Abstract
Aims
The objectives of this study were to determine the effect of brain trauma on the multiple pathways of metabolism of valproate and to evaluate the use of the urinary 6β-hydroxycortisol to cortisol ratio in predicting changes in hepatic metabolism induced by brain injury.
Methods
Fourteen patients with severe head injuries received a 15 mg kg−1 loading dose and a maintenance dose of valproate to maintain therapeutic plasma concentrations. A minimum of one steady state trough blood sample and one dosage interval urine were collected during days 3–6 and during days 7–14 post-injury. Total and unbound valproate plasma concentrations were determined by gas chromatography-flame ionization detection (GC-FID) with and without ultrafiltration. Urinary valproate metabolites were measured by gas chromatography|mass spectrometry (GC-MS) (n = 10). Urinary 6β-hydroxycortisol and cortisol concentrations were determined by high performance liquid chromatography (h.p.l.c.) (n = 14). Total intrinsic clearance (CLint) for valproate and individual formation clearances (CLf) to its major metabolites were calculated. Data obtained during baseline (days 3–6) were averaged for each patient and were compared with averaged data obtained from days 7 to 14 using a paired t-test.
Results
Statistically significant increases in the CLint, CLf of VPA glucuronide, 2-ene-VPA, and 4-OH-VPA pathways and the 6β-hydroxycortisol to cortisol ratio were found. The percent change in the 6β-hydroxycortisol to cortisol ratio correlated significantly with the changes in the CLint of valproate.
Conclusions
Brain trauma results in induction of multiple pathways of valproate metabolism and increases in the 6β-hydroxycortisol to cortisol ratio, suggesting a non-specific enzyme induction in response to head injury.
Keywords: valproate, 6-β-hydroxycortisol, cortisol, brain injury, metabolism
Introduction
Traumatic brain injury results in a time-dependent increase in the plasma clearance of several compounds. In a study evaluating the use of phenytoin for prevention of post-traumatic seizures, unusually large doses of intravenous phenytoin were needed during the first 2–3 weeks post-injury in order to maintain therapeutic plasma concentrations [1]. Subsequent studies demonstrated that alterations in both protein binding and hepatic metabolism were involved. The free fraction of phenytoin was increased two-fold [2–4], while the free fraction of valproate increased six to seven fold [5] after acute head trauma secondary to decreased albumin concentrations. Increases in the intrinsic metabolic clearance of phenytoin [2], antipyrine [6], and lorazepam [6] have been demonstrated suggesting that multiple metabolic pathways are induced in patients after head injuries. Generally, significant increases in hepatic metabolism occur 7 days post-trauma [2, 6].
Valproate is a short chain fatty acid that is metabolized by the body in the same manner as endogenous fatty acids with greater than 97% eliminated by hepatic metabolism [7]. Approximately 85% of the dose is recoverable in urine as 13 metabolites using a gas chromatography/mass spectrometry assay (GC-MS) [8]. Major pathways of valproate biotransformation include β-oxidation and conjugation with glucuronic acid. Minor metabolism occurs by cytochrome P450 (CYP) dependent oxidation and desaturation reactions [7]. Valproate is a substrate for CYP2C9, CYP2C19 and CYP2A6 [9] but not for CYP3A4 [10], the most abundant CYP isozyme in the human liver. Determining the effect of brain trauma on valproate metabolism will provide information on a broad spectrum of metabolic enzymes involved in endogenous and xenobiotic biotransformation.
The measurement of cortisol metabolism to 6β-hydroxycortisol has been proposed as a non invasive method for evaluating hepatic metabolism and a clinical test to detect enzyme induction [11–16]. 6β-hydroxycortisol is formed by the glucocorticoid inducible CYP3A4 in human liver [15]. This ratio has been shown to be several fold higher in patients on known enzyme inducing drugs: rifampicin, phenytoin and carbamazepine [13–15]. Under conditions of enzymatic induction by rifampicin, the 24 h urinary ratio of 6β-hydroxycortisol/cortisol increased several fold and correlated with CYP3A4 levels in human liver [15]. The intra-subject correlation (before and during induction) was considerably stronger than the intersubject correlation [13, 16]. Conversely, there was no change in either the 6β-hydroxycortisol/cortisol ratio or antipyrine metabolism after treatment with oxcarbamazepine, a non-inducer of hepatic metabolism [17]. Even though the 6β-hydroxycortisol/cortisol ratio does not provide conclusive results when used to compare liver CYP3A4 activity among individuals, the ratio is a useful marker of the extent of hepatic metabolic induction for drugs that are metabolized by CYP3A4 [18].
