Abstract
The plasmid-encoded toxin (Pet) from enteroaggregative Escherichia coli is a serine protease autotransporter that acts as an enterotoxin and cytotoxin. When applied to epithelial cells in culture, purified toxin induces cell elongation and rounding, followed by exfoliation of cells from the substratum. These effects are accompanied by loss of actin stress fibers and electrophysiologic changes. Although it has been hypothesized that Pet has an intracellular site of action, evidence for this is indirect. In addition, Pet has recently been shown to cleave spectrin in vitro and in vivo. If Pet requires intracellular localization to execute its toxic effects, then intracellular expression of the protein could induce cytopathic effects similar to those observed when the toxin is applied to the cell surface. To test this hypothesis, we expressed the mature Pet toxin (comprising only the passenger domain of the Pet precursor) in the cytoplasm of HEp-2 cells by using mammalian expression vectors. Separately, we expressed the Pet passenger domain mutated at the catalytic serine (PetS260A), a construct that has been reported to lack toxic effects. Forty-eight hours after transient transfection of pcDNA3.1-pet in HEp-2 cells, we observed cell elongation and other morphological changes similar to those induced by applied toxin. Cells transfected with pcDNA3.1 vector alone appeared normal, while cells expressing the PetS260A mutant displayed similar (though less pronounced) changes compared with those in cells expressing pcDNA3.1-pet. Notably, intracellular expression of Pet was accompanied by condensation of the spectrin cytoskeleton. These studies corroborate an intracellular site of action for the Pet toxin, further implicate a role for spectrin in Pet intoxication, and provide a powerful tool for Pet structure and function analyses.
Enteroaggregative Escherichia coli (EAEC) is an emerging diarrheal pathogen, whose pathogenesis is thought to comprise colonization of the intestinal mucosa with the release of secretogenic enterotoxins and cytotoxins (5, 8, 16). Organ culture experiments reveal heavy adherence of EAEC on the mucosal surface with exfoliation of epithelial cells (13). We have previously reported that exfoliation of colonic epithelial cells and mucosal damage induced by prototype EAEC strain 042 requires secretion of a 104-kDa toxin called Pet (plasmid-encoded toxin), which is encoded on the 60-MDa plasmid of strain 042 and in a subset of other EAEC strains (10). Pet is secreted by the autotransporter mechanism and belongs to a growing class of Enterobacteriaceae autotransporter proteins that display a protease motif (GDSGS; catalytic serine residue underlined) at a homologous position. This class of proteins, called the SPATEs (14), includes the putative virulence factors SepA, SigA, and Pic of Shigella flexneri (1, 4, 12), Sat of uropathogenic E. coli (11), EspP of Shiga-toxin producing E. coli (7), EspC of enteropathogenic E. coli (15, 21), and Pet and Pic of EAEC (3, 10, 12). The contributions of these proteins to virulence are unclear, but Sat, EspP, and Pet have been shown to elicit cytopathic effects on epithelial cells in vitro (14).
The mechanism of action of the Pet toxin has been partially elucidated. Pet has been reported to enter epithelial cells in culture (18), and the effects of Pet are almost completely abolished by the incubation of cells with brefeldin A. These data suggest that Pet has an intracellular site of action. Moreover, Villaseca et al. have shown that Pet cleaves erythroid spectrin in vitro and that Pet intoxication is accompanied by degradation of spectrin species and clumping of spectrin in intoxicated HEp-2 cells (22). Whether Pet cleaves spectrin in vivo has not yet been directly demonstrated.
