aA and αA strains in the H99 background was equivalent in murine inhalation and rabbit meningitis models of cryptococcosis. Further studies conducted under a broad range of conditions may be necessary to reveal differences between these strains, but at present it appears that mating type does not play a predominant role in virulence of C. neoformans var. grubii strains.
The observation that mating type does not appear to play a dominant role in virulence of serotype A strains raises an interesting question: why are the majority of clinical serotype A isolates of the α mating type? Sexual reproduction is expected to generate a 1a:1α ratio of spores, and this ratio is seen in our laboratory crosses. If sexual reproduction is the primary method by which the infectious propagule is generated, then a higher proportion of clinical isolates would be expected to be of the a mating type. Some serotype AD strains can filament and produce spores that germinate poorly (<5%) and typically are still AD hybrids, and thus this cannot account for the predominance of mating type α, serotype A clinical isolates (34). An alternative possibility is that spores could be produced via the asexual process of haploid fruiting. In serotype D strains, haploid fruiting is linked to the α mating type (59). If this were also true in serotype A, it could account for an increased prevalence of αA clinical isolates. However, we have not yet observed haploid fruiting of any serotype A isolates, and as-yet-unknown environmental conditions may be necessary. It is also possible that aA strains are less virulent than αA strains in humans yet due to host organism differences, this lack of virulence is not detected in the mouse or rabbit models.
The fact that the majority of clinical serotype A isolates are α mating type could also be due to the relative abundance of αA strains in the environment. Analysis of ∼1,500 serotype A strains worldwide has identified only two aA strains, one a clinical isolate from Africa and the other an environmental isolate from soil near an aviary in Italy. Because aA strains appear to be rare, sexual reproduction to produce the infective propagule may take place only in geographically restricted areas where both mating types are present. Alternatively, aA strains might not survive well in the environment. For example, α strains may be more resistant than a strains to predation by amoebas and nematodes, resulting not only in increased virulence of some α strains (42, 50) but also in a decreased a strain population size.
With the characterization of a sexual cycle in C. neoformans var. grubii, sexual reproduction has been defined or reported for all C. neoformans varieties. However, the function of the sexual cycle in C. neoformans and its role in pathogenicity of this organism still remain unclear. The observation that the aA strain 125.91 mates with only a few αA strains might suggest that C. neoformans is evolving to be asexual and that the a and α mating types are increasingly genetically isolated. This situation may be similar to that observed with other fungal pathogens and parasites. The ability of the human pathogen Histoplasma capsulatum to undergo a sexual cycle is lost with laboratory passage of isolates (62). Loss of sexual reproduction upon prolonged culture has also been reported for C. neoformans var. neoformans strains (63). The obligate diploid C. albicans is primarily clonal (17) and may only rarely utilize its recently discovered sexual cycle (25, 38, 40). Recent studies of the parasite Toxoplasma gondii reveal that clinical isolates are largely clonal and restricted to three highly related sibling clades that descended from a single recent sexual cross (<10,000 years ago) that enabled efficient oral transmission without the sexual phase (51). In addition, completion of the sexual cycle in T. gondii can yield recombinant progeny with dramatically enhanced virulence (18). In the oomycete Phytophera infestans, mating-competent strains are geographically restricted and produce recombinant progeny that emigrate to cause clonal outbreaks of fungal disease on plants (16). Our studies suggest that the sexual cycle of C. neoformans may similarly be geographically restricted and may infrequently lead to the production of recombinants with altered fates that could be important in the evolution of this human pathogen.
Acknowledgments
We thank Arturo Casadevall, Vishnu Chaturvedi, Mahmoud Ghannoum, Harish Gugnani, Sam Messer, Juneann Murphy, Michael Pfaller, and Wiley Schell for strains. We thank James Fraser, Christina Hull, Alex Idnurm, and Klaus Lengeler for helpful comments and discussions; Cristl Arndt for technical assistance; and other members of the Heitman lab for their support.
This work was supported in part by NIAID R01 grant AI50113 to Joseph Heitman, NIAID R01 AI28388 grant to John Perfect, and an NIAID program project grant AI44975 to the Duke University Mycology Research Unit. Gary Cox is a Burroughs Wellcome New Investigator, and Joseph Heitman is a Burroughs Wellcome Scholar in Molecular Pathogenic Mycology and an Associate Investigator of the Howard Hughes Medical Institute.