The objectives of this study were twofold. First, to determine the effect of brain trauma on the multiple pathways of metabolism of valproate and second, to evaluate the use of the urinary 6β-hydroxycortisol to cortisol ratio in prediction of brain trauma induced changes in hepatic metabolism.
Methods
Subjects
Subjects were enrolled in an on-going study evaluating the use of valproate for prophylaxis of post-traumatic seizures. Eligibility criteria included at least one of the following: cortical contusions, depressed skull fracture, subdural, epidural or intracerebral haematoma, penetrating head wounds and early seizures (within 24 h of injury). Exclusion criteria were evidence of hepatic dysfunction, coagulation abnormality, prior head injury, any prior antiseizure medication or pregnancy. Subjects identified for this part of the study, were excluded if they received steroids, oral contraceptives or drugs with known enzyme inducing or inhibiting properties. The institutional review board of the University of Washington approved the study and written consent was obtained by the subjects or their legal guardians.
Study design
Subjects received an intravenous loading dose of valproate (15 mg kg−1) within 24 h of injury and thereafter a maintenance dose of valproate sufficient to maintain plasma concentrations of valproate within the therapeutic range (346–693 μmol l−1). When the patient could tolerate oral dosing, intravenous valproate was withdrawn and oral therapy was initiated at the same dose. Trough valproate plasma concentrations were obtained daily in the morning for the first 7 days and then 3 times weekly. Steady state plasma concentrations are reached within 2 days of a dosage change. A minimum of one steady state dosage interval urine was obtained during days 3–6 (week 1) and one during days 7–14 (week 2) post-injury. Steady state was verified with more than one sample only during week 1 when daily blood samples were taken.
Assays
Total and unbound plasma concentrations of valproate were determined in a blood sample obtained the same day as the urine collection by a capillary gas chromatography method [19]. Extraction and trimethylsilyl (TMS) derivitization procedures were similar to the published procedure using chloroform as an extraction solvent and 1-methyl-1-cyclohexane carboxylic acid as an internal standard. Unbound plasma concentrations of valproate were obtained after ultrafiltration using the Centrifree Micropartition system-1 (Amicon, Danvers, MA). Both interday and intraday coefficient of variation (CVs) were less than 5% for total and unbound plasma concentrations of valproate.
In 10 patients, total urinary concentrations of valproate metabolites (unconjugated and glucuronide conjugate) were measured by GC-MS and selected ion monitoring of the [M-15]+ ion of the TMS derivatives. Data on the procedures for the assay including sensitivity, precision and reproducibility of the 13 valproate metabolites were described in detail elsewhere [8]. In general, intra-day precision is less than 6% for any of the individual metabolites accounting for greater than 5% of the valproate dose.
The urine samples obtained from the 10 patients used for evaluation of the metabolism of valproate and urines obtained from an additional four subjects receiving valproate therapy were used to assess the suitability of the 6β-hydroxycortisol/cortisol ratio as a marker of metabolic induction. Urinary 6β-hydroxycortisol and cortisol were assayed by a modification of a previously described high pressure liquid chromatography method [13]. Intra-day CVs were less than 5% for both cortisol and 6β-hydroxycortisol Interday CVs were less than 10% for both compounds. For the assays described above, urine and plasma samples for each subject with assayed in duplicate during the same assay run to decrease possible effects of interday variability.