DH5α (2) and HB101 (6) were used in this work and were grown aerobically at 37°C in Luria-Bertani (LB) medium; when required, ampicillin was added at a concentration of 100 μg/ml. Site-directed mutagenesis was performed on the pet gene in minimal clone pCEFN1 by using the QuikChange mutagenesis kit (Stratagene, La Jolla, Calif.) as described previously (10). The oligonucleotide primers used in the reaction were 5′-CACTAACTTAACCACTAATGGTGACGCTGGATCAGGCGTGTATG (forward) and 5′-CATACACGCCTGATCCAGCGTCACCATTAGTGGTTAAGTTAGTG (reverse). The primers encompassed bases 1033 to 1076 of the pet sequence (accession no. AF056581). By this procedure, adenine and guanine at pet nucleotides 1058 and 1059 were substituted for with guanine and cytosine, respectively, resulting in substitution of alanine for serine at residue 260. For construction of pcDNA3.1-pet and pcDNA3.1-S260A, the passenger domains of Pet and its S260A mutant (PetS260A) were amplified by PCR for cloning into pcDNA3.1 (Invitrogen, Carlsbad, Calif.) digested with NheI and XhoI. The PCR primers were 5′-GCAGGCTAGCATGGCCAATATGGATATATCTAAAG-3′(forward), and 5′-CTGACTCGAGTCAGTTGACCTCTGCAAGGAAG-3′(reverse). The forward primer contained an NheI cleavage site (underlined) and an ATG start codon (boldface), and the reverse primer contained an XhoI cleavage site (underlined) and the reverse complement sequence of a TGA stop codon (boldface). Amplifications were performed by using 0.2 mM deoxynucleoside triphosphate (dNTP), 0.2 μM each primer, 500 ng of purified plasmid pCEFN1 or pS260A as a template, 2.5 mM MgCl2, 1× buffer, and 1 U of Pfu Turbo DNA polymerase (Stratagene). Reaction mixtures were subjected to initial denaturation for 2 min at 94°C, followed by 35 amplication cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min 30 s; the last extension step was performed at 72°C for 5 min. PCR products were purified and digested with NheI and XhoI and ligated into pcDNA3.1 vector (Invitrogen).
HEp-2 cells were propagated in humidified 5% CO2 at 37°C in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (vol/vol) fetal bovine serum (FBS; Invitrogen), 1% (wt/vol) nonessential amino acids (NEAA), 50 U of penicillin G sodium per ml, and 50 μg of streptomycin per ml. Transfection was performed according to the manufacturer's protocols (Invitrogen) with 4 μg of plasmid and 40 μg of Lipofectin added to 300 μl of serum-free DMEM and incubated at room temperature for 30 min prior to transfection. A similar protocol was used to transfect cells in eight-well chamber slides, using 0.25 μg of plasmid and 2.5 μg of Lipofectin per well. To produce stably transfected cell lines, 107cells were washed three times with cold PBS and resuspended in a final volume of 700 μl in a Genepulse cuvette (Bio-Rad, Hercules, Calif.). Twelve micrograms of plasmid was added to the cell suspension, and the cuvette was placed on ice for 10 min and then pulsed twice at 1 kV, 25 μF, and 200 Ω using the GENE pulser II electroporation system (Bio-Rad). After 10 min on ice, the cell suspension was transferred to a cell culture flask; fresh DMEM supplemented with 10% (vol/vol) FBS, 1% (wt/vol) NEAA, 50 U of penicillin per ml, and 50 μg of streptomycin per ml were added; and the flask was incubated for 24 h at 37°C in 5% CO2. After this time, the medium was changed, and Geneticin (Sigma Chemical Co., St. Louis, Mo.) was added to a final concentration of 400 μg/ml. Medium was changed subsequently every 5 days, maintaining Geneticin at 400 μg/ml. Eighteen days after transfection, colonies were subcultured into the same medium containing Geneticin at 400 μg/ml.
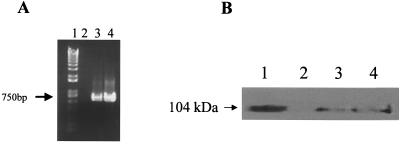
To verify expression of Pet and PetS260A within transfected cells, total RNA was isolated from cells 48 h after transfection by pcDNA3.1 vector alone, pcDNA3.1-pet, and pcDNA3.1-S260A. Reverse transcription-PCR (RT-PCR) revealed the expected 750-bp product indicative of pet and pet-S260A transcripts (Fig. 1A). No product was observed from cells harboring the pcDNA3.1 vector alone (Fig. 1A, lane 2). A Western blot revealed a protein of the same size as the secreted native Pet protein (Fig. 1B). No such protein was visualized in cells transfected with the pcDNA3.1 vector alone (Fig. 1B, lane 2).
FIG. 1.