Editor: T. R. Kozel
REFERENCES
- 1.Alspaugh, J. A., J. R. Perfect, and J. Heitman. 1997. Cryptococcus neoformans mating and virulence are regulated by the G-protein α subunit GPA1 and cAMP. Genes Dev. 11:3206-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anya, A. O. 1976. Physiological aspects of reproduction in nematodes. Adv. Parasitol. 14:267-351. [DOI] [PubMed] [Google Scholar]
- 3.Arkhipova, I., and M. Meselson. 2000. Transposable elements in sexual and ancient asexual taxa. Proc. Natl. Acad. Sci. USA 97:14473-14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barton, N. H., and B. Charlesworth. 1998. Why sex and recombination? Science 281:1986-1990. [PubMed] [Google Scholar]
- 5.Bell, G. 1982. The masterpiece of nature: the evolution and genetics of sexuality. University of California Press, Berkeley.
- 6.Burt, A. 2000. Sex, recombination, and the efficacy of selection—was Weismann right? Evolution Int. J. Org. Evolution 54:337-351. [DOI] [PubMed] [Google Scholar]
- 7.Butlin, R. 2002. Evolution of sex: the costs and benefits of sex: new insights from old asexual lineages. Nat. Rev. Genet. 3:311-317. [DOI] [PubMed] [Google Scholar]
- 8.Calderone, R. A. 2002. Candida and candidiasis. American Society for Microbiology, Washington, D.C.
- 9.Casadevall, A., and J. R. Perfect. 1998. Cryptococcus neoformans. American Society for Microbiology, Washington, D.C.
- 10.Chan, R. K., and C. A. Otte. 1982. Isolation and genetic analysis of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and alpha factor pheromones. Mol. Cell. Biol. 2:11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corley, L. S., J. R. Blankenship, and A. J. Moore. 2001. Genetic variation and asexual reproduction in the facultative parthenogenetic cockroach Nauphoteta cinerea: implications for the evolution of sex. J. Evolutionary Biol. 14:68-74. [DOI] [PubMed] [Google Scholar]
- 12.Cruz, M. C., L. M. Cavallo, J. M. Gorlach, G. Cox, J. R. Perfect, M. E. Cardenas, and J. Heitman. 1999. Rapamycin antifungal action is mediated via conserved complexes with FKBP12 and TOR kinase homologs in Cryptococcus neoformans. Mol. Cell. Biol. 19:4101-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson, R. C., J. R. Blankenship, P. R. Kraus, M. de Jesus Berrios, C. M. Hull, C. D'Souza, P. Wang, and J. Heitman. 2002. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology 148:2607-2615. [DOI] [PubMed] [Google Scholar]
- 14.Dietzel, C., and J. Kurjan. 1987. The yeast SCG1 gene: a G alpha-like protein implicated in the a- and alpha-factor response pathway. Cell 50:1001-1010. [DOI] [PubMed] [Google Scholar]
- 15.Franzot, S. P., J. S. Hamdan, B. P. Currie, and A. Casadevall. 1997. Molecular epidemiology of Cryptococcus neoformans in Brazil and the United States: evidence for both local genetic differences and a global clonal population structure. J. Clin. Microbiol. 35:2243-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodwin, S. B., B. A. Cohen, and W. E. Fry. 1994. Panglobal distribution of a single clonal lineage of the Irish potato famine fungus. Proc. Natl. Acad. Sci. USA 91:11591-11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graser, Y., M. Volovsek, J. Arrington, G. Schonian, W. Presber, T. G. Mitchell, and R. Vilgalys. 1996. Molecular markers reveal that population structure of the human pathogen Candida albicans exhibits both clonality and recombination. Proc. Natl. Acad. Sci. USA 93:12473-12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grigg, M. E., S. Bonnefoy, A. B. Hehl, Y. Suzuki, and J. C. Boothroyd. 2001. Success and virulence in Toxoplasma as the result of sexual recombination between two distinct ancestries. Science 294:161-165. [DOI] [PubMed] [Google Scholar]
- 19.Hawkesworth, D. L., P. M. Kirk, B. C. Sutton, and D. N. Pegler. 1995. Ainsworth and Bisby's dictionary of the fungi, 8th ed. International Mycology Institute, Surrey, United Kingdom.