Data analysis
Total plasma clearance (CLp) of valproate was determined as the ratio of the dosing rate to the trough total valproate concentration. In order to separate the effects of protein binding from change in hepatic metabolism of valproate, the intrinsic plasma clearance (CLint) of valproate was calculated from the ratio of the dosing rate to the unbound valproate concentration. Three assumptions are made in this method of calculating CLint. First, the fraction of valproate absorbed is close to unity when valproate was administered orally. Secondly, valproate is a low extraction hepatically cleared drug. Finally, valproate trough plasma concentrations can be used to estimate average steady state concentrations. Ideally, a complete area under the concentration-time curve is required to determine the average steady state concentration. In cases where intensive sampling is not possible, trough plasma concentration can be used to estimate average steady state concentrations when the dosage interval is less than the elimination half-life of the compound [20]. Based on the validity of the above assumptions [21], a trough valproate plasma concentration can be used to estimate CLint.
The calculation of intrinsic formation clearance (CLf) requires grouping of metabolites according to define primary metabolic pathways.
Conjugation: VPA glucuronide
β-oxidation: 2(E)-ene-VPA, 3-OH-VPA, 3-oxo-VPA
4-ene-pathway: 4-ene-VPA, 3′-oxo-4ene-VPA, 2(E),4-diene-VPA, and 4,5-diOH-VPA-lactone
ω-1 oxidation: 4-OH-VPA, 4-OXO-VPA, PSA
ω oxidation: 5-OH-VPA, PGA
The CLf of each primary metabolic pathway of valproate was calculated as the excretion rate of urinary metabolites excreted (nmol h−1) in that pathway divided by the unbound valproate concentration in plasma (μmol). In the patients where more than one urine was obtained during week 2, the results were averaged for both the valproate metabolite data and the 6β-hydroxycortisol/cortisol ratio. Statistically significant differences were determined by paired t-test, rejecting the null hypothesis when P≤0.05.
Pearson product-moment correlation coefficients were calculated to determine the strength of relationships between patient factors (age, gender, Glasgow Coma Scale, Injury Severity Scale) and CLint and the 6β-hydroxycortisol/cortisol ratio. Again, difference were declared significance if P≤0.05.
Results
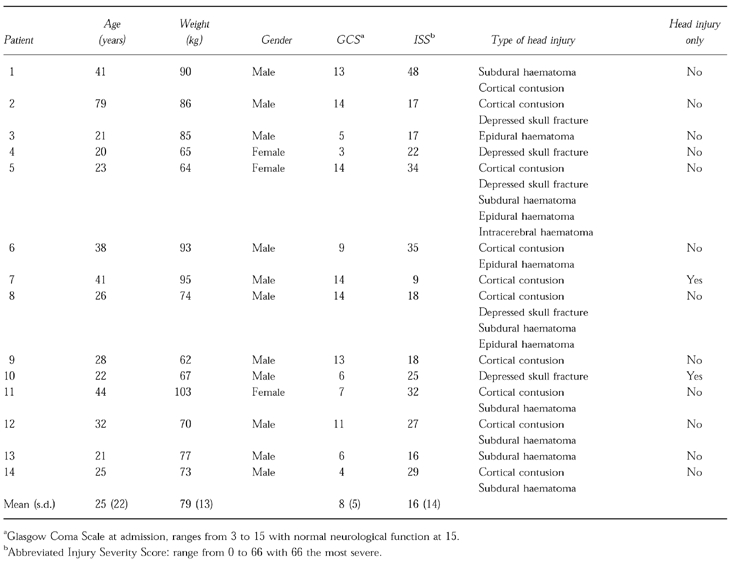
A description of the subjects is given in Table 1. Subjects were predominantly young males with multiple system injuries. During the study, subjects were receiving a variety of medications as part of their routine clinical care. These included antibiotics, analgesics, sucralfate, ranitidine, and β-adrenoceptor blockers. Three patients (1, 11, 14) received tube feedings. Renal and hepatic function remained without normal ranges.
Table 1.
Patient demographics.