(A) RT-PCR to detect pet and pet-S260A transcripts. 48 h after transfection, total RNA was isolated from cells transfected by pcDNA3.1 vector alone, pcDNA3.1-pet, and pcDNA3.1-S260A using Trizol (Invitrogen, Grand Island, N.Y.). RT was performed with Moloney murine leukemia virus reverse transcriptase (Invitrogen) according to the manufacturer's protocols, and RT-PCR was performed as previously described (19). The primers employed were 5′-CAGGCTGACCTGTTAAGTGCATCACTATTC-3′ (forward) and 5′-CTCACCCAGGGCGTTCTCTGCATTGAGTTG-3′ (reverse). Lanes: 1, size standard (1 Kb-Plus DNA Ladder from Gibco/BRL); 2, cells transfected with pcDNA3.1 vector alone; 3, cells transfected with pcDNA3.1-pet; 4, cells transfected with pcDNA3.1-S260A. (B) Detection of Pet or PetS260A expression by Western blot analysis with anti-Pet antibodies. Cells were lysed in Laemmli sample loading buffer (Bio-Rad), and proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (8% [wt/vol] polyacrylamide) and transferred to polyvinylidene difluoride membrane overnight. The membrane was incubated with rabbit anti-Pet polyclonal antibodies (diluted 1:8,000) as described by Eslava et al. (10). The secondary antibody was horseradish peroxidase-labeled goat anti-rabbit (KPL). Pet was visualized with Supersignal West Femto Maximum (Pierce) as a substrate according to manufacturer's protocols. Lanes: 1, purified native Pet control; 2, lysate of HEp-2 cells transfected with pcDNA3.1 vector alone; 3, lysate of cells transfected with pcDNA3.1-pet; 4, lysate of cells transfected with pcDNA3.1-S260A.
Cells transfected with pcDNA3.1-pet, pcDNA3.1-S260A, or pcDNA3.1 vector alone were examined under phase-contrast light microscopy at time points up to 48 h after transfection (Fig. 2). Cells transfected with pcDNA3.1 vector alone appeared healthy throughout this interval. In contrast, by 48 h, cells transfected with pcDNA3.1-pet diminished in number; some remaining cells were elongated, with some lengthened up to three times the diameter of healthy cells. A small subset of surviving cells appeared dense and rounded, similar in appearance to HEp-2 cells intoxicated by native Pet toxin. Observation of transfected cells over this period suggested that the sequence of events comprised elongation, followed by rounding, and ultimately resulting in exfoliation from the substratum. In wells transfected with pcDNA3.1-S260A, cells appeared generally similar to those transfected with pcDNA3.1-pet, although there appeared to be fewer cells that were severely elongated.
FIG. 2.
Phase-contrast microscopy of HEp-2 cells 48 h after transfection with pcDNA3.1 vector alone (a), pcDNA3.1-pet (b), (c) or pcDNA3.1-S260A. (d) HEp-2 cells incubated with purified Pet at a concentration of 0.02 μg/μl in an eight-well chamber slide for 3 h. Panels e and f are higher-magnification views of panels b and c, respectively. Bars in panels a to d, 100 μm. Bars in panels e and f, 10 μm. Black arrows indicate elongated cells, which are more pronounced in Pet-transfected cells. White arrows indicate rounded cells, seen predominantly in Pet-transfected wells. The open triangle in panel f indicates normal cells seen in PetS260A-transfected wells; normal cells are rarely seen in Pet-transfected wells.
We counted the cells remaining 48 h after transfection in an eight-well chamber format (Fig. 3). The number of normal-appearing cells in wells transfected with pcDNA3.1 vector alone was significantly greater than that in wells transfected with pcDNA3.1-pet or pcDNA3.1-S260A (P < 0.05). The numbers of cells at the beginning of each experiment were similar.
FIG. 3.
Quantification of normal cells in wells transfected with Pet or PetS260A. Forty-eight hours after transfection with vector pcDNA3.1, pcDNA3.1-pet, or pcDNA3.1-S260A, normal-appearing cells were counted in transfected wells under phase-contrast microscopy by a blinded observer. Mean numbers of normal-appearing cells from three transfected wells are presented with standard deviation. Thirty-five percent of cells expressed GFP after 48 h of transient transfection when pGFP control plasmid was used alone to test the transfection rate.
One potential mechanism of intoxication after pet transfection is release of toxin from transfected cells and intoxication of neighboring cells. To investigate whether Pet or PetS260A was released from transfected cells in an active form, we aspirated supernatants from the cell culture flask at 36, 40, or 48 h after transfection, centrifuged the supernatants to remove cell debris, and added 400 μl to culture wells containing 50% confluent normal HEp-2 cells. This experiment did not reveal any evidence of altered cell morphology despite 8 h of incubation (data not shown).