- 20.Heitman, J., B. Allen, J. A. Alspaugh, and K. J. Kwon-Chung. 1999. On the origins of congenic MATα and MATa strains of the pathogenic yeast Cryptococcus neoformans. Fungal Genet. Biol. 28:1-5. [DOI] [PubMed] [Google Scholar]
- 21.Hickey, D. A. 1982. Selfish DNA: a sexually-transmitted nuclear parasite. Genetics 101:519-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hull, C. M., R. C. Davidson, and J. Heitman. 2002. Cell identity and sexual development in Cryptococcus neoformans are controlled by the mating-type-specific homeodomain protein Sxi1α. Genes Dev. 16:3046-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hull, C. M., and J. Heitman. 2002. Fungal mating: Candida albicans flips a switch to get in the mood. Curr. Biol. 12:R782-R784. [DOI] [PubMed]
- 24.Hull, C. M., and J. Heitman. 2002. Genetics of Cryptococcus neoformans. Annu. Rev. Genet. 36:557-615. [DOI] [PubMed] [Google Scholar]
- 25.Hull, C. M., R. M. Raisner, and A. D. Johnson. 2000. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science 289:307-310. [DOI] [PubMed] [Google Scholar]
- 26.Keller, S. M., M. A. Viviani, M. C. Esposto, M. Cogliati, and B. L. Wickes. 2003. Molecular and genetic characterization of a serotype A MATa Cryptococcus neoformans isolate. Microbiology 149:131-142. [DOI] [PubMed] [Google Scholar]
- 27.Kwon-Chung, K. J. 1976. Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia 68:821-833. [PubMed] [Google Scholar]
- 28.Kwon-Chung, K. J. 1975. A new genus, Filobasidiella, the perfect state of Cryptococcus neoformans. Mycologia 67:1197-1200. [PubMed] [Google Scholar]
- 29.Kwon-Chung, K. J., J. E. Bennett, and J. C. Rhodes. 1982. Taxonomic studies on Filobasidiella species and their anamorphs. Antonie Leeuwenhoek 48:25-38. [DOI] [PubMed] [Google Scholar]
- 30.Kwon-Chung, K. J., J. C. Edman, and B. L. Wickes. 1992. Genetic association of mating types and virulence in Cryptococcus neoformans. Infect. Immun. 60:602-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon-Chung, K. J., and J. E. Bennett. 1992. Cryptococcosis. Lea and Febiger, Malvern, Pa.
- 32.Kwon-Chung, K. J., and J. E. Bennett. 1978. Distribution of alpha and a mating types of Cryptococcus neoformans among natural and clinical isolates. Am. J. Epidemiol. 108:337-340. [DOI] [PubMed] [Google Scholar]
- 33.Kwon-Chung, K. J., and W. B. Hill. 1981. Sexuality and pathogenicity of Filobasidiella neoformans (Cryptococcus neoformans), p. 243-250. In R. Vanbreuseghem and C. DeVroy (ed.), Sexuality and pathogenicity of fungi. Masson, Paris, France.
- 34.Lengeler, K. B., G. M. Cox, and J. Heitman. 2001. Serotype AD strains of Cryptococcus neoformans are diploid or aneuploid and are heterozygous at the mating-type locus. Infect. Immun. 69:115-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lengeler, K. B., D. S. Fox, J. A. Fraser, A. Allen, K. Forrester, F. S. Dietrich, and J. Heitman. 2002. Mating-type locus of Cryptococcus neoformans: a step in the evolution of sex chromosomes. Eukaryot. Cell 1:704-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lengeler, K. B., P. Wang, G. M. Cox, J. R. Perfect, and J. Heitman. 2000. Identification of the MATa mating-type locus of Cryptococcus neoformans reveals a serotype A MATa strain thought to have been extinct. Proc. Natl. Acad. Sci. USA 97:14455-14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lenski, R. E. 2001. Genetics and evolution. Come fly, and leave the baggage behind. Science 294:533-534. [DOI] [PubMed] [Google Scholar]
- 38.Magee, B. B., and P. T. Magee. 2000. Induction of mating in Candida albicans by construction of MTLa and MTLα strains. Science 289:310-313. [DOI] [PubMed] [Google Scholar]
- 39.McDade, H. C., and G. M. Cox. 2001. A new dominant selectable marker for use in Cryptococcus neoformans. Med. Mycol. 39:151-154. [DOI] [PubMed] [Google Scholar]
- 40.Miller, M. G., and A. D. Johnson. 2002. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110:293-302. [DOI] [PubMed] [Google Scholar]
- 41.Montagna, M. T. 2002. A note on the isolation of Cryptococcus neoformans serotype A MATa strain from the Italian environment. Med. Mycol. 40:593-595. [DOI] [PubMed] [Google Scholar]
- 42.Mylonakis, E., F. M. Ausubel, J. R. Perfect, J. Heitman, and S. B. Calderwood. 2002. Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc. Natl. Acad. Sci. USA 99:15675-15680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rispe, C., J.-S. Pierre, J.-C. Simon, and P.-H. Gouyon. 1998. Models of sexual and asexual coexistence in aphids based on constraints. J. Evolutionary Biol. 11:685-701. [Google Scholar]
- 44.Roux, K. H., and K. H. Hecker. 1997. One-step optimization using touchdown and stepdown PCR. Methods Mol. Biol. 67:39-45. [DOI] [PubMed] [Google Scholar]
- 45.Schein, J. E., K. L. Tangen, R. Chiu, H. Shin, K. B. Lengeler, W. K. MacDonald, I. Bosdet, J. Heitman, S. J. Jones, M. A. Marra, and J. W. Kronstad. 2002. Physical maps for genome analysis of serotype A and D strains of the fungal pathogen Cryptococcus neoformans. Genome Res. 12:1445-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmeding, K. A., S. C. Jong, and R. Hugh. 1984. Biochemical variation of Cryptococcus neoformans. Mycopathologia 84:121-131. [DOI] [PubMed] [Google Scholar]
- 47.Schmeding, K. A., S. C. Jong, and R. Hugh. 1981. Sexual compatibility between serotypes of Filobasidiella neoformans (Cryptococcus neoformans). Curr. Biol. 5:133-138. [Google Scholar]
- 48.Sia, R. A., K. B. Lengeler, and J. Heitman. 2000. Diploid strains of the pathogenic basidiomycete Cryptococcus neoformans are thermally dimorphic. Fungal Genet. Biol. 29:153-163. [DOI] [PubMed] [Google Scholar]
- 49.Smith, J. M. 1978. The evolution of sex. Cambridge University Press, Cambridge, United Kingdom.