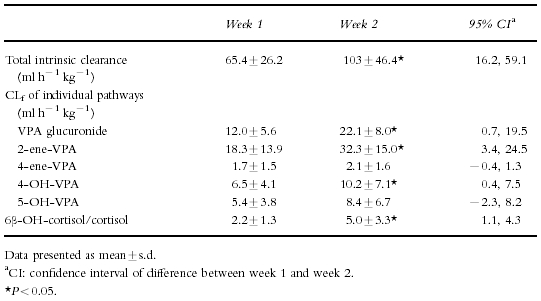
Baseline (day 3) CLp of valproate was 10.4±2.8 ml h−1 kg−1. Plasma clearances of valproate reported in healthy adults or patients with epilepsy on valproate monotherapy are similar [21]. CLp increased to 14.2±5.0 ml h−1 kg−1 during week 2. There was a significant increase in the CLint of valproate from 65.4±26.2 ml h−1 kg−1 to 103±46.4 ml h−1 kg−1 from week 1 to week 2 post trauma (Table 2). CLint increased by greater than 20% in 11 of 14 subjects with no change in CLint in the other 3. Glucuronidation was increased in 9 of 10 patients, β-oxidation in 8 of 10 patients while the cytochrome P450 dependent pathways increased in only 6 of 10 patients. Statistically significant increases in the formation clearances of valproate glucuronide, β-oxidation (2-ene-VPA formation) and 4-OH-VPA (P < 0.05) were observed from week 1 to week 2.
Table 2.
Time dependent changes in the total and intrinsic formation clearances of individual pathways of valproic acid and the 6β-OH-cortisol/cortisol ratio.
The urinary 6β-hydroxycortisol/cortisol ratio increased from 2.2±1.3 to 5.0±3.3 from week 1 to week 2 post-trauma. The ratio increased by greater than 15% in 12 of 14 subjects. There was no correlation between the CLint of valproate and the ratio during either week 1 or week 2 (r2 = 0.029 and r2 = 0.007) consistent with the lack of CYP3A4 metabolism of valproate. However, the percent change in the ratio correlated significantly with the percent change in the intrinsic clearance of valproate (r2 = 0.287, P = 0.048) from week 1 to week 2 suggesting non-specific induction. There was no consistent increase in either the ratio or the CLint in the subjects (Figure 1). None of the patient characteristics (initial Glasgow coma scale, Injury Severity Score, age and gender) correlated with the baseline CLint of valproate, the 6β-hydroxycortisol/cortisol ratio or the percentage change with trauma from week 1 to week 2.
Figure 1.
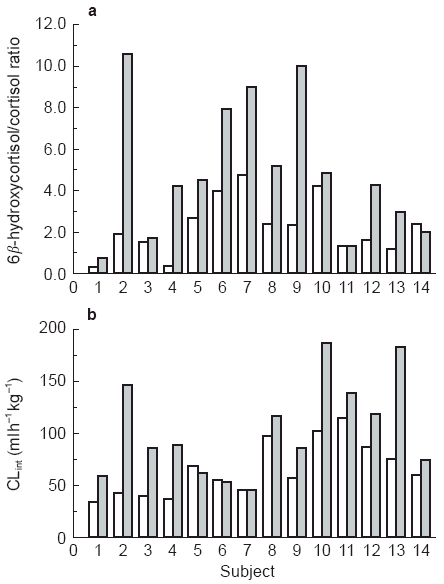
Individual subject data for 6β-hydroxycortisol/cortisol ratio (a) and valproate intrinsic clearance (CLint) (b). □ week 1,  week 2.
week 2.
Discussion
Traumatic head injuries cause a non-specific induction of a variety of hepatic enzymes. Glucuronidation, mitochondrial β-oxidation and metabolism catalyzed by multiple CYPs enzymes all appear to be affected. However the extent and enzyme specificity of induction varies from patient to patient. The mechanism of the increased hepatic metabolism is unknown. The acute phase response associated with trauma is under the regulatory control of the cytokines and in the last twenty years, there have been many reports demonstrating the acute depression of hepatic metabolism in animals and cell culture systems [22–27]. Clinically traumatic head injury appears to have the opposite effect on drug metabolism. Maximal increases in plasma clearance of valproate occur two to three weeks after the trauma and return to baseline values by week 4 (unpublished observations). Clearance of valproate on day 3 post-trauma was similar to values reported in previous studies [21] suggesting that an initial inhibition followed by a subsequent loss of inhibition can not explain the results. As the effect of head injury occurs only several days post-trauma, it is possible that the in vitro and in vivo models can demonstrate only the acute effects of immunostimulation and may not predict the long term effects demonstrated in this study and by other investigators.