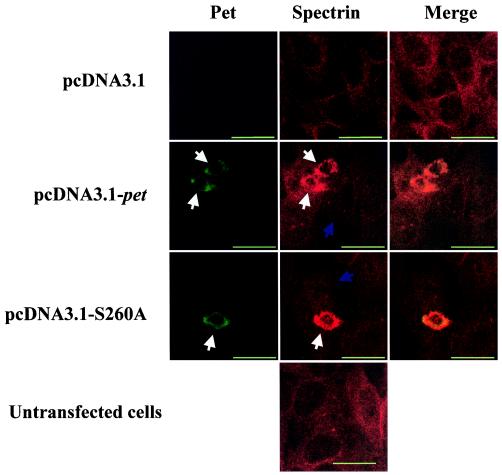
Having established an intracellular expression system for Pet, we sought to compare the cytoskeletal effects of the toxin expressed intracellularly with those induced by the native toxin added extracellularly. We and others have shown that intoxication with Pet is accompanied by dissolution of actin microfilaments (17). Loss of the actin cytoskeleton is manifested by elongation, followed by rounding and exfoliation. Villaseca et al. have shown that Pet is able to cleave spectrin in vitro and that cells intoxicated with Pet demonstrate redistribution and condensation of the spectrin membrane cytoskeleton (22). To assess the effects of intracellular Pet on the spectrin cytoskeleton, immunofluorescence was performed 48 h after transfection of HEp-2 cells in eight-well chamber slides. Spectrin was stained overnight at 4°C with mouse anti-alpha II spectrin monoclonal antibody (Chemicon, Temecula, Calif.) and visualized with goat anti-mouse antiserum conjugated with Alexa Fluor 647 (Molecular Probes) (Fig. 4). Untransfected cells or those transfected with pcDNA3.1 vector alone demonstrated diffuse spectrin staining, compatible with normal localization of spectrin to the membrane cytoskeleton. In contrast, cells transfected with pcDNA3.1-pet displayed condensation of spectrin into large intracellular aggregates distributed throughout the cytoplasm. Cells transfected with pcDNA3.1-S260A also displayed condensation of the spectrin skeleton.
FIG. 4.
Distribution of Pet and spectrin in transfected cells. Confocal microscopy was performed on normal and transfected HEp-2 cells incubated with mouse anti-alpha II spectrin antibody, followed by secondary antimouse Ig antibody labeled with Alexa Fluor 647. Zeiss LSM410 confocal laser-scanning microscopy with a ×63, NA 1.4 objective was used. Alexa Fluor 488 was excited with the 488-nm lines of a 50-mW KrAr laser and detected through 515- to 565-nm band-pass filter. Alexa Fluor 647 signal was excited with the 633-nm lines of a 6-mW HeNe laser and detected through 670- to 810-nm band-pass filter. The diameter of the detector pinhole corresponded to one Airy unit at 590 nm, which corresponds to an optical thickness of 1 μm along the z axis. White arrows indicate contracted cells with condensed spectrin; blue arrows indicate cells with normal distribution of spectrin. All bars are 25 μm.
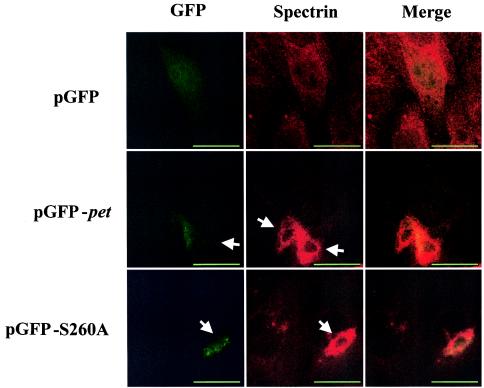
To ensure that the abnormal cells were those expressing the toxin constructs, we cloned pet and petS260A passenger domain genes into expression vector pGFP, producing protein fusions of the toxins with green fluorescent protein (GFP). HEp-2 cells were transiently transfected and stained for spectrin distribution at 32 and 48 h after transfection. GFP expression alone resulted in multiple cells with diffuse green fluorescence; there were no apparent alterations in cell morphology (Fig. 5). However, cells expressing the Pet-GFP fusion appeared condensed compared with nonfluorescing cells in the same field. Immunofluorescence staining for spectrin revealed aggregates of spectrin in Pet-GFP-expressing cells, similar to the effects observed after transient pcDNA3.1-pet transfection. Spectrin aggregates were not uniformly distributed throughout the cell, and the distribution of GFP roughly corresponded to the location of the spectrin aggregates. Cells expressing PetS260A-GFP again manifested a roughly similar phenotype: cells with green fluorescence appeared smaller and rounded with condensation of spectrin.