- 50.Steenbergen, J. N., H. A. Shuman, and A. Casadevall. 2001. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl. Acad. Sci. USA 98:15245-15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su, C., D. Evans, R. H. Cole, J. C. Kissinger, J. W. Ajioka, and L. D. Sibley. 2003. Recent expansion of Toxoplasma through enhanced oral transmission. Science 299:414-416. [DOI] [PubMed] [Google Scholar]
- 52.Sukroongreung, S., K. Kitiniyom, C. Nilakul, and S. Tantimavanich. 1998. Pathogenicity of basidiospores of Filobasidiella neoformans var. neoformans. Med. Mycol. 36:419-424. [PubMed] [Google Scholar]
- 53.Taylor, J. W., D. M. Geiser, A. Burt, and V. Koufopanou. 1999. The evolutionary biology and population genetics underlying fungal strain typing. Clin. Microbiol. Rev. 12:126-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toffaletti, D. L., T. H. Rude, S. A. Johnston, D. T. Durack, and J. R. Perfect. 1993. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J. Bacteriol. 175:1405-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viviani, M. A., M. C. Esposto, M. Cogliati, M. T. Montagna, and B. L. Wickes. 2001. Isolation of a Cryptococcus neoformans serotype A MATa strain from the Italian environment. Med. Mycol. 39:383-386. [DOI] [PubMed] [Google Scholar]
- 56.Wang, P., C. B. Nichols, K. B. Lengeler, M. E. Cardenas, G. M. Cox, J. R. Perfect, and J. Heitman. 2002. Mating-type-specific and nonspecific PAK kinases play shared and divergent roles in Cryptococcus neoformans. Eukaryot. Cell 1:257-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, P., J. R. Perfect, and J. Heitman. 2000. The G-protein β subunit GPB1 is required for mating and haploid fruiting in Cryptococcus neoformans. Mol. Cell. Biol. 20:352-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Welch, D. B., and M. S. Meselson. 2001. Rates of nucleotide substitution in sexual and anciently asexual rotifers. Proc. Natl. Acad. Sci. USA 98:6720-6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wickes, B. L., M. E. Mayorga, U. Edman, and J. C. Edman. 1996. Dimorphism and haploid fruiting in Cryptococcus neoformans: association with the alpha-mating type. Proc. Natl. Acad. Sci. USA 93:7327-7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wickes, B. L., and K. J. Kwon-Chung. 2002. Genetic basis of pathogenicity in Cryptococcus neoformans, p. 25-49. In R. A. Calderone and R. L. Cihlar (ed.), Fungal pathogenesis: principles and clinical applications, vol. 14. Marcel Dekker, New York, N.Y.
- 61.Williams, G. C. 1975. Sex and recombination. Princeton University Press, Princeton, N.J.
- 62.Woods, J. P. 2002. Histoplasma capsulatum molecular genetics, pathogenesis, and responsiveness to its environment. Fungal Genet. Biol. 35:81-97. [DOI] [PubMed] [Google Scholar]
- 63.Xu, J. 2002. Estimating the spontaneous mutation rate of loss of sex in the human pathogenic fungus Cryptococcus neoformans. Genetics 162:1157-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu, J., T. G. Mitchell, and R. Vilgalys. 1999. PCR-restriction fragment length polymorphism (RFLP) analyses reveal both extensive clonality and local genetic differences in Candida albicans. Mol. Ecol 8:59-73. [DOI] [PubMed] [Google Scholar]
- 65.Xu, J., and T. G. Mitchell. 2002. Strain variation and clonality in Candida spp. and Cryptococcus neoformans, p. 739-749. In R. A. Calderone and R. L. Cihlar (ed.), Fungal pathogenesis: principles and clinical applications, vol. 14. Marcel Dekker, New York, N.Y.