In conclusion, the effect of head injury on the metabolism of valproate and cortisol to 6β-hydroxycortisol demonstrates a non-enzyme-specific induction. The ratio of 6β-hydroxycortisol and cortisol may be a useful tool in predicting injury induced changes in hepatic metabolism.
Acknowledgments
This work was supported in part by the National Institutes of Health Grant No. NS19643 and the AACP Grant Program for New Investigators. We gratefully acknowledge the technical assistance of Eric Kantor and thank Dr Rene Levy for the generous gift of the valproate metabolite standards.
References
- 1.Temkin NR, Dikmen SS, Wilensky AJ, Keihm J, Chabal S, Winn HR. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323:497–502. doi: 10.1056/NEJM199008233230801. [DOI] [PubMed] [Google Scholar]
- 2.Boucher BA, Rodman JH, Jaresko GS, Rasmussen SN, Watridge CB, Fabian TC. Phenytoin pharmacokinetics in critically ill trauma patients. Clin Pharmacol Ther. 1988;44:675–683. doi: 10.1038/clpt.1988.211. [DOI] [PubMed] [Google Scholar]
- 3.Griebel ML, Kearns GL, Fiser DH, Woody RC, Turley CP. Phenytoin protein binding in pediatric patients with acute traumatic injury. Crit Care Med. 1990;18:385–391. doi: 10.1097/00003246-199004000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Anderson GD, Pak C, Doane KW, et al. Revised Winter-Tozer equation for normalized phenytoin concentrations in trauma and elderly patients with hypoalbuminemia. Ann Pharmacother. 1997;31:279–284. doi: 10.1177/106002809703100301. [DOI] [PubMed] [Google Scholar]
- 5.Anderson GD, Gidal BE, Hendryx RJ, et al. Decreased plasma protein binding of valproate in patients with acute head trauma. Br J Clin Pharmacol. 1994;37:559–562. doi: 10.1111/j.1365-2125.1994.tb04304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boucher BA, Kuhl DA, Fabian TC, Robertson JT. Effect of neurotrauma on hepatic drug clearance. Clin Pharmacol Ther. 1991;50:487–497. doi: 10.1038/clpt.1991.173. [DOI] [PubMed] [Google Scholar]
- 7.Baillie TA, Sheffels PR. Valproate: Chemistry and biotransformation. In: Levy RH, Mattson RH, Meldrum MB, editors. Antiepileptic drugs. 4. New York: Raven Press; 1995. pp. 589–604. [Google Scholar]
- 8.Rettenmeier AW, Howald WN, Levy RH, et al. Quantitative metabolic profiling of valproic acid in humans using automated gas chromatographic/mass spectrometric/techniques. Biomed Environ Mass Spec. 18:192–199. doi: 10.1002/bms.1200180308. [DOI] [PubMed] [Google Scholar]
- 9.Sadeque AJM, Korzekwa KR, Gonzalez FJ, Rettie AE. Identification of human liver cytochrome P450 isozymes responsible for the formation of 4ene-valproic acid (abstract) ISSX Proceedings. 1995;8:87. [Google Scholar]
- 10.Sadeque AJM, Korzekwa KR, Gonzalez F, Rettie AE. Metabolism of valproic acid by human liver microsomes(abstract) ISSX Proceedings. 1994;6:273. [Google Scholar]
- 11.Saenger P. 6-beta-hydroxycortisol in random urine samples as an indicator of enzyme induction . Clin Pharmacol Ther. 1983;34:818–821. doi: 10.1038/clpt.1983.255. [DOI] [PubMed] [Google Scholar]
- 12.Desager JP, Dumont E, Harvengt C. The urinary 6β-hydroxycortisol excretion in man on inducers and inhibitors of the hepatic mixed function oxidase. Pharmac Ther. 1987;33:197–199. doi: 10.1016/0163-7258(87)90051-9. [DOI] [PubMed] [Google Scholar]