FIG. 5.
Immunofluorescence assay of the pGFP control alone and pGFP-pet- and pGFP-S260A-transfected HEp-2 cells stained with mouse anti-alpha II spectrin antibody and secondary anti-mouse Ig antibody labeled with Alexa Fluor 647. GFP C-terminal fusions were constructed from Pet and the PetS260A mutant with the primers 5′-AGCGGCCGCATGGCCAATATGGATATATCTAAAG (forward) and 5′-GGTTGACCTCTGCAAGGAAGGCTTTATAG (reverse). The forward primer contained a NotI cleavage site (underlined) and an ATG start codon (boldface). Amplication was performed as described above, and then a 3′ adenine was added to PCR products by incubation at 72°C for 20 min with 0.2 mM dNTP, 2.5 mM MgCl2, 1× reaction buffer, and 0.2 U of Taq DNA polymerase (Promega, Madison, Wis.). Images were taken with a Zeiss LSM410 confocal microscope. White arrows indicate contracted cells with condensed spectrin. Dotted lines in the spectrin column delineate a single normal cell for size comparison with transfected cells. Bar, 25 μm.
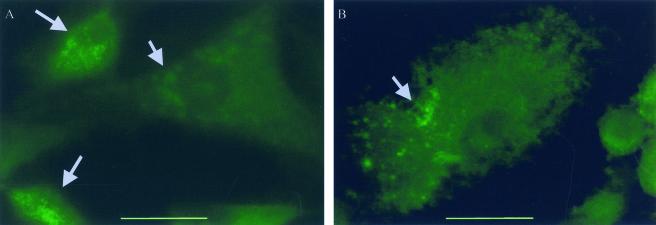
We were surprised by the finding of cellular alterations in cells transfected by pcDNA3.1-S260A, (which does not intoxicate cells when applied externally). To further assess the effect of toxin production within the cell, we established stably transfected cell lines. Eighteen days after transfection, nontransfected cells were killed by the addition of Geneticin. Cell colonies in flasks transfected with pcDNA3.1 vector alone were reproducibly much more numerous than in flasks transfected by pcDNA3.1-pet or pcDNA3.1-S260A (data not shown). Cells stably expressing Pet or PetS260A constructs were subcultured and examined for spectrin distribution by immunofluorescence in eight-well chamber slides (Fig. 6). The results were largely similar to those observed with transiently transfected cells. pcDNA3.1-pet-transfected cells demonstrated elongation and rounding. A percentage of cells transfected with pcDNA3.1-pet revealed marked spectrin condensation (1.76% ± 1.2%). In contrast, cell lines transfected with pcDNA3.1-S260A revealed less (0.11% ± 0.05%; P < 0.05) spectrin condensation than pcDNA3.1-pet-transfected cells. The cells shown in Fig. 6 are typical of cells transfected with Pet (Fig. 6A) and S260A (Fig. 6B) constructs; the degree of spectrin condensation appeared more dramatic in Pet-transfected cells, although this was difficult to quantify within individual cells. Cells stably transfected with pcDNA3.1 vector alone were indistinguishable from untransfected control cells (data not shown) and revealed no detectable spectrin condensation.
FIG. 6.
Spectrin visualization in stably transfected HEp-2 cells. Spectrin was visualized by staining with mouse anti-alpha II spectrin antibodies, followed by secondary anti-mouse Ig antibody labeled with Alexa Fluor 488. Images were taken with a Zeiss epifluorescence microscope (bar, 25 μm). (A) Transfection with pcDNA3.1-pet. (B) Transfection with pcDNA3.1-S260A. Gray arrows indicate accumulations of condensed spectrin.
We and others have previously shown that the EAEC Pet toxin is internalized into epithelial cells and that Pet intoxication is accompanied by breakdown of cellular spectrin and loss of actin microfilaments (17, 22). Pet mutants in the catalytic serine residue were devoid of any detectable toxic activity. Thus, the data are consistent with a model in which Pet enters the cytoplasm and therein cleaves cytoskeletal spectrin, resulting in its morphological effects. However, it has not yet been proven definitively that Pet acts intracellularly or that cleavage of spectrin is its fundamental mode of action. Using a number of mammalian expression systems, we have shown here that when expressed intracellularly, the Pet passenger domain (corresponding to the mature toxin) induces damage to the epithelial cell similar to that seen after application of the purified toxin to cultured cells. When expressed transiently or stably in expression vector pcDNA3.1, Pet elicited elongation, rounding, and exfoliation of HEp-2 cells, a cell line normally susceptible to Pet intoxication. Pet expression led to a decreased number of epithelial cells in the culture vessel, but the surviving cells were more likely than vector-transfected cell lines to manifest morphological changes. Transfection with GFP fusion constructs revealed that cells with GFP fluorescence were invariably altered in their morphology. Interestingly, transfected cells that persisted on the substratum, although altered in morphology, generally expressed only dim fluorescence, perhaps suggesting that a low level of Pet expression can be tolerated by the cell. Localization of GFP revealed diffuse distribution of the Pet-GFP fusion protein.
Despite similarities between Pet intoxication and its intracellular expression, our experiments also highlighted several differences. Although we observed spectrin condensation, spectrin aggregates were diffusely distributed through the cytoplasm, whereas spectrin blebs are typically peripherally located in intoxicated cells (22; R. Cappello and J. P. Nataro, unpublished observations). The explanation for this difference is not apparent from our studies, but we hypothesize that normal trafficking of Pet after external application may result in a different distribution of the toxin within the cells compared with intracellular expression.
A second unexpected difference pertained to the effects of the catalytic serine mutant PetS260A. Previous studies have reported that PetS260A is devoid of toxic effects (17, 22), whereas the current analysis suggested that PetS260A expressed within the cell induced significant morphological changes. Navarro-Garcia et al. have recently shown that Pet is able to bind spectrin within the 8th internal repeat (A. Canizalez-Roman, A., J. Luna, and F. Navarro-Garcia, Abstr. 102nd Gen. Meet. Am. Soc. Microbiol., abstr. B-128, p. 53-54, 2002.), close to the calmodulin binding domain (9, 20). This binding would presumably be independent of serine-mediated catalysis, and so the mild effects of PetS260A could be the result of spectrin binding, possibly coupled with calmodulin displacement. Alternatively, PetS260A could have some residual protease activity (although this is not observed in vitro), or the toxin may possess additional mechanisms by which to damage epithelial cells. Further investigations using our experimental systems will test these hypotheses.
Our studies have corroborated the importance of intracellular delivery of Pet into epithelial cells, and this system provides an important tool for further studies of the SPATE proteins. Intracellular expression of other SPATE proteins will illuminate functional differences among these toxins. For example, we have recently found that expression of EspC within cells also results in cytopathic effects (B. Sui, F. Navarro-Garcia, and J. Nataro, unpublished observations). Our data also suggest that the Pet toxin can fold into an active form in the cytoplasm of epithelial cells, obviating the need for translocation through the bacterial outer membrane. These data suggest that all sequences required for proper folding are contained within the passenger domain itself. Ongoing experiments in our laboratory will utilize the systems described herein to elucidate Pet structure-function relationships and to characterize the events surrounding intoxication.
Acknowledgments
This work was supported by Public Health Service grant AI 43615 to J.P.N.