- 13.Roots I, Holbe R, Hovermann W, Nigam S, Heinemeyer G, Hildebrandt AG. Quantitative determination by HPLC of urinary 6β-hydroxycortisol, an indicator of enzyme induction by rifampicin and antiepileptic drugs. Eur J Clin Pharmacol. 1979;16:63–71. doi: 10.1007/BF00644969. [DOI] [PubMed] [Google Scholar]
- 14.Bienvenu T, Rey E, Pons G, d’Athis P, Olive G. A simple non-invasive procedure for the investigation of cytochrome P450IIIA dependent enzymes in humans. Int J Clin Pharmacol Ther Toxicol. 1991;29:441–445. [PubMed] [Google Scholar]
- 15.Ged C, Rouillon JM, Picard L, et al. The increase in urinary excretion of 6β-hydroxycortisol as a marker of human hepatic cytochrome P450IIIA induction. Br J Clin Pharmacol. 1989;28:373–387. doi: 10.1111/j.1365-2125.1989.tb03516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohnhaus EE, Gerber-Taras E, Park BK. Enzyme inducing drug combinations and their effects on liver microsomal enzyme activity in man. Eur J Clin Pharmacol. 1983;24:247–250. doi: 10.1007/BF00613826. [DOI] [PubMed] [Google Scholar]
- 17.Larkin JG, McKee PJ, Forrest G, et al. Lack of enzyme induction with oxcarbamazepine (600 mg daily) in healthy subjects. Br J Clin Pharmacol. 1991;31:65–71. doi: 10.1111/j.1365-2125.1991.tb03858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watkins PB. Noninvasive tests of CYP3A enzymes. Pharmacogenetics. 1994;4:171–184. doi: 10.1097/00008571-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Rettenmeier AW, Gordon WP, Prickett KS, et al. Metabolic fate of valproic acid in the rhesus monkey: formation of a toxic metabolic, 2-n-propyl-4-pentenoic acid. Drug Metab Dispos. l986;14:443–453. [PubMed] [Google Scholar]
- 20.Winters ME. Basic clinical pharmacokinetics. 3. Vancouver WA: Applied Therapeutics; 1996. Interpretation of plasma drug concentrations; pp. 73–92. [Google Scholar]
- 21.Levy RH, Shen DD. Valproate: Absorption, distribution and excretion. In: Levy RH, Mattson RH, Meldrum MB, editors. Antiepileptic drugs. 4. New York: Raven Press; 1995. pp. 605–620. [Google Scholar]
- 22.Griffeth LK, Rosen GM, Tschanz C, Rauckman EJ. Effects of model traumatic injury on hepatic drug metabolism in the rat. I. In vivo antipyrine metabolism. Drug Metab Disp. 1983;11:517–525. [PubMed] [Google Scholar]
- 23.Griffeth LK, Rosen GM, Rauckman EJ. Effects of model traumatic injury on hepatic drug metabolism in the rat. II. In vivo metabolism of hexobarbital and zoxazolamine. Drug Metab Disp. 1984;12:582–587. [PubMed] [Google Scholar]
- 24.Griffeth LK, Rosen GM, Rauckman EJ. Effects of model traumatic injury on hepatic drug metabolism in the rat. IV. Glucuronidation. Drug Metab Disp. 1985;13:391–397. [PubMed] [Google Scholar]
- 25.Griffeth LK, Rosen GM, Rauckman EJ. Effects of model traumatic injury on hepatic drug metabolism in the rat. V. Sulfation and acetylation. Drug Metab Disp. l985;13:398–405. [PubMed] [Google Scholar]
- 26.Toler SM, Young AB, McClain CJ, Shedlofsky SI, Bandyopadhyay AM, Blouin RA. Head injury and cytochrome P450 enzymes. Differential effect on mRNA and protein expression in the Fischer-344 Rat . Drug Metab Disp. 1993;21:1064–1069. [PubMed] [Google Scholar]
- 27.Renton K. Alteration of drug biotransformation during the operation of host defense mechanisms in humans. In: Jeffrey EH, editor. Human drug metabolism: From molecular biology to Man. Boca Raton, FL: CRC Press; pp. 143–151. [Google Scholar]