Editor: A. D. O'Brien
REFERENCES
- 1.Al-Hasani, K., I. R. Henderson, H. Sakellaris, K. Rajakumar, T. Grant, J. P. Nataro, R. Robins-Browne, and B. Adler. 2000. The sigA gene which is borne on the she pathogenicity island of Shigella flexneri 2a encodes an exported cytopathic protease involved in intestinal fluid accumulation. Infect. Immun. 68:2457-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. A. Smith, J. G. Seidman, and K. Struhl. (ed.) 1989. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 3.Behrens, M., J. Sheikh, and J. P. Nataro. 2002. Regulation of the overlapping pic/set locus in Shigella flexneri and enteroaggregative Escherichia coli. Infect. Immun. 70:2915-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjelloun-Touimi, Z., P. J. Sansonetti, and C. Parsot. 1995. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol. Microbiol. 17:123-135. [DOI] [PubMed] [Google Scholar]
- 5.Bhan, M. K., P. Raj, M. M. Levine, J. B. Kaper, N. Bhandari, R. Srivastava, R. Kumar, and S. Sazawal. 1989. Enteroaggregative Escherichia coli associated with persistent diarrhea in a cohort of rural children in India. J. Infect. Dis. 159:1061-1064. [DOI] [PubMed] [Google Scholar]
- 6.Boyer, H. W., and R. Roulland-Dussoix. 1969. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J. Mol. Biol. 41:459-468. [DOI] [PubMed] [Google Scholar]
- 7.Brunder, W., H. Schmidt, and H. Karch. 1997. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol. Microbiol. 24:767-778. [DOI] [PubMed] [Google Scholar]
- 8.Cravioto, A., A. Tello, A. Navarro, J. Ruiz, H. Villafan, F. Uribe, and C. Eslava. 1991. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet 337:262-264. [DOI] [PubMed] [Google Scholar]
- 9.De Matteis, M. A., and J. S. Morrow. 2000. Spectrin tethers and mesh in the biosynthetic pathway. J. Cell Sci. 113:2331-2343. [DOI] [PubMed] [Google Scholar]
- 10.Eslava, C., F. Navarro-Garcia, J. R. Czeczulin, I. R. Henderson, A. Cravioto, and J. P. Nataro. 1998. Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect. Immun. 66:3155-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guyer, D. M., I. R. Henderson, J. P. Nataro, and H. L. Mobley. 2000. Identification of sat, an autotransporter toxin produced by uropathogenic Escherichia coli. Mol. Microbiol. 38:53-66. [DOI] [PubMed] [Google Scholar]
- 12.Henderson, I. R., J. Czeczulin, C. Eslava, F. Noriega, and J. P. Nataro. 1999. Characterization of Pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect. Immun. 67:5587-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson, I. R., S. Hicks, F. Navarro-Garcia, W. P. Elias, A. D. Phillips, and J. P. Nataro. 1999. Involvement of the enteroaggregative Escherichia coli plasmid-encoded toxin in causing human intestinal damage. Infect. Immun. 67:5338-5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson, I. R., and J. P. Nataro. 2001. Virulence functions of autotransporter proteins. Infect. Immun. 69:1231-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mellies, J. L., F. Navarro-Garcia, I. Okeke, J. Frederickson, J. P. Nataro, and J. B. Kaper. 2001. espC pathogenicity island of enteropathogenic Escherichia coli encodes an enterotoxin. Infect. Immun. 69:315-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nataro, J. P., T. Steiner, and R. L. Guerrant. 1998. Enteroaggregative Escherichia coli. Emerg. Infect. Dis. 4:251-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarro-Garcia, F., C. Sears, C. Eslava, A. Cravioto, and J. P. Nataro. 1999. Cytoskeletal effects induced by Pet, the serine protease enterotoxin of enteroaggregative Escherichia coli. Infect. Immun. 67:2184-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navarro-Garcia, F., A. Canizalez-Roman, J. Luna, C. Sears, and J. P. Nataro. 2001. Plasmid-encoded toxin of enteroaggregative Escherichia coli is internalized by epithelial cells. Infect. Immun. 69:1053-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheikh, J., S. Hicks, M. Dall'Agnol, A. D. Phillips, and J. P. Nataro, J. P. 2001. Roles for Fis and YafK in biofilm formation by enteroaggregative Escherichia coli Mol. Microbiol. 41:983-997. [DOI] [PubMed] [Google Scholar]
- 20.Stabach, P. R., C. D. Cianci, S. B. Glantz, Z. Zhang, and J. S. Morrow. 1997. Site-directed mutagenesis of alpha II spectrin at codon 1175 modulates its mu-calpain susceptibility. Biochemistry 7:57-65. [DOI] [PubMed] [Google Scholar]
- 21.Stein, M., B. Kenny, M. A. Stein, and B. B. Finlay. 1996. Characterization of EspC, a 110-kilodalton protein secreted by enteropathogenic Escherichia coli which is homologous to members of the immunoglobulin A protease-like family of secreted proteins. J. Bacteriol. 178:6546-6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villaseca, J. M., F. Navarro-Garcia, G. Mendoza-Hernández, J. P. Nataro, A. Cravioto, and C. Eslava. 2000. Pet toxin from enteroaggregative Escherichia coli produces cellular damage associated with fodrin disruption. Infect. Immun. 68:5920-5927. [DOI] [PMC free article] [PubMed] [Google Scholar]